Abstract
The envelope of human parainfluenza virus type 3 (HPF3) contains two viral glycoproteins, the hemagglutinin-neuraminidase (HN) and the fusion protein (F). HN, which is responsible for receptor attachment and for promoting F-mediated fusion, also possesses neuraminidase (receptor-destroying) activity. We reported previously that 4-guanidino-neu5Ac2en (4-GU-DANA) and related sialic acid-based inhibitors of HPF3 neuraminidase activity also inhibit HN-mediated receptor binding and fusion processes not involving neuraminidase activity. We have now examined this mechanism, as well as neuraminidase's role in the viral life cycle, using a neuraminidase-deficient HPF3 variant (C28a) and stable cell lines expressing C28a or wild-type (wt) HN. C28a, which has a wt F sequence and two point mutations in the HN gene corresponding to two amino acid changes in the HN protein, is the first HPF3 variant with insignificant neuraminidase activity. Cells expressing C28a HN did not bind erythrocytes at 4°C unless pretreated with neuraminidase, but no such pretreatment was required for hemadsorption activity (HAD) at 22 or 37°C. HAD was blocked by 4-GU-DANA, attesting to the ability of this compound to inhibit HN's receptor-binding activity. C28a or wt plaque enlargement, a process that involves cell-cell fusion and does not depend on virion release, is diminished by the presence of 4-GU-DANA, confirming the inhibitory effect of 4-GU-DANA on the fusogenic function of C28a HN. In C28a-infected cell monolayers, virion release and thus multicycle replication are severely restricted. This defect was corrected by supplementation of exogenous neuraminidase and also by the addition of 4-GU-DANA; neuraminidase destroys the receptors whereby newly formed C28a virions would remain attached to the cell surface, whereas 4-GU-DANA prevents the attachment itself, obviating the need for receptor cleavage. In accord with the ability of 4-GU-DANA to prevent attachment, the neuraminidase inhibitory effect of 4-GU-DANA on wt HPF3 did not diminish virion release into the medium. Thus, it is by inhibition of viral entry and syncytium formation that sialic acid analogs like 4-GU-DANA may counteract wt HPF3 infection.
Infection by human parainfluenza virus type 3 (HPF3) is mediated by its two envelope glycoproteins, HN (hemagglutinin-neuraminidase) and the fusion protein, F. HN, recognizing the sialic acid-containing cellular receptors on cell surfaces, is responsible for binding the virus to the host cell and for promoting F-mediated fusion whereby the virus penetrates the host cell. In addition to its receptor attachment and fusion functions, HN possesses neuraminidase activity and thus the ability to cleave the sialic acid moiety of those receptors. By virtue of this ability, HN is thought to promote the release of newly formed virions from the cell surface, thus allowing these virions to penetrate additional cells (8). HPF3 infection can also be propagated without the release of complete virions. As the viral envelope proteins accumulate on the infected cell's membrane, the infected cell can fuse with neighboring cells, leading to syncytium formation. Although this process does not require virion release, the level of viral neuraminidase activity influences its outcome by modulating the number of receptors available on adjacent cells (8, 18).
In the influenza virus, HA (hemagglutinin) is responsible for receptor binding and fusion, while the release of newly budded virions is attributable to the other envelope protein, neuraminidase (NA). For variants deficient in NA activity, the spread of infection is limited primarily by aggregation of progeny virions (6, 13, 23). It has thus been postulated (29) that in the case of the minority of viruses requiring neuraminidase activity for virion release, cellular receptors are incorporated into the viral envelope at the time of budding, with the consequence that the incorporated receptor can then bind to another virion's HA or HN, and in the absence of sufficient neuraminidase activity, virions remain attached to one another. Structural information about NA's active site permitted the synthesis of powerful NA inhibitors; one of these, the sialic acid analog 4-guanidino-neu5Ac2en (4-GU-DANA; zanamivir), proved to be a clinically effective anti-influenza agent (5). 4-GU-DANA also inhibits HPF3 neuraminidase activity (4); we found, however, that in both influenza virus and HPF3, 4-GU-DANA exerts effects that cannot be explained by inhibition of neuraminidase activity. Thus, in cells expressing influenza virus HA as the only viral protein, 4-GU-DANA blocked fusion with red blood cells (RBC) (4), indicating that 4-GU-DANA not only has an affinity for the active site of NA but also exerts a direct effect on the other envelope protein, HA. In our studies on HPF3, results in several different experimental systems supported the postulate that 4-GU-DANA inhibits HN-mediated attachment and fusion processes not involving neuraminidase activity (4). New experimental systems for examining this mechanism, as well as for elucidating the role of neuraminidase in the HPF3 life cycle, have now been provided by isolation of a neuraminidase-deficient HPF3 variant, C28a, and by the generation of cell lines stably expressing C28a or wild-type (wt) HN.
HPF3 variants that we have previously isolated included one, C28, with a partial (60 to 70%) deficiency in neuraminidase activity (8). Analysis of its growth in infected monolayers showed that C28 virion release began with a significant delay, which was eliminated by exogenous neuraminidase. A more serious defect was now seen in the C28a variant, which, owing to a second mutation, is virtually devoid of neuraminidase activity. The drastic and prolonged restriction of C28a virion release, and its correction by exogenous neuraminidase supplementation, confirmed the role of HN's neuraminidase activity in destroying the receptors whereby virions would remain attached to the cell surface. With the assumption that agents which prevent the attachment itself would abrogate the need for receptor cleavage, we used 4-GU-DANA, shown here to block erythrocyte binding to cells expressing C28a HN. The addition of 4-GU-DANA to C28a-infected cells indeed resulted in an even greater yield of virions in the medium than did neuraminidase supplementation.
No crystallographic information about the location and number of active sites on any paramyxovirus HN was available at the time of our 1999 (12) and 2000 (4) reports on the abilities of neuraminidase inhibitors DANA, 4-GU-DANA, and 4-amino-DANA to also inhibit HN-receptor interaction. Since this dual action must be related to the fact that both neuraminidase and receptor-binding activities involve recognition of sialosides, we postulated either that these sialic acid analogs have an affinity for both the receptor-binding and neuraminidase active sites or that one site is responsible for both activities (4, 12). Evidence which appears to favor the second postulate emerged in the intervening year: according to studies of Crennel et al. (3) on the crystal structure of Newcastle disease virus (NDV) HN, a single site (with two conformationally switchable states) provides both the binding and the hydrolytic function.
MATERIALS AND METHODS
Virus.
Stocks of wt and variant HPF3 were made in CV-1 cells from virus that was plaque purified four times. Virus was collected 36 to 48 h postinfection and stored at −80°C. HPF3 variants were isolated during growth of virus in neuraminidase-treated cells as previously described (19). For isolation of the variant C28a, supernatant fluid from cultures infected with the C28 variant was collected and used in plaque assays. For C28a, the plaques were qualitatively different from those formed by wt or C28 HPF3. Throughout the clearly demarcated area of each plaque, no unfused cells could be detected microscopically, and intact, entirely normal cells surrounded the sharply bordered plaque. This distinct morphology was used to identify this variant. Large plaques were picked and plaque purified four times, and a single plaque was used to infect each CV-1 cell monolayer for preparation of stocks of variant viruses.
Cells.
HeLa cell lines and CV-1 (African green monkey kidney) cells were maintained with Eagle minimal essential medium supplemented with 10% fetal bovine serum and antibiotics. The generation of monoclonal cell lines stably expressing HN-green fluorescent protein (GFP) of wt and C28a HPF3 was as described elsewhere (7). Briefly, the full-length cDNAs encoding either wt HN or the C28a variant of HN were obtained by PCR amplification of the full-length HN cDNA (19) and subcloned into the corresponding sites of pGFP-C3 (Clontech, Palo Alto, Calif.) to obtain plasmids pwtHN-EGFP and pC28aHN-EGFP, with the amplified HNs fused to the 5′ end of the EGFP gene. Lentivirus vectors pseudotyped with vesicular stomatitis virus G glycoprotein were used for the expression of the EGFP-tagged HNs, with the fused genes under the control of the early cytomegalovirus promoter. Clonal populations stably expressing HN-GFP (wt and C28a variant HNs) or expressing GFP only were selected.
Chemicals.
4-GU-DANA was a gift from Glaxo Wellcome Research and Development Ltd. (Stevenage, United Kingdom).
Neuraminidase assay.
The fluorimetric assay of neuraminidase in sonicated HPF3 preparations (4) was based on the methods of Warner and O'Brien (32) and of Potier et al. (24). Reaction mixtures, containing 100 mM malate buffer (pH 4.75) and 20 mM (4-methylumbelliferyl-α-d-N-acetylneuraminic acid) in a total volume of 25 to 50 μl, were incubated at 37°C for 15 to 20 min. To determine the rate of product formation, which was constant during these periods, samples were taken at four to five time points, mixed with 100 mM methylenediamine, and read in a Sequoia-Turner fluorimeter at 365-nm excitation wavelength and 450-nm emission wavelength. The amount of reaction product denoted by these readings was determined from fluorescence versus concentration curves determined with commercially obtained 4-methylumbelliferone. Fluorescence resulting from the spontaneous hydrolysis of the substrate, corrected for as described by Potier et al. (24), was always less than 25% of the total. Specific activity is expressed as nanomoles of product formed per minute per milligram of protein.
Sequence analysis.
The F and HN genes of the C28a variant of HPF3 were sequenced after reverse transcription-PCR amplification of each gene as described previously (19). The variant genes were sequenced in parallel with the wt genes, and the process was repeated twice, starting with isolation of RNA from freshly infected cells.
Plaque assays, plaque reduction assays, and plaque size assessment.
For virus titering, supernatant fluid from infected or mock-infected cells was serially diluted in serum-free medium, and 100 μl of each serial dilution was added per well to confluent CV-1 cell monolayers in 48-well plates. Cells were incubated at 37°C with intermittent rocking. After 90 min, minimum essential medium containing 0.5% agarose was added to the dishes, and incubation continued for 24 h at 37°C. After removal of the agarose overlay, the cells were fixed with methanol for 15 min and immunostained for plaque detection as described previously (12). For the purpose of determining plaque area, plaque diameters were measured at a magnification of ×7 to ×45, using a zoom stereomicroscope equipped with a micrometer.
HAD assay.
Monolayers of 293T cells expressing C28a variant HN, wt HN, or GFP were seeded on 24-well plates. On the following day, confluent monolayers (4 × 105 to 6 × 105 cells/well) were washed with cold, serum-free medium and incubated for 1 h at 37°C with 1 ml of medium containing 0 or 0.1 U of Clostridium perfringens neuraminidase. After three rinses with phosphate-buffered saline, 300 μl of a 0.5% suspension of freshly obtained human RBC was added to every well, and the wells were incubated at 4, 22, or 37°C for 2 h in the absence or presence of the indicated concentrations of 4-GU-DANA. Nonadherent cells were removed by washing with cold medium; the extent of RBC adsorption was estimated. For quantitation of hemadsorption activity (HAD), the adherent RBC were lysed in 50 mM NH4Cl and transferred into 96-well plates, and the optical density at 540 nm was read on a Biotek Instruments enzyme-linked immunosorbent assay reader.
RESULTS
Isolation of a new HPF3 variant derived from the neuraminidase-deficient variant C28.
In previous studies on the role of neuraminidase in the HPF3 life cycle, we isolated a variant, C28, with less than half as much neuraminidase activity as wt HN (8). Cloning and sequencing of the F and HN genes revealed a single amino acid change in the HN protein, with no alterations in the F sequence. This variant was characterized by a delay in the release of virus particles into the supernatant, by the formation of large plaques, and by causing more extensive fusion through infected cell monolayers. The addition of exogenous bacterial neuraminidase abolished the delay in release of viral particles, indicating that HPF3 viral neuraminidase activity is important for the release of newly formed virions from infected cells.
C28a, a variant of C28 that we have now isolated, was identified on the basis of its ability to form large, isolated plaques in cell monolayers infected with C28. The C28a plaques were qualitatively different from those formed by wt or C28. Throughout the clearly demarcated area of each plaque, no unfused cells could be detected microscopically, and intact, entirely normal cells surrounded the sharply bordered plaque.
The variant has two amino acid changes in HN that render it neuraminidase deficient.
The F and HN genes of C28a were sequenced after reverse transcription-PCR amplification of each mRNA (19). The variant has a wt F gene sequence and has two point mutations in the HN gene corresponding to two amino acid changes in the HN glycoprotein. In addition to the C28 mutation at nucleotide 724 in variant C28 that changes aspartic acid 216 to an asparagine, C28a has a second mutation at nucleotide 409 that changes proline 111 to a serine.
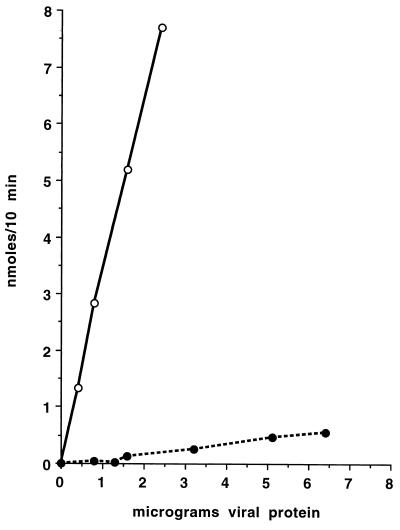
This second mutation results in an essentially complete loss of neuraminidase activity. With the thiobarbiturate assay, no activity could be detected in C28a preparations. The more sensitive fluorimetric assay, using 4-methylumbelliferyl-α-d-N-acetylneuraminic acid as the substrate, was then applied to comparing the neuraminidase activities of wt and C28a preparations. Figure 1 shows that in wt preparations, product formation (nanomoles per 10 min) was proportional to the micrograms of protein added; specific activity thus determined was 330 nmol/min/mg of protein. For the C28a preparation, activity was too low to allow an accurate relation between product formation and protein concentration to be established: specific activity was 8.4 nmol/min/mg of protein, i.e., 1.9% of that for wt HPF3. In two additional pairs of preparations that we compared, the percentages were 0.7 and 0.8, respectively. Since the low values for C28a were close to the subtracted blank, we cannot be certain whether they denote finite or zero activity. Previous assays of intact HN-expressing cells showed that recombinant wt HN-GFP molecules retained their neuraminidase activity and that C28a HN-GFP-expressing cells had no detectable neuraminidase activity (7). The neuraminidase activity of wt HN-expressing cells, like that of wt HPF3 viral preparations (4), was inhibited by 4-GU-DANA.
FIG. 1.
Comparison of the neuraminidase activities of wt HPF3 and its C28a variant. Neuraminidase activities are shown as a function of amount of protein in the wt (open circles) and C28a (closed circles) preparations assayed.
The neuraminidase-deficient variant C28a spreads only from cell to cell.
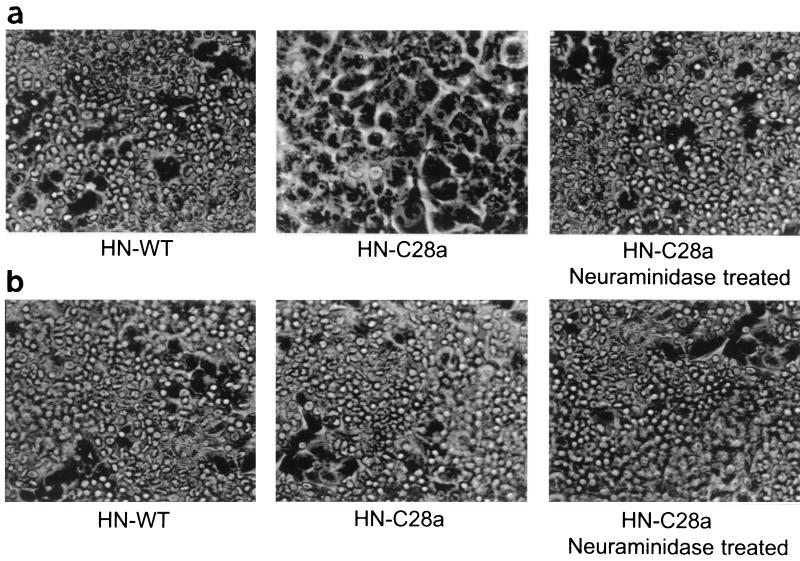
Figure 2 shows a cell monolayer in liquid culture infected with C28a (Fig. 2a) compared to a cell monolayer infected with wt HPF3 (Fig. 2b). It can be seen that even after 48 h of infection, C28a spreads only concentrically in the form of large plaques and does not cause fusion throughout the monolayer. Figure 2a documents the ability of C28a to plaque in liquid culture. Addition of exogenous neuraminidase allows C28a to fuse large areas of the cell monolayer and to spread throughout the monolayer, as shown in Fig. 2c. This is to be expected, since neuraminidase is essential for HPF3 release from the infected cell and therefore for multicycle replication (8).
FIG. 2.
Comparison of cell fusion mediated by C28a, with and without exogenous neuraminidase, and wt HPF3. Cells were infected with C28a (a), with wt HPF3 (b), or with C28a in the presence of exogenous neuraminidase (c) and photographed after 48 h.
Receptor-binding properties of C28a HN.
In our previous studies, the receptor-binding capacity of wt HN was assessed by hemadsorption assays applied to cells persistently infected with HPF3. No persistently infected cells could be obtained with the neuraminidase-deficient C28a variant. Nor could infected monolayers or plaques be used to reliably compare HAD by wt and C28a, since the latter (as described above) does not spread through monolayers, and the plaques it forms are very different from those of the wt. A suitable experimental system was provided by the generation in the course of our recent investigations (7) of stable cell lines expressing HN (of wt and C28a) linked to GFP positioned at the amino terminus of HN. RBC binding was assessed microscopically and then also by a quantitative assay. For these experiments, the cells were grown overnight at 37°C and placed at 4 or 22°C just for the period of the assay.
As determined by confocal microscopy of these cell lines, the level of surface expression of C28a HN is the same as or somewhat higher than that of wt HN (7). Nevertheless, the photographs in Fig. 3a show that the C28a HN-expressing cells did not bind RBC at 4°C. After pretreatment with exogenous neuraminidase (and removal of the added neuraminidase by washing), the C28a HN-expressing cells were able to bind RBC as extensively as the wt HN-expressing cells, indicating that the deficient neuraminidase activity of C28a HN was, directly or indirectly, responsible for its failure to bind to the sialic acid containing receptors on RBC at 4°C. At 22°C, on the other hand, C28a HN-expressing cells exhibited HAD even without neuraminidase treatment (Fig. 3b).
FIG. 3.
Effects of temperature and neuraminidase pretreatment on HAD by cells expressing C28a or wt HN. HAD assays at 4°C (a) and 22°C (b) were carried out as described in Materials and Methods, with and without neuraminidase pretreatment as indicated.
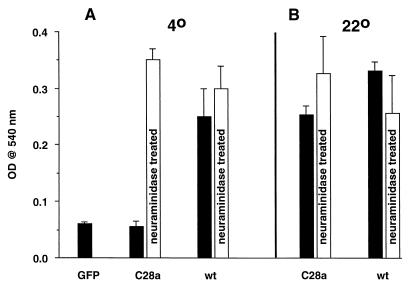
The above results were confirmed with the quantitative HAD assay. Figure 4 shows that values for the C28a HN-expressing cells at 4°C were negligible (as low as for cells expressing GFP only), but that pretreatment with neuraminidase resulted in HAD levels comparable to those of the wt HN-expressing cells. However, at 22°C (Fig. 4b), the HAD of C28a HN-expressing cells was comparable to that of wt HN-expressing cells and was not appreciably enhanced by prior neuraminidase treatment. These results cannot be explained by the assumption that C28a exhibits some neuraminidase activity during the HAD assay at 22°C; if this were so, then desialylation by this neuraminidase would have been accomplished during the 20-h period at 37°C which preceded the HAD assays, and the assay results would have been positive at 4°C as well as at 22°C.
FIG. 4.
Quantitation of the effect of temperature and neuraminidase pretreatment on HAD by cells expressing C28a or wt HN. HAD assays at 4°C (a) and 22°C (b), after (light columns) and without (dark columns) neuraminidase pretreatment, were carried out as described in Materials and Methods. Heights of the columns denote means (bars show SD) of four to seven results. The values for GFP-expressing cells (first column) were the same as those for cell-free or RBC-free blanks. OD, optical density.
A likely explanation of these results for the neuraminidase-deficient variant C28a is that at 4°C the expressed HN tends to bind to sialoglycosides on the cell surface, so that only after the cleavage of these sialic groups by exogenous neuraminidase do enough HN sites become free to react with RBC receptors and to result in significant HAD. At the higher temperature of 22°C, the dynamics of the interaction of expressed HN with neighboring sialoglycosides is such that RBC receptors can successfully compete for HN's binding sites even without neuraminidase pretreatment. Results similar to those at 22°C were obtained in assays of C28a HN-expressing cells at 37°C (not shown). However, in wt HN-expressing cells at this temperature, the neuraminidase activity and thus the receptor-destroying activity becomes much higher than at 22°C, with a consequent lowering of HAD.
Effect of 4-GU-DANA on receptor binding by C28a HN-expressing cells.
Recent investigations in this laboratory have shown that 4-GU-DANA, as well as DANA and 4-amino-DANA, inhibit not only the neuraminidase activity of wt HPF3 but also HN's receptor-binding and fusogenic functions (4, 12). For example, the addition of these compounds to wt-infected cells after the 90-min adsorption period inhibits plaque enlargement; this was also true for cells infected with C28a. The present confirmation of these results with different preparations showed that 1 mM 4-GU-DANA, added after the adsorption period, reduced the areas of wt and C28a plaques by 97 and 80%, respectively. We had also shown that 4-GU-DANA blocks HAD at 4°C by cells persistently infected with wt HPF3. This finding, we postulated, denotes a direct interference by 4-GU-DANA with HN-receptor interaction and is unrelated to the neuraminidase-inhibitory potential of this compound. Further evidence for this postulate was now sought by testing the effect of 4-GU-DANA on HAD in cells expressing the HN of the neuraminidase-deficient variant C28a.
Table 1 shows results obtained at a temperature, 22°C, where C28a HN-expressing cells exhibit HAD activity without neuraminidase pretreatment. This activity, as well as the similar activity found after neuraminidase pretreatment, was inhibited 50 and 92% at 4-GU-DANA concentrations of 1.0 and 2.5 mM, respectively. In wt HN-expressing cells, 0.5 mM 4-GU-DANA was sufficient to block HAD; again, the same results were obtained after neuraminidase treatment.
TABLE 1.
Inhibition by 4-GU-DANA of HAD on cells expressing HN of the C28a variant and of wt HPF3a
| Expressed HN | Neuraminidase treatment | Temp (°C) | HAD (optical density [103])
|
|||
|---|---|---|---|---|---|---|
| 0 mM | 0.5 mM | 1.0 mM | 2.5 mM | |||
| C28a | − | 22 | 159 ± 29 | 169 ± 13 | 72 ± 4 | 8 ± 1 |
| C28a | + | 22 | 236 ± 67 | 205 ± 7 | 126 ± 7 | 18 ± 3 |
| wt | − | 22 | 292 ± 88 | 16 ± 1 | 14 ± 1 | 15 ± 2 |
| wt | + | 22 | 325 ± 50 | 27 ± 2 | 17 ± 2 | 13 ± 4 |
| C28a | + | 4 | 291 ± 9 | 218 ± 11 | 65 ± 12 | 67 ± 18 |
| wt | − | 4 | 212 ± 17 | 55 ± 6 | 46 ± 8 | 20 ± 3 |
| wt | + | 4 | 208 ± 36 | 71 ± 5 | 96 ± 8 | 21 ± 1 |
At 4°C, C28a HN-expressing cells do not exhibit HAD unless pretreated with exogenous neuraminidase. The effect of 4-GU-DANA on these cells was thus tested after such pretreatment. As shown in Table 1, 1.0 to 2.5 mM 4-GU-DANA inhibited the HAD activity of C28a HN-expressing cells by over 75%. In wt HN-expressing cells, 0.5 and 2.5 mM 4-GU-DANA caused 70 and 90% inhibition of HAD, respectively; these results were in accord with our previous demonstration of the inhibition by 4-GU-DANA of HAD on monolayers of cells persistently infected with wt HPF3 (4).
C28a failure to release from infected cells into the supernatant fluid; effects of neuraminidase and 4-GU-DANA.
Virion release, expressed as PFU per milliliter of supernatant fluid, was determined at 24, 48, and 72 h after infection of CV-1 cell monolayers with wt HPF3 or its neuraminidase-deficient variant C28a at a multiplicity of infection (MOI) of 0.002. Table 2 shows that the release of C28a virions was severely restricted and that this severe restriction could be overcome by addition of C. perfringens neuraminidase after the adsorption period. This effect is attributable to exogenous neuraminidase destroying the receptors to which HN binds, thus enhancing the elution of progeny C28a virions from the cell surface. As an alternative to neuraminidase supplementation, virion aggregation might also be preventable by agents that inhibit the binding of HN to the cell surface receptor. To test this hypothesis we used 4-GU-DANA since this compound blocked HAD on cells expressing C28a HN (Table 1), indicating that it can compete for the receptor binding site on HN. C28a virion release was enhanced appreciably by 0.5 mM 4-GU-DANA and much more by 5 mM 4-GU-DANA (Table 2).
TABLE 2.
Defective C28a virion release and its correction with exogenous neuraminidase or 4-GU-DANAa
| Virus | Addition | Virion release (103 PFU/ml)
|
||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| wt | None | 85 ± 37 | 2,750 ± 750 | 3,900 ± 1,600 |
| C28a | None | 0.18 ± 0.05 | 10.25 ± 3.0 | 9.5 ± 0.5 |
| C28a | Neuraminidase | 1.15 ± 0.41 | 210 ± 30 | 570 ± 160 |
| C28a | 0.5 mM 4-GU-DANA | 0.42 ± 0.1 | 67.5 ± 20 | 55.0 ± 15.0 |
| C28a | 5.0 mM 4-GU-DANA | 5.5 ± 0.27 | 350 ± 3.0 | 6,250 ± 5.0 |
Monolayers of CV-1 cells in six-well plates were infected with C28a or wt at an MOI of 0.002. After the 90-min adsorption period, the medium was removed and 6 ml of medium containing 0 or 0.1 U of C. perfringens neuraminidase or the indicated concentrations of 4-GU-DANA was added. Values for virion release, determined at 24, 48, and 72 h after infection, are means ± SD of results for three to seven samples from each of two duplicate wells.
It should be noted that virion yield in the experiments represented in Table 2 may depend not only on release per se but also on virion production (i.e., the number of virions available for release), which may be affected by the prolonged presence of 4-GU-DANA or exogenous neuraminidase. It seemed desirable to confirm the results under conditions that avoid this complication. In the following experiments, therefore, we added 4-GU-DANA or neuraminidase on the second day of infection only and determined the number of PFU accumulating during the ensuing 2 h.
Table 3 shows that during this short period, C28a virion release was enhanced by neuraminidase or by 0.5 mM 4-GU-DANA; 5 mM 4-GU-DANA was required to obtain values comparable to the control values for wt virions. Neuraminidase addition had no significant effect on wt PFU, while 0.5 or 5.0 mM 4-GU-DANA caused an approximately twofold increase. The concentrations of 4-GU-DANA used were sufficient to completely inhibit the endogenous neuraminidase activity of wt HPF3 (4); however, any negative effect of the lack of neuraminidase activity on wt virion release is counterbalanced by the fact that these concentrations of 4-GU-DANA also inhibit HN's attachment function. Consequently, progeny wt virions fail to bind to the cellular receptor, and thus receptor cleavage by neuraminidase is not required for virion elution in the presence of 4-GU-DANA.
TABLE 3.
Effects of 4-GU-DANA and neuraminidase during a 2-h period of release of C28a and wt virionsa
| Virus | Addition present during 2-h period | Virion release (103 PFU/ml) after 2 h |
|---|---|---|
| C28a | None | 1.5 ± 0.25 |
| C28a | 0.5 mM 4-GU-DANA | 22 ± 7 |
| C28a | 5.0 mM 4-GU-DANA | 370 ± 85 |
| C28a | Neuraminidase | 5.2 ± 0.8 |
| wt | None | 460 ± 50 |
| wt | 0.5 mM 4-GU-DANA | 900 ± 300 |
| wt | 5.0 mM 4-GU-DANA | 1,030 ± 190 |
| wt | Neuraminidase | 520 ± 100 |
Twenty-four hours after infection of CV-1 cell monolayers in six-well plates with C28a or wt (MOI = 0.1), the medium was removed and replaced by medium containing the indicated additions. Values for virion release during the ensuing 2-h period at 37°C are means ± SD of four results from two separate experiments.
DISCUSSION
We had previously shown that a single amino acid substitution in HN, resulting in a variant, C28, with neuraminidase activity decreased to 30% of the wt level, promotes increased membrane fusion through an increase in available sialic acid receptors and delays release (8). A second mutation in C28a, resulting in insignificant neuraminidase activity, blocks release more severely and thus completely prevents spread beyond the plaque by curtailing multicycle replication. In addition to the mutation at nucleotide 724 in variant C28 that changes aspartic acid 216 to an asparagine, C28a has a second mutation at nucleotide 409 that changes proline 111 to a serine.
The aspartic acid at position 216 is absolutely conserved among paramyxoviruses, and the region of HN containing this amino acid has been shown by us and others to be important for enzymatic activity in paramyxoviruses (8, 10, 11, 15, 26, 27, 33). The aspartic acid 216-to-asparagine change in C28 HN and C28a HN alters the amino acid that was predicted by comparison with influenza virus NA (2) to be the catalytic aspartic acid. The second mutation in C28a, changing proline 111 to a serine, is responsible for eliminating the residual neuraminidase activity of HN. This amino acid forms part of a short stretch of residues in the stalk region of HN that is conserved among the paramyxoviruses. In fact, proline 111 is absolutely conserved among paramyxovirus HN proteins. Wang and Iorio (31) showed that for NDV, mutation of several of the conserved residues in the HN stalk markedly altered neuraminidase activity without affecting receptor binding, and they noted that neuraminidase activity is highly sensitive to stalk alterations. Mutation of the NDV HN residue corresponding to the HPF3 proline 111 that is altered in C28a (proline 93 in NDV) reduced NDV HN's neuraminidase activity to 0.05% of wt levels. This finding confirms the importance of stalk region residues, and of proline 111, in paramyxovirus neuraminidase activity.
Isolation of C28a, the first HPF3 mutant with insignificant neuraminidase activity, made it possible to document the inhibitory effect of 4-GU-DANA on HN functions that do not involve neuraminidase activity, as well as to elucidate the nature of defects consequent to neuraminidase deficiency. The blockage by 4-GU-DANA of HAD on cells expressing C28a HN demonstrated the ability of this compound to inhibit the receptor binding activity of HN. In cells infected with C28a, as in wt HPF3-infected cells, the addition of 4-GU-DANA after the adsorption period greatly reduced the area of plaques formed; since HPF3 plaque enlargement proceeds by cell-cell fusion rather than depending on virion release, this finding indicates that 4-GU-DANA curtails the capacity of the neuraminidase-deficient (as well as wt) HN to promote F protein-mediated fusion.
In the course of characterizing C28a, we found that cells infected with this variant of HPF3 were not able to adsorb RBC at 4°C unless pretreated with neuraminidase. This defect, not seen in HAD assays at 22 or 37°C, is difficult to interpret, since the fact that wt HN-expressing cells are HAD positive at a temperature, 4°C, where neuraminidase is inactive indicates that HN's neuraminidase activity plays no direct role in its receptor binding function. However, the possibility of an indirect effect of neuraminidase on HN's binding function is suggested by the following observations of other enveloped viruses.
Recent studies on NDV (9) showed that inactivating mutations in HN's neuraminidase active site also abolished its attachment function and that this function could be partially rescued by exogenous neuraminidase or coexpression with wt HN. In another study on NDV, elimination of oligosaccharides on HN by site-directed mutagenesis significantly increased HN's ability to attach to RBC receptors (16). For influenza B viruses, the attachment function of HA, greatly enhanced by bacterial neuraminidase (1), was found to be inhibited by N-acetyl glycoside groups at amino acid residues 160 and 217 (14). In influenza A virus (fowl plague virus [FPV]) (21, 22), desialidation by NA of oligosaccharides located in the vicinity of HA's binding site was found to be a precondition of receptor-binding activity. HAD by cells expressing FPV HA from which these oligosaccharides were removed by site-specific mutagenesis did not require NA; however, the extent of their HAD (especially at low HA expression) was significantly increased by the addition of neuraminidase (21). The authors concluded that the lack of HAD on wt HA cells is due primarily to sialoglycosides on specific sites of HA itself, but that the presence of various sialoglycoproteins on the cell surface with an affinity to HA is a contributory factor. This second factor alone can account for the inability of our C28a HN-expressing cells to adsorb RBC at 4°C. The fact that neuraminidase pretreatment (which circumvents the defect at 4°C) is not necessary for HAD by C28a HN-expressing cells at 22°C is consistent with this assumption; at this higher temperature, the attachment of HN to sialoglycoproteins on the transfected cells' surface is subject to an increased dissociation rate, which may allow RBC receptors to successfully compete for HN.
A characteristic of C28a HPF3 is that cell monolayers infected with this variant virus fail to release virions, and thus multicycle replication is severely restricted. This restriction was corrected by supplementation of neuraminidase which, cleaving the sialic acid moieties of the cellular receptor, allows virion elution from the cell surface. The addition of 4-GU-DANA, we found, was an alternative way of overcoming the same defect, though via a different mechanism. By inhibiting HN-receptor interaction, 4-GU-DANA prevents virion aggregation, thus obviating the need for the receptor-destroying neuraminidase activity. Pertinent in this connection are studies on influenza virus variants suggesting that the balance between receptor-binding and neuraminidase activities is critical for viral propagation (17). Direct experimental evidence for this was obtained by Wagner et al. (30) in studies using FPV recombinants in which different NA subtypes were combined with an HA mutant that had increased receptor-binding capacity. The extent of this restriction of virion release in the presence of the mutant HA depended on the nature of the accompanying NA in that the high-activity NA partially overcame the high binding affinity of the mutant HA, whereas the low-activity NA subtype did not. The present finding that the severely restricted release of the neuraminidase-deficient C28a virions could be overcome by inhibiting HN's receptor-binding capacity is suggestive of the possibility that productive HPF3 infection is dependent on the balance between HN's receptor-destroying and receptor-binding activities. The same principle may underlie our previous finding that variants with decreased neuraminidase activity (8), as well as variants with increased receptor-binding avidity (19, 20), emerge in HPF3-infected cell cultures in the presence of exogenous neuraminidase which serves to remove a portion of the available sialic acid receptors. The fact that these two distinct types of variants emerged under the same selective pressure indicates that either loss of neuraminidase activity or increased binding activity can compensate for receptor scarcity.
Recent experiments using site-directed mutations in the globular domain of NDV HN that result in undetectable neuraminidase activity showed that these mutant HNs are also devoid of receptor-binding activity (9). In considering the question of whether this loss of binding activity is a direct or indirect consequence of inactivating mutations in the neuraminidase active site, the authors provide a cogent review of previous findings that speak against or in favor of the topological separation of the neuraminidase and receptor-binding sites. It is the notion of a single site that received strong support from the recent elucidation of the crystal structure of NDV HN (3). It follows that inhibitors directed to this single site should minimize both the hydrolytic and the binding function of HN. The previously reported inhibition by DANA of the neuraminidase but not the hemagglutinating activity of NDV (25) does not rule out the single-site hypothesis, because the two assays differ substantially with respect to the amount and affinity of ligands that compete with DANA (3). There are no other literature reports on testing DANA or other inhibitors for a dual effect on NDV. Thus, our studies of the effect of DANA and its analogs on HPF3 (4, 12) appear to have been the first to indicate that a single substance can inhibit both the neuraminidase and the binding activity of a paramyxovirus HN. Extension of this evidence in the present study included the finding that in cells expressing wt HPF3 HN on their surface, 4-GU-DANA inhibits both neuraminidase activity and HAD; in addition, the ability of 4-GU-DANA to also block HAD on cells expressing C28a HN demonstrated that the negative effect of 4-GU-DANA on HN's receptor-binding function is not a secondary consequence of its inhibition of neuraminidase activity.
4-GU-DANA, specifically designed to inhibit the activity of influenza virus NA (28), has been shown to prevent the release of progeny influenza virions and thus curtail the spread of infection (5). This particular mode of counteracting infection is probably not applicable to HPF3; our results indicate that by interfering with HN-receptor interaction, 4-GU-DANA can prevent virion aggregation in the first place, so that its potential to prevent virion release (by inhibiting neuraminidase activity) has no practical consequence. Preventing virion release would probably also not be the salient antiviral action of alternative, as yet unavailable sialic acid analogs synthesized on the basis of structural information about the active site of HPF3 HN. However, by interfering with HN's receptor-binding and fusogenic functions, such drugs may be effective inhibitors of viral entry and also minimize the cytopathological consequences (such as syncytium formation) of HPF3 infection if it does occur.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 31971 to A.M. from the National Institutes of Health.
We thank Rob Fenton, Glaxo Wellcome Research and Development Ltd. (Stevenage, United Kingdom), for helpful discussions and for generously providing zanamivir, and we thank Richard Peluso for helpful discussions.
REFERENCES
- 1.Brassard D L, Lamb R A. Expression of influenza B virus hemagglutinin containing multibasic residue cleavage sites. Virology. 1997;236:234–248. doi: 10.1006/viro.1997.8749. [DOI] [PubMed] [Google Scholar]
- 2.Colman P M. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci. 1994;3:1687–1696. doi: 10.1002/pro.5560031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crennell S, Takimoto T, Portner A, Taylor G. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat Struct Biol. 2000;7:1068–1074. doi: 10.1038/81002. [DOI] [PubMed] [Google Scholar]
- 4.Greengard O, Poltoratskaia N, Leikina E, Zimmerberg J, Moscona A. The anti-influenza virus agent 4-GU-DANA (zanamivir) inhibits cell fusion mediated by human parainfluenza virus and influenza virus HA. J Virol. 2000;74:11108–11114. doi: 10.1128/jvi.74.23.11108-11114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayden F G, Osterhaus A D, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 6.Hofling K, Brossmer R, Klenk H D, Herrler G. Transfer of an esterase-resistant receptor analog to the surface of influenza C virions in reduced infectivity due to aggregate formation. Virology. 1996;218:127–133. doi: 10.1006/viro.1996.0172. [DOI] [PubMed] [Google Scholar]
- 7.Horga M A, Gusella G L, Greengard O, Poltoratskaia N, Porotto M, Moscona A. Mechanism of interference mediated by human parainfluenza virus type 3 infection. J Virol. 2000;74:11792–11799. doi: 10.1128/jvi.74.24.11792-11799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huberman K, Peluso R, Moscona A. The hemagglutinin-neuraminidase of human parainfluenza virus type 3: role of the neuraminidase in the viral life cycle. Virology. 1995;214:294–300. doi: 10.1006/viro.1995.9925. [DOI] [PubMed] [Google Scholar]
- 9.Iorio R, Field G M, Sauvron J M, Mirza A M, Deng R, Mahon P J, Lanjedik J P. Structural and functional relationship between the receptor recognition and neuraminidase activities of the Newcastle disease virus hemagglutinin-neuraminidase protein: receptor recognition is dependent on neuraminidase activity. J Virol. 2001;75:1918–1927. doi: 10.1128/JVI.75.4.1918-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iorio R, Glickman R. Fusion mutants of Newcastle disease virus selected with monoclonal antibodies to the hemagglutinin-neuraminidase. J Virol. 1992;66:6626–6633. doi: 10.1128/jvi.66.11.6626-6633.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio R M, Sydall R J, Glickman R L, Riel A M, Sheehan J P, Bratt M A. Identification of amino acid residues important to the neuraminidase activity of the HN glycoprotein of Newcastle disease virus. Virology. 1989;173:196–204. doi: 10.1016/0042-6822(89)90235-3. [DOI] [PubMed] [Google Scholar]
- 12.Levin Perlman S, Jordan M, Brossmer R, Greengard O, Moscona A. The use of a quantitative fusion assay to evaluate HN-receptor interaction for human parainfluenza virus type 3. Virology. 1999;265:57–65. doi: 10.1006/viro.1999.0024. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo C, Nobusawa E, Nakajima K. An analysis of the role of neuraminidase in the receptor-binding activity of influenza B virus: the inhibitory effect of Zanamivir on haemadsorption. J Gen Virol. 1999;80:2969–2976. doi: 10.1099/0022-1317-80-11-2969. [DOI] [PubMed] [Google Scholar]
- 15.Lyn D, Mazanec M B, Nedrud J G, Portner A. Location of amino acid residues important for the structure and biological function of the haemagglutinin-neuraminidase glycoprotein of Sendai virus by analysis of escape mutants. J Gen Virol. 1991;72:817–824. doi: 10.1099/0022-1317-72-4-817. [DOI] [PubMed] [Google Scholar]
- 16.McGinnes L, Morrison T. The role of individual oligosaccharide chains in the activities of the HN glycoprotein of Newcastle disease virus. Virology. 1995;212:398–410. doi: 10.1006/viro.1995.1497. [DOI] [PubMed] [Google Scholar]
- 17.McKimm-Breschkin J, Sahasrabudhe A, Blick T J, McDonald M, Colman P M, Hart G J, Bethell R C, Varghese J N. Mutations in a conserved residue in the influenza virus neuraminidase active site decreases sensitivity to Neu5Ac 2en-derived inhibitors. J Virol. 1998;72:2456–2462. doi: 10.1128/jvi.72.3.2456-2462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moscona A, Peluso R W. Fusion properties of cells infected with human parainfluenza virus type 3: receptor requirements for viral spread and virus-mediated membrane fusion. J Virol. 1992;66:6280–6287. doi: 10.1128/jvi.66.11.6280-6287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscona A, Peluso R W. Relative affinity of the human parainfluenza virus 3 hemagglutinin-neuraminidase for sialic acid correlates with virus-induced fusion activity. J Virol. 1993;67:6463–6468. doi: 10.1128/jvi.67.11.6463-6468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscona A, Peluso R W. Analysis of human parainfluenza virus 3 receptor binding variants: evidence for the use of a specific sialic acid-containing receptor. Microb Pathog. 1996;20:179–184. doi: 10.1006/mpat.1996.0016. [DOI] [PubMed] [Google Scholar]
- 21.Ohuchi M, Feldmann A, Ohuchi R, Klenk H D. Neuraminidase is essential for fowl plague virus hemagglutinin to show hemagglutinating activity. Virology. 1995;212:77–83. doi: 10.1006/viro.1995.1455. [DOI] [PubMed] [Google Scholar]
- 22.Ohuchi M, Ohuchi R, Feldmann A, Klenk H D. Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J Virol. 1997;71:8377–8384. doi: 10.1128/jvi.71.11.8377-8384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature-sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 24.Potier M, Mameli L, Belislem M, Dallaire L, Melanxon S B. Fluorimetric assay of neuraminidase with a sodium 4-methylumbelliferyl-a-d-N-acetylneuraminidase substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 25.Sastre A, Cobaleda C, Cabezas J A, Villar E. On the inhibition mechanism of the sialidase activity from Newcastle disease virus. Biol Chem. 1991;372:923–927. doi: 10.1515/bchm3.1991.372.2.923. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan J, Iorio R. A single amino acid substitution in the hemagglutinin-neuraminidase of Newcastle disease virus results in a protein deficient in both functions. Virology. 1992;189:778–781. doi: 10.1016/0042-6822(92)90605-o. [DOI] [PubMed] [Google Scholar]
- 27.Shioda T, Wakao S, Suzu S, Shibuta H. Differences in bovine parainfluenza 3 virus variants studied by sequencing of the genes of viral envelope proteins. Virology. 1988;162:388–396. doi: 10.1016/0042-6822(88)90479-5. [DOI] [PubMed] [Google Scholar]
- 28.von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Dyason J C, Jin B, Phan T V, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 29.Yang P, Bansal A, Liu C, Air G M. Hemagglutinin specifiity and neuraminidase coding capacity of neuraminidase-deficient influenza viruses. Virology. 1997;229:155–165. doi: 10.1006/viro.1996.8421. [DOI] [PubMed] [Google Scholar]
- 30.Wagner R, Wolff T, Herwig A, Pleschka S, Klenk H D. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics. J Virol. 2000;74:6316–6323. doi: 10.1128/jvi.74.14.6316-6323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Iorio R M. Amino acid substitutions in a conserved region in the stalk of the Newcastle disease virus HN glycoprotein spike impair its neuraminidase activity in the globular domain. J Gen Virol. 1999;80:749–753. doi: 10.1099/0022-1317-80-3-749. [DOI] [PubMed] [Google Scholar]
- 32.Warner T G, O'Brien J S. Synthesis of 2′-(4-methylumbelliferyl)-alpha-d-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979;18:2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- 33.Waxham M N, Aronowski J. Identification of amino acids involved in the sialidase activity of the mumps virus hemagglutinin-neuraminidase protein. Virology. 1988;167:226–232. doi: 10.1016/0042-6822(88)90072-4. [DOI] [PubMed] [Google Scholar]