Abstract
Background and Aims:
The lack of a dedicated pain service catering to the postsurgical period has resulted in the origination of the pain–period gap. This has led to a resurgence of transitional pain service (TPS). Our objective was to evaluate the feasibility of TPS in pain practice among postsurgical cancer patients and its prevention of persistent postsurgical pain (PPSP), culminating in chronic pain catastrophising.
Methods:
The protocol for this meta-analysis was registered in the International Prospective Register of Systematic Reviews (ID: CRD42023407190). This systematic review included articles involving all adult cancer patients undergoing cancer-related surgery experiencing pain, involving pharmacological, non-pharmacological and interventional pain modalities after an initial systematic pain assessment by pain care providers across diverse clinical specialities, targeting multimodal integrative pain management. Meta-analysis with meta-regression was conducted to analyse the feasibility of TPS with individual subgroup analysis and its relation to pain-related patient outcomes.
Results:
Three hundred seventy-four articles were evaluated, of which 14 manuscripts were included in the meta-analysis. The lack of randomised controlled trials evaluating the efficacy of TPS in preventing PPSP and pain catastrophising led to the analysis of its feasibility by meta-regression. The estimate among study variances τ2 was determined and carried out along with multivariate subgroup analysis. A regression coefficient was attained to establish the correlation between the feasibility of TPS and its patient outcome measures and opioid-sparing.
Conclusion:
TPS interventions carried out by multidisciplinary teams incorporating bio-physical-psychological pain interventions have resulted in its successful implementation with improved pain-related patient outcomes mitigating the occurrence of PPSP.
Keywords: Acute pain service, chronic postsurgical pain, opioid-sparing, onco-anaesthesia, pain catastrophising, palliative care, persistent postsurgical pain, transitional pain service
INTRODUCTION
Most cancer centres have well-established acute pain services (APSs) and chronic pain services. The most vulnerable time patients experience postsurgical pain is after hospital discharge and while resuming their routine activities. Ironically, the transition between discharge from hospital to home and follow-up visits to hospital results in a pain gap. The pain gap could be attributable to the absence of a dedicated pain service, and the inability to address this gap has contributed to the evolution of persistent postsurgical pain (PPSP). The failure to recognise the pain and period gap has resulted in poor quality of life and physiological implications with adverse physical and psychological effects.[1,2] This is where the need for a dedicated transitional pain service (TPS) emerges.[2,3]
TPS strives to bridge the ‘pain gap’ and the ‘period gap’ (hospital care progressing to home care and transitioning back), providing a care continuum among postsurgical patients and modulating pain trajectories.[2,3,4,5,6,7] TPS has evolved as a new paradigm for preventing PPSP transformation by including multidisciplinary integrative pain modulation and intervention pathways utilising bio-psychosocial interventions.[4] This review aims to evaluate the feasibility of TPS in bridging the perioperative pain gap and prevention of PPSP after major cancer surgery.
METHODS
Protocol for the review was registered prospectively in the International Prospective Register of Systematic Reviews (PROSPERO) database (ID: CRD42023407190) reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement.[8] The analysis included patients undergoing pain-eliciting oncological treatment and surgical procedures. The TPS intervention is used to bridge perioperative analgesia and prevent PPSP. Standard-of-care cancer pain practice involving APSs and chronic pain clinics in the perioperative period was included for comparison.
Study design
This systematic review included randomised clinical trials and observational cohort studies. Relevant manuscripts about editorial reviews, letters to the editor and narrative review articles were not considered. Isolated case reports/series, institution protocols, educational media, non-indexed internet publications, abstract-only papers and studies on human volunteers were excluded.
All the manuscripts were evaluated in their full available version. Emphasis was placed upon extracting high-quality data and rigorous internal independent quality assessment utilising inter-rater reliability agreement between two authors regarding the risk of bias (ROB) based on the Kappa statistical table.[9,10]
Search strategy and data collection
The search strategies were defined to include title, abstract and full text published to include publications on TPS from January 2012 till March 2023, published in English about ‘transitional pain service’ with full text available for retrieval. The keywords included ‘TPS’, ‘TPS feasibility’, ‘PPSP’, ‘Pain catastrophising’ and ‘2012-2023’. Web-based tools Zotero 5.0 and Rayyan Qatar Computing Research Institute (QCRI) were utilised to conduct systematic reviews and compilations, and a comprehensive and robust exploration of the research topic was used along with a back-reference search[11,12] [Appendix 1]. Two independent authors interpreted and validated the data. The disagreements were resolved after discussion. In addition, the agreement was subjected to intraclass coefficient (ICC) (interobserver correlation by an ICC) and the ‘Cohen’s kappa’ value towards literature search with selection of primary studies for inclusion in the meta-analysis and inter-rater reliability was derived to be 0.79, which was considered to be ‘substantial agreement’.[9,10] Both authors agreed upon including 14 studies in a meta-analysis from among the 27 studies [Figure 1].
Figure 1.
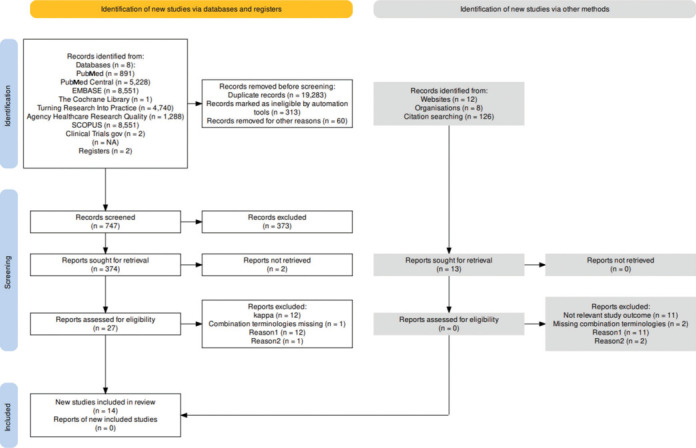
PRISMA flow chart deriving review synthesis. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Primary outcome
The primary outcome was to evaluate the feasibility of TPS in preventing PPSP after cancer surgery, thereby bridging both the pain and period gap in the context of an effective pain service.
Subgroup analysis and synthesis of secondary outcomes
A meta-analysis was performed to assess the feasibility of TPS in the context of PPSP. Employing the R open-source scripting software version 3.2.5 for statistical analysis (v3.2.5; RCore Team 2021), the effect size was realised through a forest plot with its associated confidence intervals (CIs). The meta-analysis protocol included integrating fixed and random effects models to account for the diversity and inconsistency (I2) embedded within the collective studies.[13] For heterogeneity among the studies,[14] the meta-regression and subgroup analysis was done to address the issue of heterogeneity.[15] Meta-regression was performed on the statistical software package using R open-source scripting software version 3.2.5 (The R Foundation, Vienna, Austria) to delineate the study characteristic with its intervention effect of TPS, and subgroup analysis was performed across various clinical settings involving diverse study populations.[16] Random effects analysis by meta-regression was principally employed as the number of studies was heterogeneous and very limited, as per the Cochrane guidelines for systematic intervention reviews.[17]
ROB was assessed by utilising the ROBVIS application tool (McGuiness LA 2019). Publication bias was assessed by a funnel plot, and its outliers were excluded from the final analysis.[18,19,20,21,22,23]
RESULTS
Database and hand search yielded 29,252 titles and abstracts [Figure 1]. Of these, 27 articles were eligible, and a full article review was independently conducted by two authors on these 27 articles. An assessment of inclusion was contemplated after the quality content of studies using the kappa index was assessed for agreement. Fourteen manuscripts qualified to be included in the meta-analysis based on randomised controlled trials (RCTs) or observational cohort studies evaluating the burden of PPSP and its prevention[24,25,26,27,28,29,30,31,32,33,34,35,36,37] [Table 1].
Table 1.
Qualitative synthesis of high-quality studies included in the systematic review
| Author | Population | Intervention | Comparator | Outcome | Study design | Results and conclusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admiraal et al.[24] (TRUSt) | 176 patients at risk for CPSP | TPS | Standard of care | Quality of recovery (primary) Opioid consumption |
RCT | Short-term outcomes are not affected Might improve long-term outcomes. Decreased opioid use |
||||||
| Liang et al.[25] | 95 patients with ankylosing spondylitis | Nurse-led multidisciplinary transitional care | Routine nursing care | Clinical outcomes (short form 36) and quality of life | RCT | Improved clinical outcomes and quality of life | ||||||
| Wang and Wu[26] | 156 patients undergoing cancer pain management | Transitional care model in cancer pain management | Standard care | Pain score Quality of life Patient satisfaction Adequacy of opioids |
RCT | Reduction in pain scores, higher satisfaction and quality of life and adequacy of opioids | ||||||
| Abid Azam et al.[27] | 382 patients undergoing multidisciplinary TPS to manage CPSP | ACT as part of multidisciplinary TPS | No ACT | Behavioural pain management and opioid consumption | RCT | ACT as part of TPS resulted in reduced opioid use, improved mood and pain interference/catastrophising | ||||||
| Featherall et al.[28] | 208 patients undergoing total joint arthroplasty | TPS | Historical control | Opioid use at 90 days (primary) Postoperative outcome scores and opioid consumption (secondary) |
RCT | TPS resulted in a reduction in opioid prescription consumption, leading to a reduction in persistent opioid use | ||||||
| Clarke et al.[29] | 251 high-risk TPS patients | TPS among opioid naïve | TPS among opioid experience | Opioid use, opioid weaning rate and pain management | POS | Successful opioid weaning in 50% of opioid naïve and 25% of opioid experienced | ||||||
| Hussain et al.[30] | 86 patients | Tele-TPS among opioid-naïve and exposed patients | Opioid tapering CBT achieving TPS efficacy on persistent opioid use and pain/behavioural outcomes | POS | 100% efficacy in opioid tapering among opioid naïve and in 52% among opioid exposed | |||||||
| Haynes et al.[31] | 31 paediatric patients | To evaluate the risk factors and clinical features of PPSP in a paediatric complex pain service after introduction of TPS | TPS-based intervention | ROS | TPS-based non-pharmacological strategies and conservative use of opioids by TPS are the best ways of preventing PPSP | |||||||
| Buys et al.[32] | Observational study among 336 veterans undergoing major joint surgery | To evaluate the reduction in opioid use by TPS | TPS reduced the onset of new chronic opioid use | ROS | Implementation of TPS resulted in opioid consumption and opioid weaning among preexisting opioid users | |||||||
| Buys et al.[33] | Observational study among 213 veterans undergoing orthopaedic surgery | To evaluate reduction in opioid usage by TPS among 72% opioid naïve | Evaluate opioid usage by TPS among 28% chronic opioid users | TPS as an emerging concept in perioperative surgical home concept | ROS | Multidisciplinary TPS for veteran population decreased by 40% without affecting the pain intensity and physical function | ||||||
| Huang et al.[34] | Single-centre, observational cohort study on 200 APS patients by telephonic interview | To evaluate the incidence of PPSP and persistent opioid use utilising the pain disability index, brief pain inventory and health outcome questionnaire- EuroQol 5 Dimension 5 Level (EQ-5D-5L) | APS–TPS combination to evaluate opioid usage | Postoperative opioid use is associated with lower mood and functional interference, leading to pain-related daily life disability | POS | Utility of TPS in modifying pain trajectories and effective opioid weaning | ||||||
| Montbriand et al.[35] | Retrospective study of 239 patients | Association of smoking status and pain along with opioid use | Non-smokers | Higher pain intensities and opioid consumption among smokers are associated with higher pack-years | ROS | TPS-initiated smoking cessation as a modifiable risk for opioid use after surgery | ||||||
| Liu et al.[36] | Prospective cohort study among 279 patients undergoing thoracic surgery | To evaluate pain trajectories among elective thoracic surgery patients until 1 year after surgery | Regional anaesthesia techniques and psychological assessed interventions for reducing pain catastrophising | Pain-related outcomes and complications among three subgroup pain trajectories constituted as mild or moderate and associated with pain catastrophising | POS | Higher preoperative pain catastrophising and occurrence of immediate postoperative pain progress to severe CPSP | ||||||
| Yu et al.[37] | TPS retrospective cohort study among 140 patients undergoing solid organ transplant surgery | Opioid consumption, pain catastrophising and psychological attributes evaluated | TPS in transplantation surgery evaluated | Association between opioid consumption, psychological characteristics and pain incorporating psychology and physiotherapy | ROS | Treatment by the multidisciplinary TPS team was associated with significant improvement in pain severity and a reduction in opioid consumption |
ACT=acceptance and commitment therapy, APS=acute pain service, CBT=cognitive behavioural therapy, CPSP=chronic postsurgical pain, POS=observational studies based on prospective cohort population, PPSP=persistent postsurgical pain, RCT=randomised controlled trial, ROS=observational studies based on mixed cohort population, TPS=transitional pain service
Using the meta-regression approach, a subgroup analysis was performed. Variables such as geographical diversity, sample size, diagnostic test utility and quality scores related to its inherent potential bias were scrutinised using meta-regression to quantify the extent and magnitude of heterogeneity within the dataset [Table 2]. The findings of this meta-regression analysis underscored the influential role of specific covariates in the observed study heterogeneity. These covariates included the size of the sample (regression coefficient for events: Qm = 128.51, P < 0.001), the techniques employed for detection (tests; Qm = 118.68, P < 0.001), the classification of study species (Qm = 11.79, P < 0.001) and countries in which the studies were conducted (Qm = 138.83, P < 0.001) (Qm represents meta-regression as a measure of the overall fit of the technologies used) [Table 2].
Table 2.
Meta-regression of factors of TPS studies: Investigating heterogeneity and effect sizes
| Group | Particulars | SE | Z | Estimated (95% CI) | Qm | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | High | 0.15 | 8.71 | 1.23 (0.99, 1.57) | 128.51 | <0.001 | ||||||
| Low | 0.15 | 7.26 | 1.08 (0.78, 1.37) | |||||||||
| Detection techniques | Observational Study (P) | 0.20 | 5.43 | 1.10 (0.70, 1.50) | 118.68 | <0.001 | ||||||
| RCT | 0.18 | 7.28 | 1.33 (0.97, 1.68) | |||||||||
| Observational study® | 0.18 | 6.01 | 1.10 (0.73, 1.45) | |||||||||
| Category of species | (ACT- TPS) | 0.43 | 3.68 | 1.57 (0.73, 2.40) | 11.79 | <0.001 | ||||||
| (APS- TPS) | 0.43 | 3.67 | 1.57 (0.73, 2.40) | |||||||||
| Pain catastrophising and psychological interventions | 0.43 | 2.74 | 1.17 (0.33, 2.01) | |||||||||
| Tele-TPS on opioid naive | 0.43 | 1.84 | 0.79 (-0.04, 1.62) | |||||||||
| TPS on opioid naive | 0.43 | 2.10 | 0.89 (0.05, 1.73) | <0.001 | ||||||||
| TPS-based weaning | 0.15 | 7.42 | 1.12 (0.82, 1.41) | |||||||||
| Transitional care nurse based | 0.43 | 3.67 | 1.57 (0.73, 2.40) | |||||||||
| Country | Australia | 0.38 | 1.29 | 0.49 (-0.25, 1.24) | 138.83 | <0.001 | ||||||
| Canada | 0.16 | 7.51 | 1.17 (0.86, 1.48) | |||||||||
| China | 0.27 | 4.89 | 1.33 (0.79, 1.86) | |||||||||
| Veteran USA | 0.38 | 4.09 | 1.57 (0.81, 2.32) | |||||||||
| The Netherlands | 0.38 | 2.21 | 0.84 (0.09, 1.59) | |||||||||
| USA | 0.22 | 5.92 | 1.31 (0.87, 1.74) |
ACT=acceptance and commitment therapy, APS=acute pain service, CI=confidence interval, SE=standard error, TPS=transitional pain service
A regression coefficient was attained to establish the correlation between the feasibility of TPS and its outcome measures on patient satisfaction and opioid consumption, enabling us to find whether a linear relationship was demonstrable. The estimate among study variances τ2 was determined using the most extreme probability assessment [Figure 2]. Effect size (sample size) was regressed against the moderator variable. Several moderators were considered, including the diagnostic assay, geographical region, year of publication and relative sample size while performing univariate meta-regression analysis. While transitioning to the multivariable meta-regression phase, only those variables that demonstrated a P value below 0.05 in the univariate analysis were retained [Table 2]. The estimate among study variances τ2 was determined, and the P value from each regression coefficient was further analysed to find differences among subgroups from TPS intervention. The final model included factors that exhibited statistical significance (P value threshold of ≤0.05) [Table 3].
Figure 2.
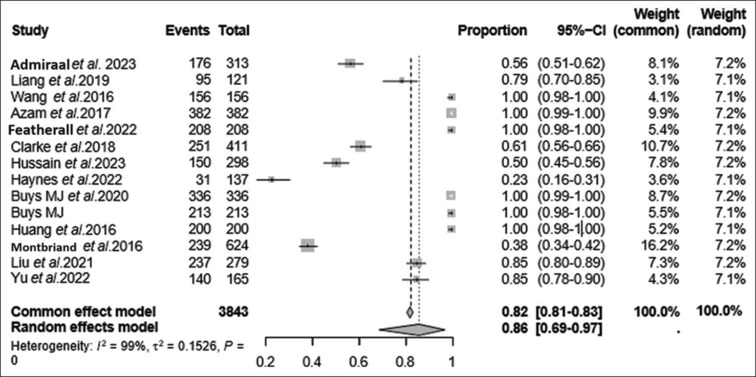
Forest plot for the studies included. The visual representation of the meta-analysis findings was accomplished through forest plots. These plots depict each study’s effect size and corresponding CIs. Within these plots, each study is portrayed as a square, indicating the point estimate of the effect size. In addition, extending from the square is a horizontal line that represents the 95% CI. Each square’s size indicates the study’s weight within the broader meta-analysis context. The diamond represents heterogeneity, and its increasing width depicts increased heterogeneity. The outcome evaluated was the feasibility of TPS, estimated at 86% (0.86 proportion depicted on the random effects model). CI = confidence interval, TPS = transitional pain service
Table 3.
Subgroup analysis stratification pattern: Analysis by consideration of various factors and variations taken into consideration
| Group | Subgroup | I2% | T2% | P | Total no. of studies | Total no. of samples | Feasibility (%) | 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | High | 99 | 0.16 | <0.01 | 7 | 1390 | 77 | (0.99, 0.96) | ||||||||
| Low | 100 | 0.14 | <0.01 | 7 | 2453 | 92 | (0.71, 1.00) | |||||||||
| Detection techniques | POS | 99 | 0.12 | <0.01 | 4 | 1188 | 80 | (0.48, 0.98) | ||||||||
| RCT | 99 | 0.12 | 0 | 5 | 1180 | 99 | (0.74, 1.00) | |||||||||
| Other observational studies (ROS) | 100 | 0.25 | <0.01 | 5 | 1475 | 79 | (0.37, 1.00) | |||||||||
| Species | Subspecific TPS | 100 | 0.18 | 0 | 9 | 2499 | 85 | (0.61, 0.69) | ||||||||
| Country | Canada | 100 | 0.13 | <0.01 | 6 | 2061 | 85 | (0.60, 0.99) | ||||||||
| China | 98 | 0.11 | <0.01 | 2 | 277 | 94 | (0.57, 1.00) | |||||||||
| USA | 100 | 0.20 | <0.01 | 3 | 842 | 93 | (0.51, 1.00) |
CI=confidence interval, POS=observational studies based on prospective cohort population, RCT=randomised controlled trial, ROS=observational studies based on mixed cohort population, TPS=transitional pain service
Risk of bias and publication bias
RCTs were evaluated utilising the ROB2 tool, and observational studies using the ROBINS-E tool [Figures 3 and 4]. The funnel plot reveals asymmetry attributed to publication bias, potentially arising from the variability that causes smaller studies to report effects that notably deviate from larger ones [Figure 5]. Among the research articles, the majority were dispersed outside the funnel, with only a few falling within it, indicating the presence of publication bias. To address the potential ramifications of publication bias, we employed meta-regression, integrating sample size as a parameter for assessing ROB. The analysis yielded outcomes that were not statistically significant (P < 0.05), thus mitigating the impact of publication bias on the study’s conclusions. The outcome indicated non-significance (P > 0.05), nullifying publication bias's impact within the study.
Figure 3.
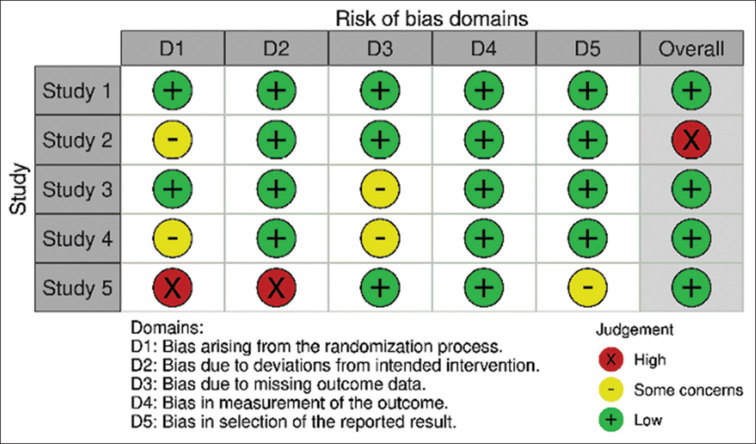
Risk of bias (ROB2) for randomised controlled studies. Risk of bias domains (ROB2) represented on the X-axis; and randomised controlled studies included in the meta-analysis represented on the Y-axis
Table 4.
Interventions in TPS
| Non-pharmacological | Pharmacological | Pain interventions | ||
|---|---|---|---|---|
| Physical therapy | Tricyclic antidepressants | Head and neck | ||
| Acupuncture, Acupressure | Amitriptyline | Cervical plexus block, TMJ injection | ||
| Myofascial trigger | SNRI | Buccal infiltration, dental intraligamental injection, inferior alveolar nerve | ||
| TENS | Venlafaxine, duloxetine | Infraorbital, mandibular nerve, suprazygomatic maxillary nerve blocks | ||
| Whole body exercise (walking, cycling) | Antiepileptics | Breast | ||
| Yoga | Levetiracetam | Thoracic paravertebral block (radiofrequency ablation, steroids) | ||
| Resistance training | Gabapentinoids | Intercostobrachial nerve, pectoralis-II, serratus anterior plane | ||
| Targeted functional exercises (shoulder exercise) | Gabapentin, pregabalin | Proximal intercostal, erector spinae blocks | ||
| Laser therapy | NMDA antagonist | Thoracic | ||
| Magnetic stimulation | Low-dose ketamine, magnesium, memantine, nitrous oxide | Thoracic epidural, thoracic paravertebral, erector spinae plane | ||
| Opioids | Intercostal, serratus anterior plane blocks | |||
| Psychological therapy | Oxycodone, tramadol, tapentadol, morphine, fentanyl patch | Upper abdomen | ||
| CBT | Opioid substitutes | Thoracic epidural, thoracic paravertebral | ||
| ACT | Buprenorphine, buprenorphine- naloxone, methadone, cannabis | Erector spinae plane, subcostal transversus abdominis plane | ||
| Pain neuroscience education | Steroids | Quadratus lumborum, rectus sheath blocks | ||
| MBI | Dexamethasone, depot methylprednisolone | Abdominopelvic | ||
| Dialectical behavioural therapy | NSAIDs | Lower thoracic/lumbar epidural, paravertebral block | ||
| Desensitisation | Randomised | Transversus abdominis plane, quadratus lumborum | ||
| Sensory discrimination training | Alpha-2-agonist | Ilioinguinal, fascia iliaca, rectus sheath blocks | ||
| Guided imagery | Clonidine, dexmedetomidine | Miscellaneous | ||
| Music therapy | Local anaesthetics | Sympathetic-mediated blocks, epidural steroid, spinal cord stimulation | ||
| Relaxation techniques | Intravenous lignocaine, liposomal bupivacaine | Intra-articular local anaesthetic, continuous wound infiltration devices | ||
| Clinically induced hypnosis | Topical | Subcutaneous infusion pumps, transdermal drug delivery and subperiosteal catheters | ||
| Capsaicin, prilocaine, eutectic mixture |
ACT=acceptance and commitment therapy, CBT=cognitive behavioural therapy, MBI=mindfulness-based intervention, NMDA=N-methyl-d-aspartate, NSAIDs=nonsteroidal anti-inflammatory drugs, SNRI=serotonin–norepinephrine reuptake inhibitor, TENS=transcutaneous electrical nerve stimulation, TMJ=temporomandibular joint, TPS=transitional pain service
Figure 5.
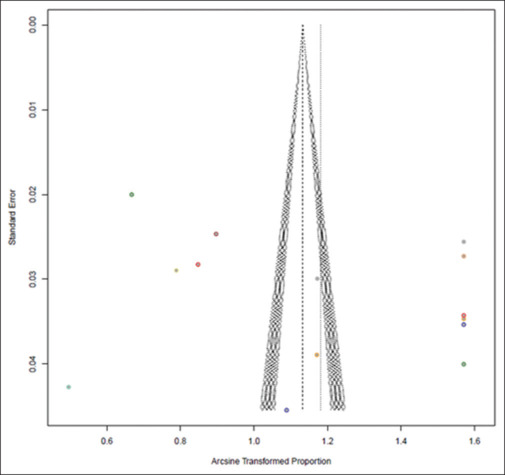
Funnel plot representing publication bias among the included studies. The X-axis is represented by the proportion of the arcsine transformation of the study fraction, and the standard error represents the Y-axis. Symmetry of funnel plot is established. The outliers in the study are represented in dotted colour, and their associated asymmetry is depicted, contributing to the heterogeneity of studies
Figure 4.
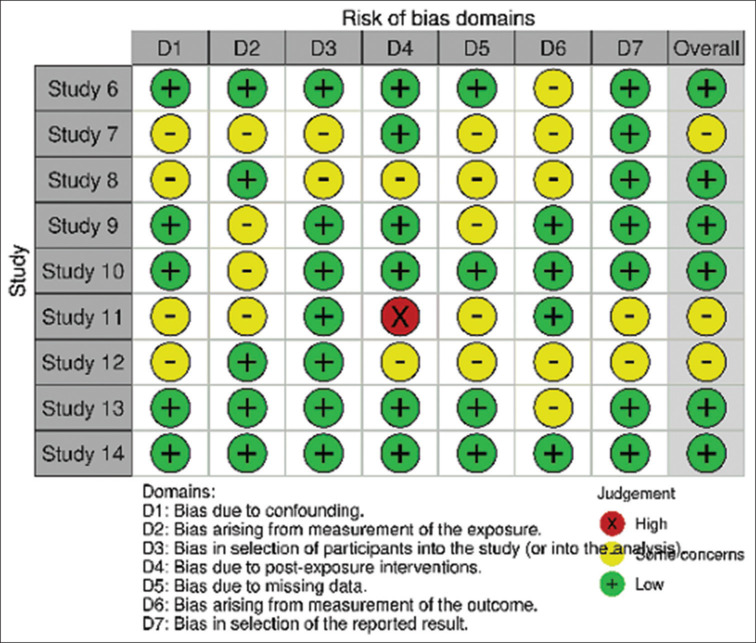
Risk of bias (ROBINS) for observational studies. Risk of bias domains (ROBINS) are represented on the X-axis; and observational studies included in the meta-analysis represented on the Y-axis
The stratification of sample sizes revealed notable disparities in feasibility rates of TPS pain-related interventions among patients. Studies falling below the median sample size reported a higher percentage of 92% (95% CI: 71%, 100%, I2 = 100, τ2 = 0.14, P < 0.01), while those exceeding the median sample size exhibited a lower feasibility percentage of 77% (95% CI: 99%, 96%, I2 = 99, τ2 = 0.16, P < 0.01) [Tables 2, 3 and Figure 6].
Figure 6.
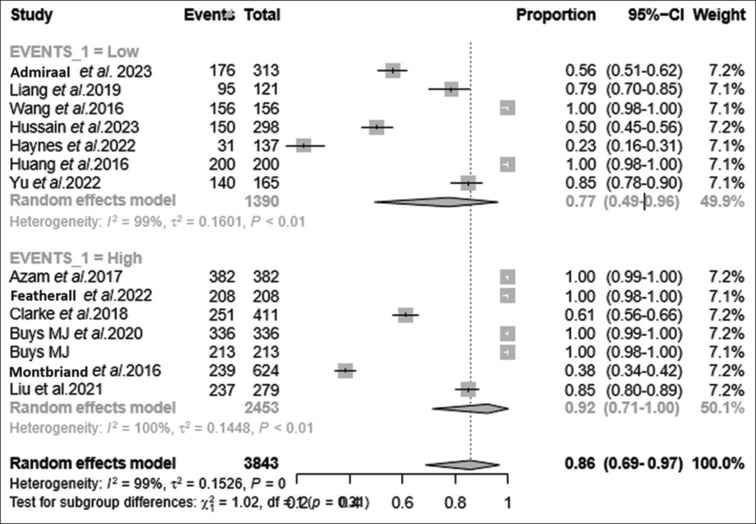
Subgroup analysis based on forest plot for event occurrence. Forest plot portraying subgrouping of sample size based on the events (high-occurrence vs. low-occurrence events) among the studies evaluated. TPS feasibility was estimated to be 77% among studies with low occurrence of events (PPSP, PCS), whereas the feasibility attained 92% among studies with high occurrence of events (PPSP, PCS). The overall feasibility of TPS was 86% on the random effects model. PCS = pain catastrophising, PPSP = persistent postsurgical pain, TPS = transitional pain service
Subgroup analysis after meta-regression
Subgroup analysis helps identify potential effect modifiers or factors influencing outcomes due to TPS, resulting in the development of tailored interventions or treatment strategies [Table 3 and Figures 6, 7]. The RCT, although showing a high effectiveness feasibility rate with 99% (95% CI: 74%, 100%, I2 = 99, τ2 = 0.12, P < 0.01), needed further investigation towards refinement in its study design. The observational studies involving prospective cohorts showed 80% feasibility efficacy (95% CI: 48%, 98%, I2 = 99, τ2 = 0.12, P < 0.001), a substantial effectiveness rate, signifying their potential utility in identifying patients who may benefit from TPS interventions. We also analysed observational studies with random mixed cohort, which showed 79% efficacy in feasibility (95% CI: 37%, 100%, I2 = 100, τ2 = 0.25, P < 0.001), again a notable effectiveness rate, also providing valuable insights into early identification of pain catastrophising within TPS feasibility studies [Table 3, Figure 7]. This refinement becomes particularly crucial when considering various countries, varied diagnostic test methodologies and distinct categorisations of study species. Focusing on subspecific TPS groups within TPS feasibility studies revealed a percentage of 85% (95% CI: 61%, 69%, I2 = 100, τ2 = 0.18, P = 0). Its high effectiveness rate emphasises the relevance and applicability of TPS interventions in addressing pain-related challenges among subspecific TPS [Table 3 and Figures 6, 7]. However, the subspeciality and subspecific TPS domains did not achieve significance with the P value. This finding highlights the need for patient-centred approaches and the customisation of TPS strategies to suit individual patient needs and preferences.
Figure 7.
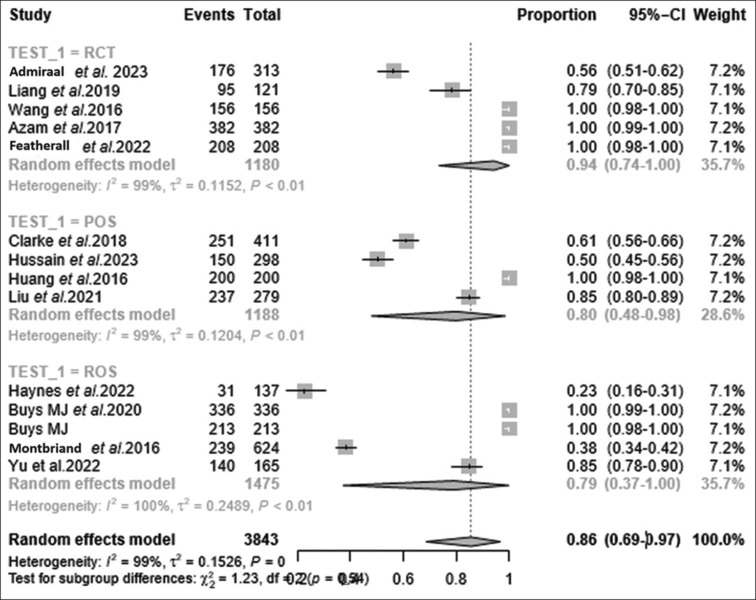
Subgroup analysis based on forest plot portraying subgrouping by detection techniques based on the study type involved. Random effects model depicting efficacy proportions for each study subtype involved. Feasibility of TPS was estimated among studies involving RCT at 94%, POS at 80% and ROS at 79%. CI = confidence interval, POS = observational studies based on prospective cohort population, RCT = randomised controlled trial, ROS = observational studies based on mixed cohort population, TPS = transitional pain service
DISCUSSION
We evaluated the feasibility of TPS and its efficacy in preventing PPSP and achieving opioid sparing during its process.
It is catastrophic that 5%–60% of postoperative patients bear the burden of PPSP across various surgeries, leading to debilitated recovery.[38] The median prevalence of PPSP is 20% in the paediatric population undergoing surgery.[39] PPSP contributes up to 25% workload of pain clinics, which could instead be diverted to TPS. The prevalence of PPSP is extremely variable (3%–85%) across multiple studies, with an incidence of about 10%.[2,40,41] Amputation (85%), thoracotomy (65%), craniotomy (65%), hernia (63%), mastectomy (57%), spine surgery (56.5%) and joint replacement (48.7%) reported the highest prevalence of PPSP.[4,42,43] PPSP has been linked with higher preoperative pain scores, lower pro-nociceptive conditioned pain modulation and enhanced temporal pain summation.[3,41,44] The prevalence of persistent pain after breast cancer treatment (surgery, hormonal, immuno-chemo-radiation) was reported to be 21.8% among breast cancer survivors, leading to a negative impact on recovery, quality of life, functional limitation and psychological distress.[1,44,45,46,47,48,49] Persistent pain following cancer surgery always needs to be differentiated from the possibility of a local recurrence. It is important to note that a high pain catastrophising score has been considered an independent risk factor for PPSP.[4,5,33,41,44,46,50]
Qualitative pain-related patient outcomes in the form of quality of recovery, patient satisfaction, quality of life, early return to intended oncological treatment (RIOT), evaluating return to baseline activities of daily routine and patient disability interference need to be considered as TPS quality indicators. Pain-psychological interventions and coping strategies by TPS prevent PPSP, with an emphasis on perioperative opioid-sparing strategies and opioid de-escalation in substance use [Table 4]. The intervention of TPS in the causation of heterogeneous outcome effects like improved pain-related patient outcomes and achieving opioid sparing, as well as considering multiple explanatory variable factors in its causation, like pain catastrophising and antecedent clinical predispositions, were analysed.
The stratification of sample sizes revealed notable disparities in the feasibility rates of TPS pain-related interventions among patients. Studies falling below the median sample size reported a higher percentage of 92%, while those exceeding the median sample size exhibited a lower feasibility percentage of 77%. The high events subgroup, with a significant effectiveness rate and a narrow CI, suggests a substantial need for TPS interventions among these patients. Conversely, despite a higher effectiveness rate, the low events subgroup indicated a wider range of effectiveness, possibly influenced by patient characteristics and treatment modalities. This variation underscores the need for tailored TPS interventions based on the severity and nature of patients’ individualised perioperative pain experiences.
Effective TPS workflow [Figure 8] implementation begins during a preoperative visit, during which patient education, multimodal prehabilitation and pain coping skills are imparted. APSs involve intraoperative regional anaesthesia techniques and the adaptation of enhanced recovery after surgery protocols, thereby facilitating early RIOT. Intensive physical therapy involving progressive resistance training, functional aerobic exercise and psychological interventions by combined APS–TPS further enhance recovery. The goal of the combined APS–TPS is to recognise acute postoperative pain persisting beyond the conventional tissue healing duration, predisposing further to PPSP and chronic pain if not intervened.[2,4]
Figure 8.
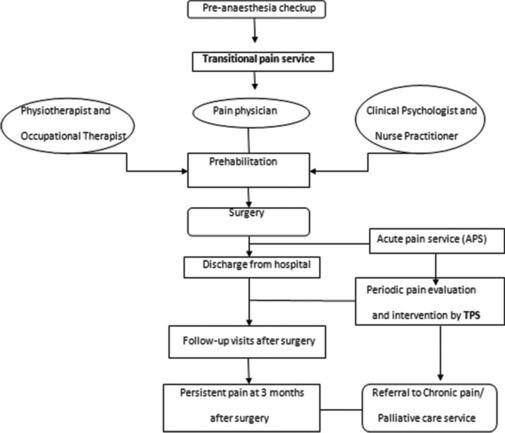
Workflow in TPS. TPS = transitional pain service
Patients on preexisting opioids presenting for surgery need titrated dose optimisation, opioid alternatives, behavioural counselling and pain coping strategies towards achieving meaningful opioid weaning.[3,5] TPS attributes the highest potential to de-escalate opioids even in complex postsurgical pain, which offers the critical window to de-escalate opioids by regional anaesthesia and non-opioid analgesic strategies. The surrogate goal of TPS would strive to prevent persistent opioid usage and mitigate opioid crisis.[5,29,32,34,51,52,53] Introduction of TPS results in overall opioid prescription reduction from 27.3% to 13.4% among both opioid-naïve and chronic opioid users.[32] Tele-TPS reduces frequent hospital visits and possesses immense future potential in cancer pain management.[2,4,5,54] Cognitive behavioural therapies, mindfulness-based interventions, mind–body exercises for stress reduction and acceptance commitment therapy (ACT) have revolutionised TPS and improved the quality of life.[29,33,34,41,52,53,54,55,56,57,58] ACT incorporates acceptance and committed action towards achieving a value-based goal by imparting pain education, pain coping skills and mindful acceptance towards the pain experience.[1,5,47,52,59,60,61] Pharmacological therapy and interventions in continuous wound infiltration, regional anaesthetic blocks, central neuraxial block, spinal cord stimulation, fascial plane blocks, targeted nerve blocks, ganglion and sympathetic blocks have all added new dimensions for pain intervention in TPS [Table 4].[1,3,5,44,62,63,64,65]
Strength and limitations
The strength of our study was evaluating the feasibility of establishing TPS. The incorporation of an independent TPS team augurs well for bridging the pain gap after cancer surgery, involving both APSs and chronic pain services. However, the review has limitations in that the administrative/economic/financial/managerial/human resource allocation and functional logistics of the institution or hospital were not considered in establishing dedicated TPS. An already overburdened pain department would be required to double up to establish TPS. Our study could not perform a sensitivity analysis, which accounts for its limitation. Further RCTs and future meta-analyses are needed to establish whether the inculcation of TPS would positively impact reduction in PPSP upon opioid sparing and by what quantitative/qualitative extent of the effect.
CONCLUSION
TPS involves individualised preoperative pain evaluation, identification of pain catastrophising, implementation of pain education, and imparting multimodal prehabilitation and early pain coping interventions to modify pain trajectory perioperatively. The feasibility of TPS has been established with meta-regression analysis by stratification of median sample sizes, with feasibility rates ranging from 77% up to 92%, achieving clinical significance for establishing a dedicated TPS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX 1
Literature search
Keywords in Mesh terminology included population-based (persistent postsurgical pain after cancer surgery), ‘cancer surgery pain’, ‘perioperative cancer pain’, ‘perioperative cancer pain intervention’, ‘persistent postsurgical pain’, ‘pain catastrophising’, ‘pain disability interference’; publication-based (transitional pain service) ‘transitional pain service’, ‘establishing dedicated transitional pain services’, ‘feasibility of transitional pain service’, ‘integrated transitional pain services’, ‘acute pain service and transitional pain service’, ‘chronic pain and transitional pain service’, ‘oncoanaesthesia and transitional pain service’, ‘pain practice and transitional pain service’; and combination terms ‘persistent postsurgical pain and transitional pain service’, ‘perioperative cancer surgery and pain catastrophising’, ‘transitional pain service and bridging pain gap in oncoanaesthesia’. Additional sources were obtained by searching the bibliography of included references.
REFERENCES
- 1.Khan JS, Ladha KS, Abdallah F, Clarke H. Treating persistent pain after breast cancer surgery. Drugs. 2020;80:23–31. doi: 10.1007/s40265-019-01227-5. [DOI] [PubMed] [Google Scholar]
- 2.Admiraal M, Hermanides J, Meinsma SL, Wartenberg HCH, Rutten MVH, Ward-van der Stam VMC, et al. Current multidisciplinary approaches to preventing chronic postoperative pain. Br J Anaesth. 2021;127:331–5. doi: 10.1016/j.bja.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Steyaert A, Lavand’homme P. Prevention and treatment of chronic postsurgical pain: A narrative review. Drugs. 2018;78:339–54. doi: 10.1007/s40265-018-0866-x. [DOI] [PubMed] [Google Scholar]
- 4.Glare P, Aubrey KR, Myles PS. The transition from acute to chronic pain after surgery. Lancet. 2019;393:1537–46. doi: 10.1016/S0140-6736(19)30352-6. [DOI] [PubMed] [Google Scholar]
- 5.Mikhaeil J, Ayoo K, Clarke H, Wąsowicz M, Huang A. Review of the transitional pain service as a method of postoperative opioid weaning and a service aimed at minimizing the risk of chronic postsurgical pain. Anaesthesiol Intensive Ther. 2020;52:148–53. doi: 10.5114/ait.2020.96018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz J, Weinrib A, Fashler SR, Katznelzon R, Shah BR, Ladak SS, et al. The Toronto General Hospital Transitional Pain Service: Development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695–702. doi: 10.2147/JPR.S91924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke H, Weinrib A, Kotteeswaran Y, Katz J, Yu A, Tanguay R. Remote buprenorphine-naloxone initiation as an essential service for people with chronic pain and opioid dependence during the COVID-19 pandemic: Case reports, clinical pathways, and implications for the future. Can J Pain. 2020;4:224–35. doi: 10.1080/24740527.2020.1795634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallgren KA. Computing inter-rater reliability for observational data: An overview and tutorial. tutor quant methods psychol. 2012;8:23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Gao Q, Yu T. Kappa statistic considerations in evaluating inter-rater reliability between two raters: Which, when and context matters. BMC Cancer. 2023;23:799. doi: 10.1186/s12885-023-11325-z. doi: 10.1186/s12885-023-11325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guimarães NS, Ferreira AJF, Ribeiro Silva RC, de Paula AA, Lisboa CS, Magno L, et al. Deduplicating records in systematic reviews: There are free, accurate, automated ways to do so. J Clin Epidemiol. 2022;152:110–5. doi: 10.1016/j.jclinepi.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 12.McKeown S, Mir ZM. Considerations for conducting systematic reviews: Evaluating the performance of different methods for de-duplicating references. Syst Rev. 2021;10:38. doi: 10.1186/s13643-021-01583-y. doi: 10.1186/s13643-021-01583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley TD, Doucouliagos H. Neither fixed nor random: Weighted least squares meta-regression. Res Synth Methods. 2017;8:19–42. doi: 10.1002/jrsm.1211. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI. Health Outcomes, Policy, and Economics (HOPE) Collaborative Group. Understanding heterogeneity in meta-analysis: The role of meta-regression. Int J Clin Pract. 2009;63:1426–34. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 16.Geissbühler M, Hincapié CA, Aghlmandi S, Zwahlen M, Jüni P, da Costa BR. Most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: A meta-epidemiological study. BMC Med Res Methodol. 2021;21:123. doi: 10.1186/s12874-021-01310-0. doi: 10.1186/s12874-021-01310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: A revised tool for assessing the risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Bero L, Chartres N, Diong J, Fabbri A, Ghersi D, Lam J, et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: Concerns arising from application to observational studies of exposures. Syst Rev. 2018;7:242. doi: 10.1186/s13643-018-0915-2. doi: 10.1186/s13643-018-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–94. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwetsloot PP, Van Der Naald M, Sena ES, Howells DW, IntHout J, De Groot JA, et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. Elife. 2017;6:e24260. doi: 10.7554/eLife.24260. doi: 10.7554/eLife.24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 24.Admiraal M, Hermanides J, Meinsma SL, Wartenberg HCH, Rutten MVH, Heine Y, et al. The effectiveness of a transitional pain service in patients undergoing surgery with an increased risk of developing chronic postsurgical pain (TRUSt study). A randomized clinical trial. J Clin Anesth. 2023;91:111262. doi: 10.1016/j.jclinane.2023.111262. doi: 10.1016/j.jclinane.2023.111262. [DOI] [PubMed] [Google Scholar]
- 25.Liang L, Pan Y, Wu D, Pang Y, Xie Y, Fang H. Effects of multidisciplinary team-based nurse-led transitional care on clinical outcomes and quality of life in patients with ankylosing spondylitis. Asian Nurs Res (Korean Soc Nurs Sci) 2019;13:107–14. doi: 10.1016/j.anr.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Wu X-C. Application of transitional care model in cancer pain management after discharge: A randomized controlled trial. Chin Nurs Res. 2016;3:86–9. [Google Scholar]
- 27.Abid Azam M, Weinrib A Z, Montbriand J, Burns L C, McMillan K, Clarke H, et al. Acceptance and commitment therapy to manage pain and opioid use after major surgery: Preliminary outcomes from the Toronto general hospital Transitional pain service. Can J Pain. 2017;1:37–49. doi: 10.1080/24740527.2017.1325317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Featherall J, Anderson JT, Anderson LA, Bayless K, Anderson Z, Brooke BS, et al. A multidisciplinary transitional pain management program is associated with reduced opioid dependence after primary total joint arthroplasty. J Arthroplasty. 2022;37:1048–53. doi: 10.1016/j.arth.2022.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Clarke H, Azargive S, Montbriand J, Nicholls J, Sutherland A, Valeeva L, et al. Opioid weaning and pain management in postsurgical patients at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2018;2:236–47. doi: 10.1080/24740527.2018.1501669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain M, Norgeot B, Zaafran A, Stark J, Caridi J, Fenoy A, et al. Virtual transitional pain service delivered via telehealth is effective in preventing new and persistent opioid use amongst postsurgical spine patients. medRxiv. 2023 2023.08.18.23294272. doi: 10.1101/2023.08.18.23294272. [Google Scholar]
- 31.Haynes N, Mclean C, Collins J, de Lima J. Persistent postoperative pain in children - An argument for a transitional pain service in pediatrics. Pain Manag Nurs. 2022;23:784–90. doi: 10.1016/j.pmn.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Buys MJ, Bayless K, Romesser J, Anderson Z, Patel S, Zhang C, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45:847–52. doi: 10.1136/rapm-2020-101797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buys MJ, Bayless K, Romesser J, Anderson Z, Patel S, Zhang C, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37:472–8. doi: 10.12788/fp.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang A, Azam A, Segal S, Pivovarov K, Katznelson G, Ladak SS, et al. Chronic postsurgical pain and persistent opioid use following surgery: The need for a transitional pain service. Pain Manag. 2016;6:435–43. doi: 10.2217/pmt-2016-0004. [DOI] [PubMed] [Google Scholar]
- 35.Montbriand JJ, Weinrib AZ, Azam MA, Ladak SSJ, Shah BR, Jiang J, et al. Smoking, pain intensity, and opioid consumption 1-3 months after major surgery: A retrospective study in a hospital-based transitional pain service. Nicotine Tob Res. 2018;20:1144–51. doi: 10.1093/ntr/ntx094. [DOI] [PubMed] [Google Scholar]
- 36.Liu C W, Page M G, Weinrib A, Wong D, Huang A, McRae K, et al. Predictors of one year chronic postsurgical pain trajectories following thoracic surgery. J Anesth. 2021;35:505–14. doi: 10.1007/s00540-021-02943-7. [DOI] [PubMed] [Google Scholar]
- 37.Yu H C, Kleiman V, Kojic K, Slepian PM, Cortes H, McRae K, et al. Prevention and management of chronic postsurgical pain and persistent opioid use following solid organ transplantation: Experiences from the Toronto General Hospital Transitional Pain Service. Transplantation. 2023;107:1398–405. doi: 10.1097/TP.0000000000004441. [DOI] [PubMed] [Google Scholar]
- 38.Levy N, Mills P, Rockett M. Postsurgical pain management: Time for a paradigm shift. Br J Anaesth. 2019;123:e182–6. doi: 10.1016/j.bja.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams G, Howard RF, Liossi C. Persistent postsurgical pain in children and young people: Prediction, prevention, and management. Pain Rep. 2017;2:e616. doi: 10.1097/PR9.0000000000000616. doi: 10.1097/PR9.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geil D, Thomas C, Zimmer A, Meissner W. Chronified pain following operative procedures. Dtsch Arztebl Int. 2019;116:261–6. doi: 10.3238/arztebl.2019.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulur P, Nelli A. Persistent postoperative pain: Mechanisms and modulators. Curr Opin Anaesthesiol. 2019;32:668–73. doi: 10.1097/ACO.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 42.Simanski CJ, Althaus A, Hoederath S, Kreutz KW, Hoederath P, Lefering R, et al. Incidence of chronic postsurgical pain (CPSP) after general surgery. Pain Med. 2014;15:1222–9. doi: 10.1111/pme.12434. [DOI] [PubMed] [Google Scholar]
- 43.Laufenberg-Feldmann R, Kappis B, Mauff S, Schmidtmann I, Ferner M. Prevalence of pain 6 months after surgery: A prospective observational study. BMC Anesthesiol. 2016;16:91. doi: 10.1186/s12871-016-0261-7. doi: 10.1186/s12871-016-0261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain: Pathophysiology and preventative pharmacologic considerations. Anesthesiology. 2018;129:590–07. doi: 10.1097/ALN.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 45.Wang K, Yee C, Tam S, Drost L, Chan S, Zaki P, et al. Prevalence of pain in patients with breast cancer post-treatment: A systematic review. Breast. 2018;42:113–27. doi: 10.1016/j.breast.2018.08.105. [DOI] [PubMed] [Google Scholar]
- 46.Habib AS, Kertai MD, Cooter M, Greenup RA, Hwang S. Risk factors for severe acute pain and persistent pain after surgery for breast cancer: A prospective observational study. Reg Anesth Pain Med. 2019;44:192–9. doi: 10.1136/rapm-2018-000040. [DOI] [PubMed] [Google Scholar]
- 47.Osypiuk K, Ligibel J, Giobbie-Hurder A, Vergara-Diaz G, Bonato P, Quinn R, et al. Qigong mind-body exercise as a biopsychosocial therapy for persistent postsurgical pain in breast cancer: A pilot study. Integr Cancer Ther. 2020;19:1534735419893766. doi: 10.1177/1534735419893766. doi: 10.1177/1534735419893766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K, Moon DH, Amdur RJ, Dagan R, Sheets NC, Shen CJ, et al. Shoulder symptoms and quality of life impact of limited neck dissection after de-intensified chemo-radiotherapy: Secondary analysis of two prospective trials. Head Neck. 2019;41:1213–19. doi: 10.1002/hed.25535. [DOI] [PubMed] [Google Scholar]
- 49.Ammitzbøll G, Andersen KG, Bidstrup PE, Johansen C, Lanng C, Kroman N, et al. Effect of progressive resistance training on persistent pain after axillary dissection in breast cancer: A randomized controlled trial. Breast Cancer Res Treat. 2020;179:173–83. doi: 10.1007/s10549-019-05461-z. [DOI] [PubMed] [Google Scholar]
- 50.Kojic K, Clarke H. Important considerations with respect to reducing the transition from acute to persistent postoperative pain. Expert Opin Pharmacother. 2021;22:779–82. doi: 10.1080/14656566.2021.1892073. [DOI] [PubMed] [Google Scholar]
- 51.Clarke H, Soneji N, Ko D T, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: Population based cohort study. BMJ. 2014;348:g1251. doi: 10.1136/bmj.g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinrib AZ, Burns LC, Mu A, Azam MA, Ladak SS, McRae K, et al. A case report on the treatment of complex chronic pain and opioid dependence by a multidisciplinary transitional pain service using the ACT Matrix and buprenorphine/naloxone. J Pain Res. 2017;10:747–55. doi: 10.2147/JPR.S124566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng H, Hanlon JG, Katznelson R, Ghanekar A, McGilvray I, Clarke H. The prescription of medical cannabis by a transitional pain service to wean a patient with complex pain from opioid use following liver transplantation: A case report. Can J Anaesth. 2016;63:307–10. doi: 10.1007/s12630-015-0525-6. [DOI] [PubMed] [Google Scholar]
- 54.Hunter OO, Mariano ER, Harrison TK. Leveraging video telehealth for the transitional pain service in response to COVID-19. Reg Anesth Pain Med. 2021;46:460–1. doi: 10.1136/rapm-2020-101742. [DOI] [PubMed] [Google Scholar]
- 55.Roditi D, Robinson ME. The role of psychological interventions in the management of patients with chronic pain. Psychol Res Behav Manag. 2011;4:41–9. doi: 10.2147/PRBM.S15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vowles KE, Fink BC, Cohen LL. Acceptance and commitment therapy for chronic pain: A diary study of treatment process in relation to reliable change in disability. J Contextual Behav Sci. 2014;3:74–80. doi: 10.1016/j.jcbs.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32:1703–11. doi: 10.1200/JCO.2013.54.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hadlandsmyth K, Dindo LN, Wajid R, Sugg SL, Zimmerman MB, Rakel BA. A single-session acceptance and commitment therapy intervention among women undergoing surgery for breast cancer: A randomized pilot trial to reduce persistent postsurgical pain. Psychooncology. 2019;28:2210–7. doi: 10.1002/pon.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veehof MM, Oskam MJ, Schreurs KMG, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: A systematic review and meta-analysis. Pain. 2011;152:533–42. doi: 10.1016/j.pain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and Commitment Therapy (ACT) for chronic pain: A systematic review and meta-analyses. Clin J Pain. 2017;33:552–68. doi: 10.1097/AJP.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 61.Macfarlane TV, Wirth T, Ranasinghe S, Ah-See KW, Renny N, Hurman D. Head and neck cancer pain: Systematic review of prevalence and associated factors. J Oral Maxillofac Res. 2012;3:e1. doi: 10.5037/jomr.2012.3101. doi: 10.5037/jomr.2012.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans SW, McCahon RA. Management of postoperative pain in maxillofacial surgery. Br J Oral Maxillofac Surg. 2019;57:4–11. doi: 10.1016/j.bjoms.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Dort JC, Farwell DG, Findlay M, Huber GF, Kerr P, Shea-Budgell MA, et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: A consensus review and recommendations from the enhanced recovery after surgery society. JAMA Otolaryngol Head Neck Surg. 2017;143:292–03. doi: 10.1001/jamaoto.2016.2981. [DOI] [PubMed] [Google Scholar]
- 64.Kuo PY, Williams JE. Pain control in head and neck cancer. In: Agulnik M, editor. Head and Neck Cancer. 1st. InTech; 2012. pp. 351–70. ISBN: 978-953-51-0236-6. Available from: http://www.intechopen.com/books/head-and-neck-cancer/pain-and symptom-control-in-head-and-neck-cancer . [Last accessed on 2024 Jun 03] [Google Scholar]
- 65.Brenin DR, Dietz JR, Baima J, Cheng G, Froman J, Laronga C, et al. Pain management in breast surgery: Recommendations of a multidisciplinary expert panel—The American Society of Breast Surgeons. Ann Surg Oncol. 2020;27:4588–602. doi: 10.1245/s10434-020-08892-x. [DOI] [PubMed] [Google Scholar]


