Abstract
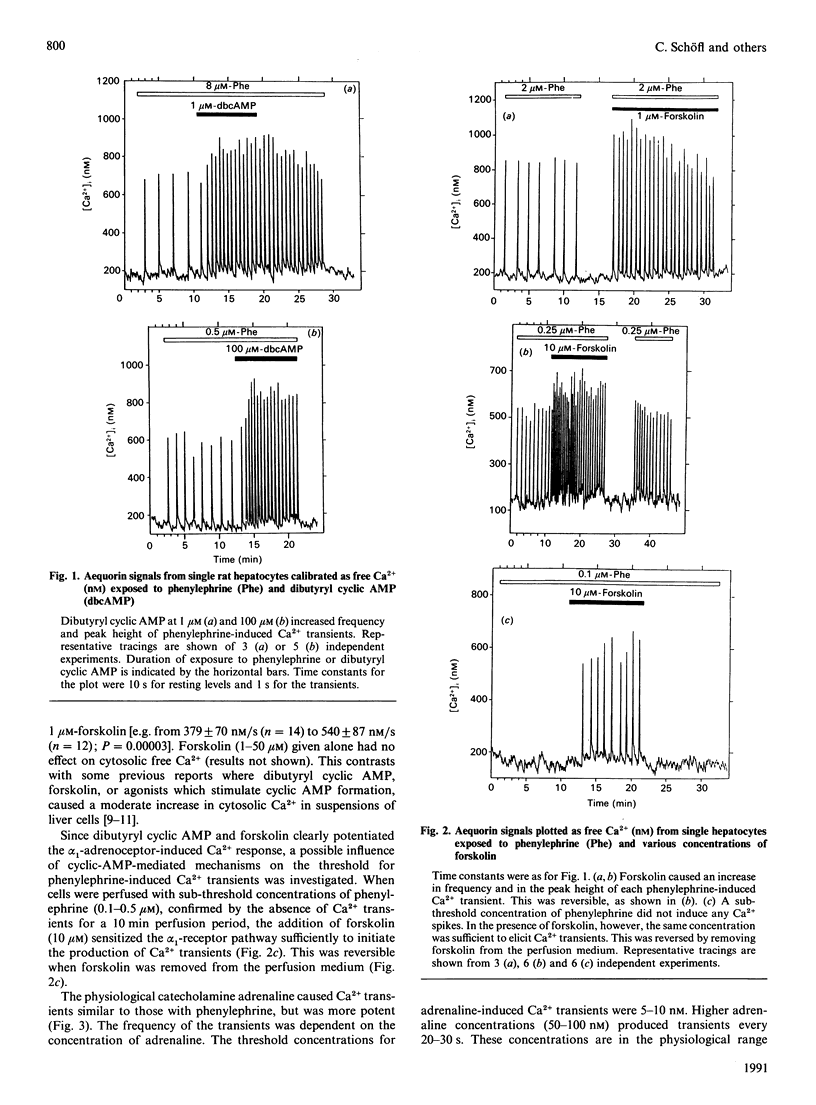
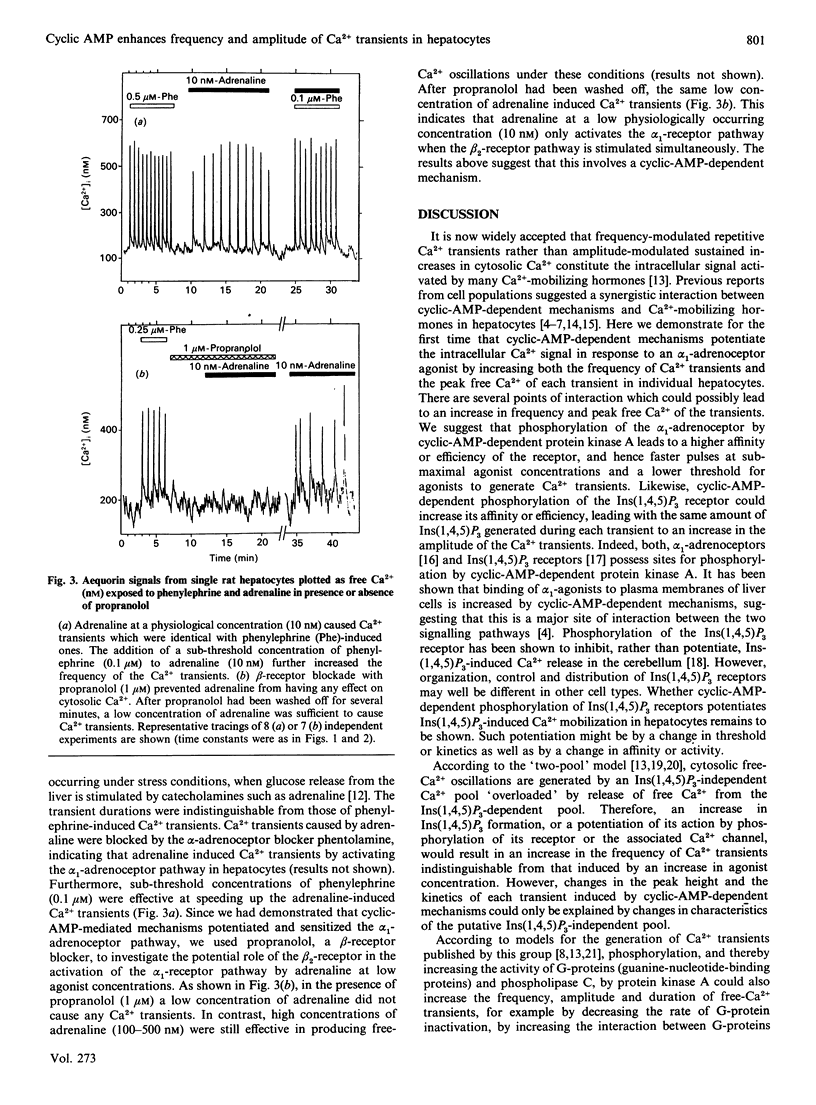
Interactions between signalling pathways such as the cyclic AMP and the Ca2+/phosphatidylinositol pathway may occur and be of major relevance in the regulation of cell function. We demonstrate here that cyclic-AMP-dependent mechanisms cause a marked increase in frequency and peak free Ca2+ of alpha 1-receptor-induced Ca2+ transients in single hepatocytes and lower the threshold for alpha 1-receptor agonists. Adrenaline at low physiological concentrations generates alpha 1-receptor-induced Ca2+ transients, which requires activation of the beta 2-receptor signalling pathway. We conclude that an interaction between the alpha 1-receptor signalling pathway and cyclic-AMP-dependent mechanisms activated by beta 2-receptor occupation is crucial to elicit a complete adrenergic response to adrenaline at physiological concentrations in rat hepatocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. Synergistic stimulation of Ca2+ uptake by glucagon and Ca2+-mobilizing hormones in the perfused rat liver. A role for mitochondria in long-term Ca2+ homoeostasis. Biochem J. 1986 Sep 15;238(3):653–661. doi: 10.1042/bj2380653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986 Aug 25;261(24):11056–11063. [PubMed] [Google Scholar]
- Bouvier M., Leeb-Lundberg L. M., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of alpha 1- and beta 2-adrenergic receptors by protein kinase C and the cyclic AMP-dependent protein kinase. J Biol Chem. 1987 Mar 5;262(7):3106–3113. [PubMed] [Google Scholar]
- Burgess G. M., Dooley R. K., McKinney J. S., Nånberg E., Putney J. W., Jr Further studies on the interactions between the calcium mobilization and cyclic AMP pathways in guinea pig hepatocytes. Mol Pharmacol. 1986 Oct;30(4):315–320. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Clutter W. E., Bier D. M., Shah S. D., Cryer P. E. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980 Jul;66(1):94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T. M., Jenkinson D. H., Koller K. Interactions between receptors that increase cytosolic calcium and cyclic AMP in guinea-pig liver cells. Br J Pharmacol. 1984 Sep;83(1):281–291. doi: 10.1111/j.1476-5381.1984.tb10144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Binet A., Claret M. Glucagon and vasopressin interactions on Ca2+ movements in isolated hepatocytes. Biochem J. 1986 Aug 1;237(3):675–683. doi: 10.1042/bj2370675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Charest R., Blackmore P. F., Exton J. H. Potentiation of alpha 1-adrenergic responses in rat liver by a cAMP-dependent mechanism. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4208–4212. doi: 10.1073/pnas.81.13.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pittner R. A., Fain J. N. Exposure of cultured hepatocytes to cyclic AMP enhances the vasopressin-mediated stimulation of inositol phosphate production. Biochem J. 1989 Jan 15;257(2):455–460. doi: 10.1042/bj2570455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Claret M. Effect of cyclic AMP-dependent hormones and Ca2+-mobilizing hormones on the Ca2+ influx and polyphosphoinositide metabolism in isolated rat hepatocytes. Biochem J. 1986 May 1;235(3):663–669. doi: 10.1042/bj2350663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


