Abstract
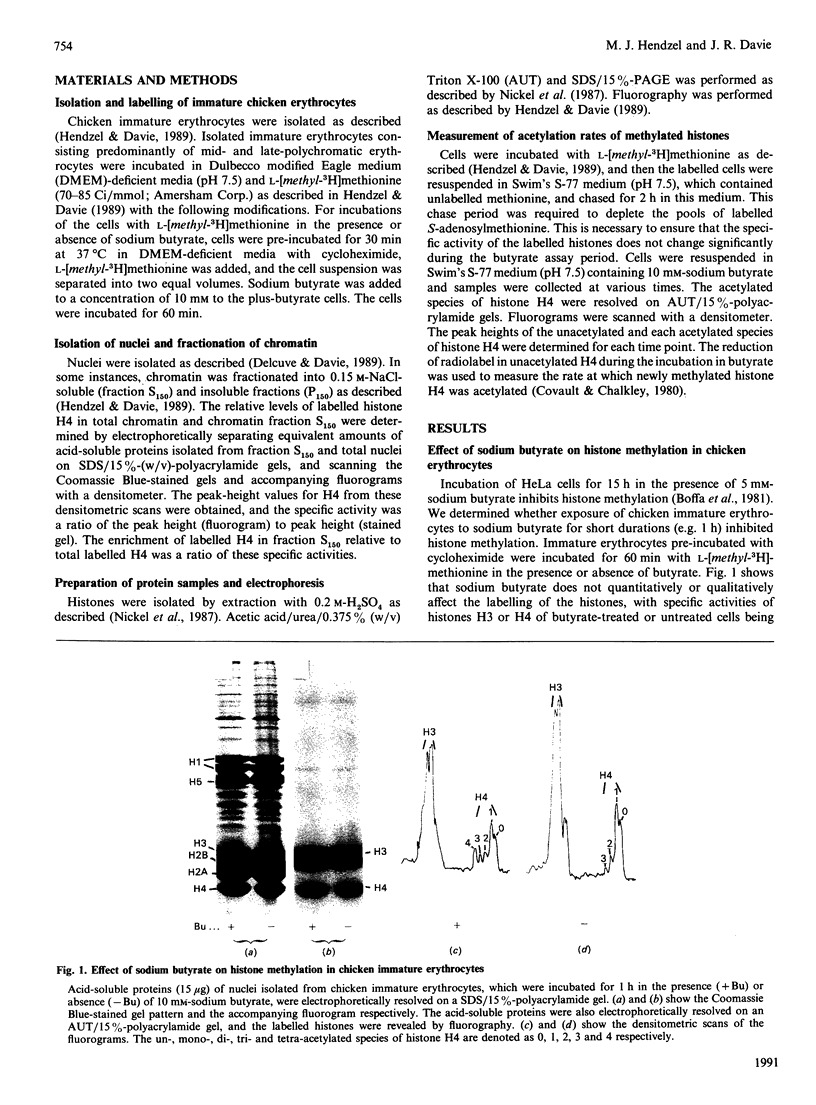
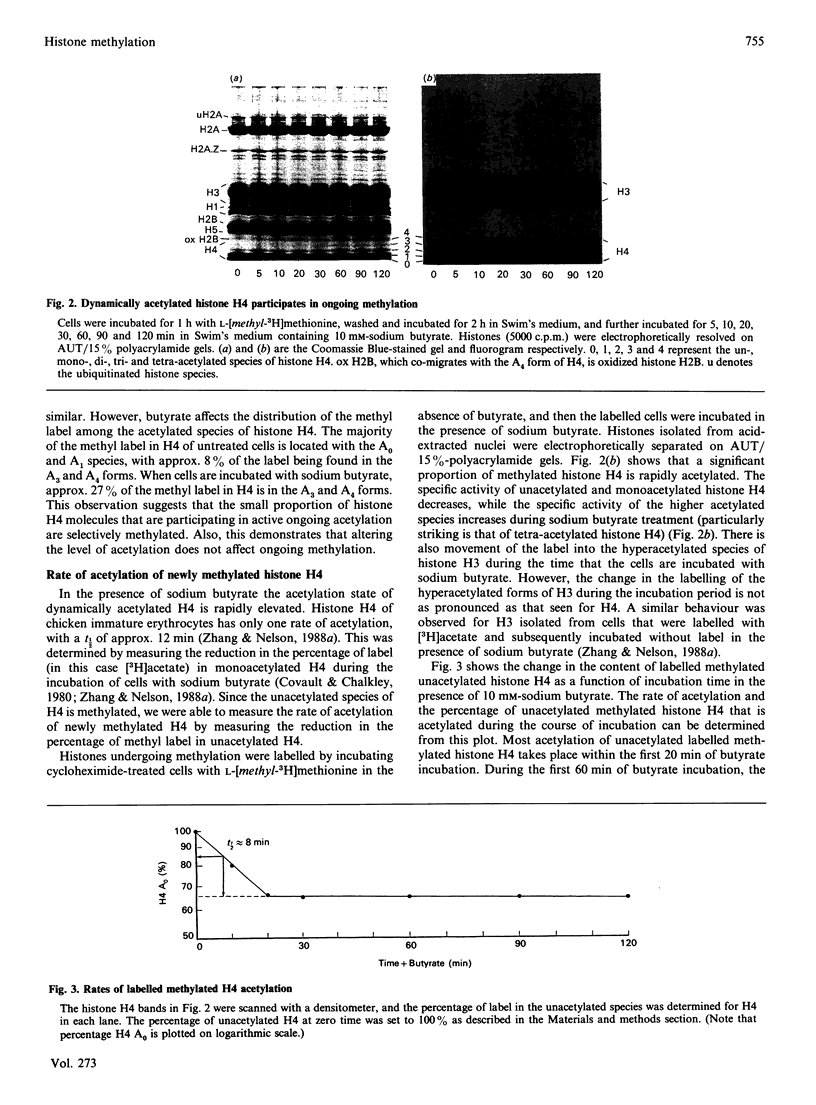
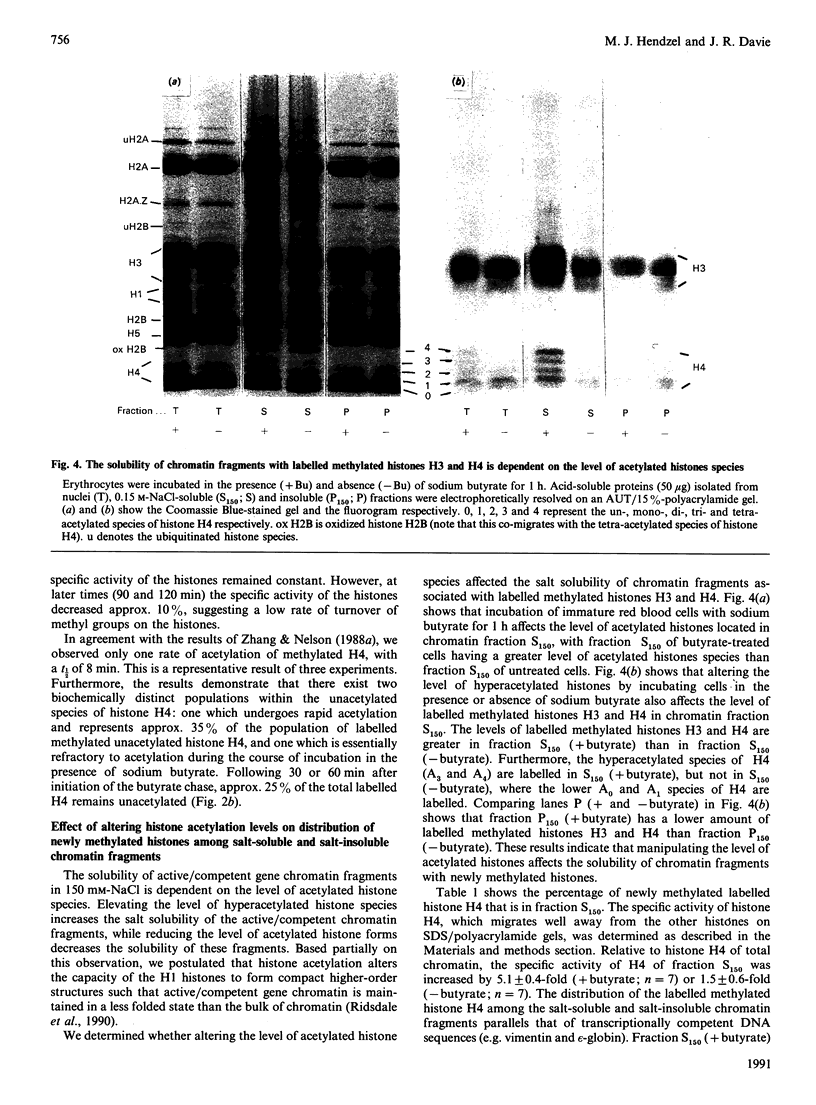
The relationship between histone acetylation and methylation in chicken immature erythrocytes was investigated. Previous studies have shown that transcriptionally active/competent gene-enriched chromatin fragments are enriched in newly methylated histones H3 and H4. Moreover, newly methylated histone H4 is hyperacetylated. Here, we show that dynamically acetylated histone H4 is selectively engaged in ongoing methylation. While sodium butyrate (an inhibitor of histone deacetylase) does not inhibit ongoing histone methylation, it does affect the acetylation state of newly methylated histone H4 when chicken immature erythrocytes are incubated in its presence or absence. Only one rate of acetylation of labelled newly methylated unacetylated histone H4 with a t1/2 of 8 min is observed. Previous studies have shown that the solubility of transcriptionally active/competent gene chromatin fragments in 0.15 M-NaCl is dependent upon the level of acetylated histone species, with induction of hyperacetylation increasing the solubility of this gene chromatin. Here, we show that the low salt solubility of chromatin fragments associated with newly methylated histones H3 and H4 is also dependent upon the level of acetylated histones. These results provide further support for the hypothesis that histones participating in ongoing methylation are associated with transcriptionally active/competent chromatin and suggest that the processes of histone H4 methylation and dynamic acetylation are partially coupled in terminally differentiated erythrocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra P., Sterner R., Clayton D. F., Allfrey V. G. Affinity chromatographic purification of nucleosomes containing transcriptionally active DNA sequences. J Mol Biol. 1987 Jul 20;196(2):379–388. doi: 10.1016/0022-2836(87)90698-x. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Gruss R. J., Allfrey V. G. Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem. 1981 Sep 25;256(18):9612–9621. [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Covault J., Chalkley R. The identification of distinct populations of acetylated histone. J Biol Chem. 1980 Oct 10;255(19):9110–9116. [PubMed] [Google Scholar]
- Delcuve G. P., Davie J. R. Chromatin structure of erythroid-specific genes of immature and mature chicken erythrocytes. Biochem J. 1989 Oct 1;263(1):179–186. doi: 10.1042/bj2630179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebralidse K. K., Grachev S. A., Mirzabekov A. D. A highly basic histone H4 domain bound to the sharply bent region of nucleosomal DNA. Nature. 1988 Jan 28;331(6154):365–367. doi: 10.1038/331365a0. [DOI] [PubMed] [Google Scholar]
- Granados E. N., Bello J. Interactions of poly(N epsilon, N epsilon, N epsilon-trimethyllysine) and poly(N delta, N delta, N delta-trimethylornithine) with polynucleotides: salt dissociation and thermal denaturation. Biochemistry. 1980 Jul 8;19(14):3227–3233. doi: 10.1021/bi00555a020. [DOI] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988 May;7(5):1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel M. J., Davie J. R. Distribution of methylated histones and histone methyltransferases in chicken erythrocyte chromatin. J Biol Chem. 1989 Nov 15;264(32):19208–19214. [PubMed] [Google Scholar]
- Ip Y. T., Jackson V., Meier J., Chalkley R. The separation of transcriptionally engaged genes. J Biol Chem. 1988 Oct 5;263(28):14044–14052. [PubMed] [Google Scholar]
- Megee P. C., Morgan B. A., Mittman B. A., Smith M. M. Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science. 1990 Feb 16;247(4944):841–845. doi: 10.1126/science.2106160. [DOI] [PubMed] [Google Scholar]
- Mold D. E., McCarty K. S., Sr A Chinese hamster ovary cell histone deacetylase that is associated with a unique class of mononucleosomes. Biochemistry. 1987 Dec 15;26(25):8257–8262. doi: 10.1021/bi00399a036. [DOI] [PubMed] [Google Scholar]
- Nickel B. E., Roth S. Y., Cook R. G., Allis C. D., Davie J. R. Changes in the histone H2A variant H2A.Z and polyubiquitinated histone species in developing trout testis. Biochemistry. 1987 Jul 14;26(14):4417–4421. doi: 10.1021/bi00388a034. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Imai B. S., Yau P., Bradbury E. M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989 May 5;57(3):449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- Oliva R., Bazett-Jones D. P., Locklear L., Dixon G. H. Histone hyperacetylation can induce unfolding of the nucleosome core particle. Nucleic Acids Res. 1990 May 11;18(9):2739–2747. doi: 10.1093/nar/18.9.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridsdale J. A., Hendzel M. J., Delcuve G. P., Davie J. R. Histone acetylation alters the capacity of the H1 histones to condense transcriptionally active/competent chromatin. J Biol Chem. 1990 Mar 25;265(9):5150–5156. [PubMed] [Google Scholar]
- Walker J., Chen T. A., Sterner R., Berger M., Winston F., Allfrey V. G. Affinity chromatography of mammalian and yeast nucleosomes. Two modes of binding of transcriptionally active mammalian nucleosomes to organomercurial-agarose columns, and contrasting behavior of the active nucleosomes of yeast. J Biol Chem. 1990 Apr 5;265(10):5736–5746. [PubMed] [Google Scholar]
- Wu R. S., Panusz H. T., Hatch C. L., Bonner W. M. Histones and their modifications. CRC Crit Rev Biochem. 1986;20(2):201–263. doi: 10.3109/10409238609083735. [DOI] [PubMed] [Google Scholar]
- Zhang D. E., Nelson D. A. Histone acetylation in chicken erythrocytes. Rates of acetylation and evidence that histones in both active and potentially active chromatin are rapidly modified. Biochem J. 1988 Feb 15;250(1):233–240. doi: 10.1042/bj2500233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. E., Nelson D. A. Histone acetylation in chicken erythrocytes. Rates of deacetylation in immature and mature red blood cells. Biochem J. 1988 Feb 15;250(1):241–245. doi: 10.1042/bj2500241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Nelson D. A. Histone acetylation in chicken erythrocytes. Estimation of the percentage of sites actively modified. Biochem J. 1986 Dec 15;240(3):857–862. doi: 10.1042/bj2400857. [DOI] [PMC free article] [PubMed] [Google Scholar]