Abstract
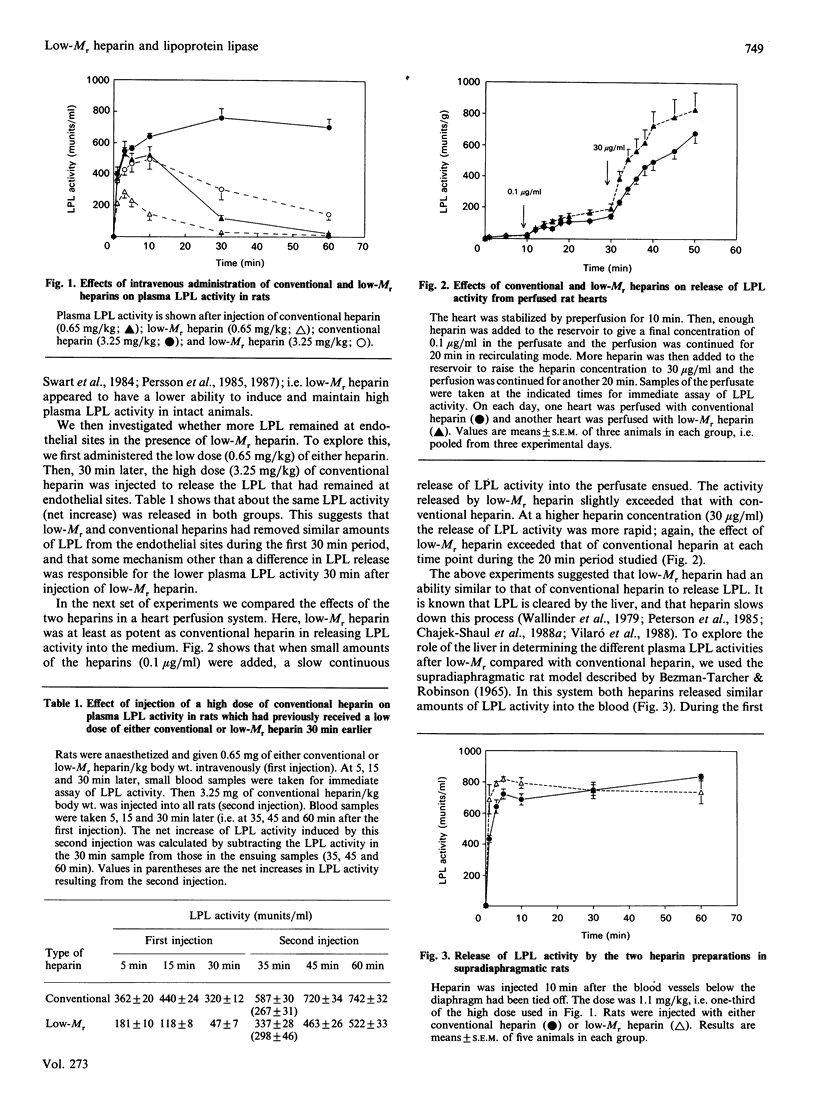
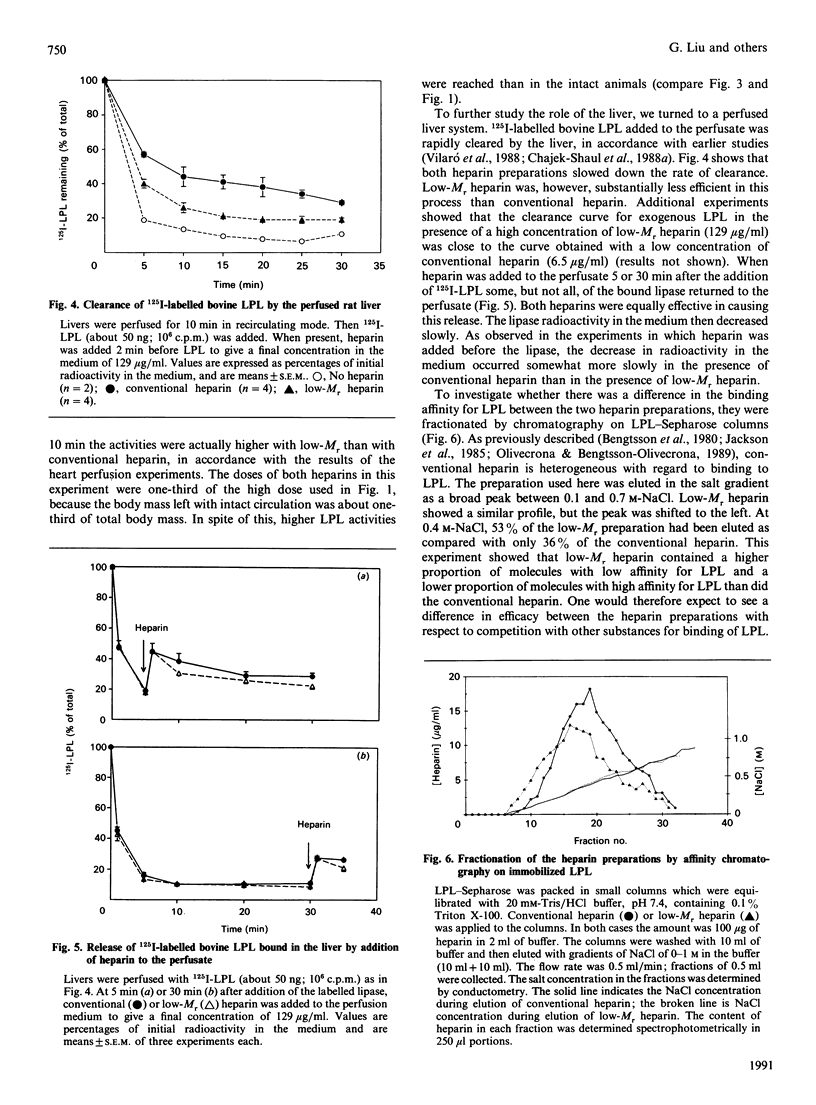
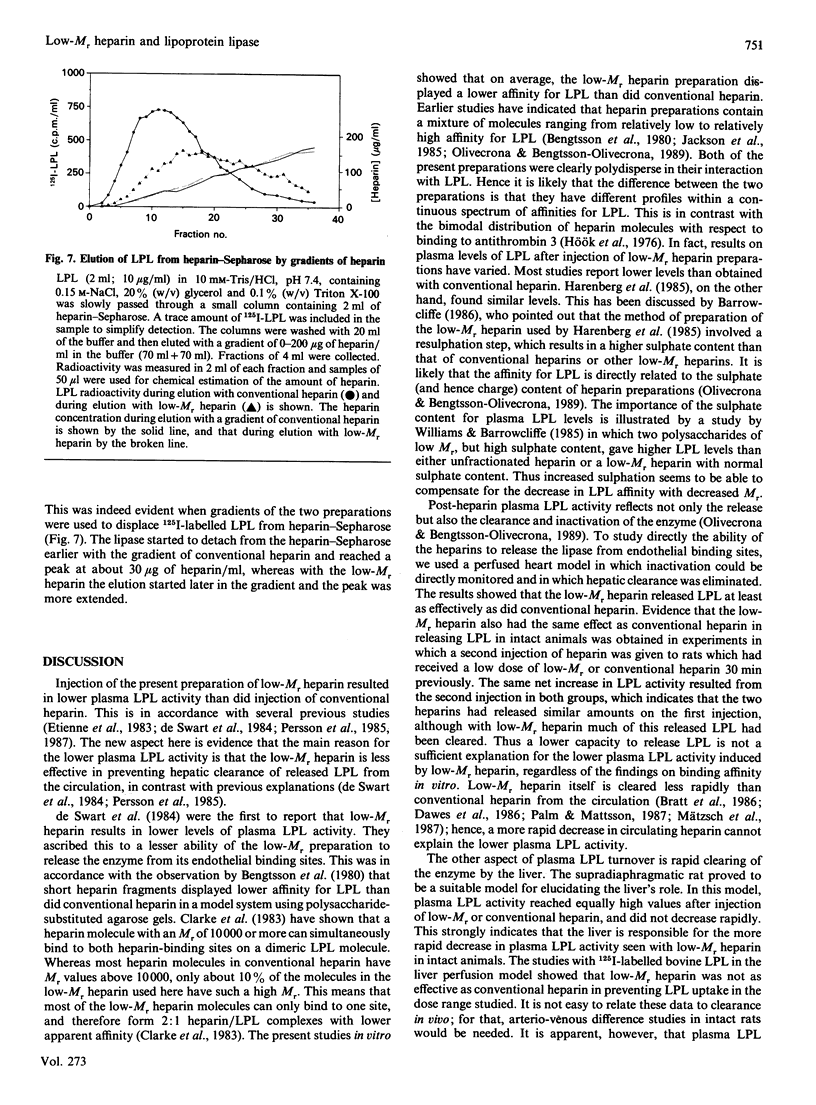
This study compares a low-Mr heparin preparation with conventional heparin with respect to its interaction with lipoprotein lipase (LPL) in vitro and its effects on the enzyme in vivo. Both heparin preparations were polydisperse in binding to LPL, but on average the low-Mr preparation showed lower affinity. Thus both conventional and low-Mr heparin bound quantitatively to immobilized LPL, and were eluted as broad peaks when a salt gradient was applied, but the peak for low-Mr heparin was shifted towards lower salt concentrations. To displace LPL from immobilized heparin a higher concentration of low-Mr than of conventional heparin was needed. Injection of the low-Mr heparin into intact rats resulted in lower plasma LPL activity than did injection of an equal mass of conventional heparin, but when the liver was excluded from the circulation both heparin preparations resulted in similar plasma LPL activities. In perfused rat hearts, low-Mr heparin had at least the same effect on the release of LPL activity as did conventional heparin. In perfused livers, on the other hand, low-Mr heparin was less effective than conventional heparin in preventing the rapid uptake of exogenous labelled LPL. Hence the apparently lower average affinity of low-Mr heparin for LPL does not result in a demonstrably lower potency to release the enzyme from endothelial binding sites in peripheral tissues, but does result in a substantially decreased effect on the hepatic clearance of the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriuoli G., Mastacchi R., Barbanti M., Sarret M. Comparison of the antithrombotic and haemorrhagic effects of heparin and a new low molecular weight heparin in rats. Haemostasis. 1985;15(5):324–330. doi: 10.1159/000215167. [DOI] [PubMed] [Google Scholar]
- Barrowcliffe T. W. Lipolytic activities of low molecular weight heparins. Thromb Res. 1986 May 15;42(4):583–584. doi: 10.1016/0049-3848(86)90222-7. [DOI] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T., Hök M., Riesenfeld J., Lindahl U. Interaction of lipoprotein lipase with native and modified heparin-like polysaccharides. Biochem J. 1980 Sep 1;189(3):625–633. doi: 10.1042/bj1890625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson G., Olivecrona T. Interaction of lipoprotein lipase with heparin-Sepharose. Evaluation of conditions for affinity binding. Biochem J. 1977 Oct 1;167(1):109–119. doi: 10.1042/bj1670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt G., Törnebohm E., Widlund L., Lockner D. Low molecular weight heparin (KABI 2165, Fragmin): pharmacokinetics after intravenous and subcutaneous administration in human volunteers. Thromb Res. 1986 Jun 1;42(5):613–620. doi: 10.1016/0049-3848(86)90340-3. [DOI] [PubMed] [Google Scholar]
- Carter C. J., Kelton J. G., Hirsh J., Cerskus A., Santos A. V., Gent M. The relationship between the hemorrhagic and antithrombotic properties of low molecular weight heparin in rabbits. Blood. 1982 Jun;59(6):1239–1245. [PubMed] [Google Scholar]
- Chajek-Shaul T., Bengtsson-Olivecrona G., Peterson J., Olivecrona T. Metabolic fate of rat heart endothelial lipoprotein lipase. Am J Physiol. 1988 Sep;255(3 Pt 1):E247–E254. doi: 10.1152/ajpendo.1988.255.3.E247. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Luscombe M., Holbrook J. J. The effect of the chain length of heparin on its interaction with lipoprotein lipase. Biochim Biophys Acta. 1983 Sep 14;747(1-2):130–137. doi: 10.1016/0167-4838(83)90131-0. [DOI] [PubMed] [Google Scholar]
- Dawes J., Bara L., Billaud E., Samama M. Relationship between biological activity and concentration of a low-molecular-weight heparin (PK 10169) and unfractionated heparin after intravenous and subcutaneous administration. Haemostasis. 1986;16(2):116–122. doi: 10.1159/000215281. [DOI] [PubMed] [Google Scholar]
- Etienne J., Millot F., Pieron R., Laruelle P. Release of LPL activity after intravenous injection of a low molecular weight heparin. Br J Clin Pharmacol. 1983 Dec;16(6):712–714. doi: 10.1111/j.1365-2125.1983.tb02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Berry M. N., Williams M. C., Severinghaus E. M. A simple and inexpensive membrane "lung" for small organ perfusion. J Lipid Res. 1974 Mar;15(2):182–186. [PubMed] [Google Scholar]
- Harenberg J., Gnasso A., de Vries J. X., Zimmermann R., Augustin J. Anticoagulant and lipolytic effects of a low molecular weight heparin fraction. Thromb Res. 1985 Sep 15;39(6):683–692. doi: 10.1016/0049-3848(85)90252-x. [DOI] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Socorro L., Fletcher G. M., Cardin A. D. Heparin binding to lipoprotein lipase and low density lipoproteins. FEBS Lett. 1985 Oct 14;190(2):297–300. doi: 10.1016/0014-5793(85)81304-1. [DOI] [PubMed] [Google Scholar]
- Kakkar V. V., Djazaeri B., Fok J., Fletcher M., Scully M. F., Westwick J. Low-molecular-weight heparin and prevention of postoperative deep vein thrombosis. Br Med J (Clin Res Ed) 1982 Feb 6;284(6313):375–379. doi: 10.1136/bmj.284.6313.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Mätzsch T., Bergqvist D., Hedner U., Ostergaard P. Effects of an enzymatically depolymerized heparin as compared with conventional heparin in healthy volunteers. Thromb Haemost. 1987 Feb 3;57(1):97–101. [PubMed] [Google Scholar]
- Palm M., Mattsson C. Pharmacokinetics of fragmin. A comparative study in the rabbit of its high and low affinity forms for antithrombin. Thromb Res. 1987 Oct 1;48(1):51–62. doi: 10.1016/0049-3848(87)90345-8. [DOI] [PubMed] [Google Scholar]
- Persson E., Nordenström J., Nilsson-Ehle P., Hagenfeldt L. Lipolytic and anticoagulant activities of a low molecular weight fragment of heparin. Eur J Clin Invest. 1985 Aug;15(4):215–220. doi: 10.1111/j.1365-2362.1985.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Persson E., Nordenström J., Nilsson-Ehle P. Plasma kinetics of lipoprotein lipase and hepatic lipase activities induced by heparin and a low molecular weight heparin fragment. Scand J Clin Lab Invest. 1987 Apr;47(2):151–155. [PubMed] [Google Scholar]
- Peterson J., Olivecrona T., Bengtsson-Olivecrona G. Distribution of lipoprotein lipase and hepatic lipase between plasma and tissues: effect of hypertriglyceridemia. Biochim Biophys Acta. 1985 Dec 4;837(3):262–270. doi: 10.1016/0005-2760(85)90049-9. [DOI] [PubMed] [Google Scholar]
- Vilaró S., Llobera M., Bengtsson-Olivecrona G., Olivecrona T. Lipoprotein lipase uptake by the liver: localization, turnover, and metabolic role. Am J Physiol. 1988 May;254(5 Pt 1):G711–G722. doi: 10.1152/ajpgi.1988.254.5.G711. [DOI] [PubMed] [Google Scholar]
- Wallinder L., Bengtsson G., Olivecrona T. Rapid removal to the liver of intravenously injected lipoprotein lipase. Biochim Biophys Acta. 1979 Oct 26;575(1):166–173. doi: 10.1016/0005-2760(79)90142-5. [DOI] [PubMed] [Google Scholar]
- Wallinder L., Peterson J., Olivecrona T., Bengtsson-Olivecrona G. Hepatic and extrahepatic uptake of intravenously injected lipoprotein lipase. Biochim Biophys Acta. 1984 Oct 4;795(3):513–524. doi: 10.1016/0005-2760(84)90181-4. [DOI] [PubMed] [Google Scholar]
- Williams S. P., Barrowcliffe T. W. The effects of post-heparin plasma lipases on anti-Xa clotting activity. Thromb Res. 1985 Feb 1;37(3):371–377. doi: 10.1016/0049-3848(85)90066-0. [DOI] [PubMed] [Google Scholar]
- de Swart C. A., Nijmeyer B., Andersson L. O., Holmer E., Verschoor L., Bouma B. N., Sixma J. J. Elimination of high affinity heparin fractions and their anticoagulant and lipase activity. Blood. 1984 Apr;63(4):836–842. [PubMed] [Google Scholar]


