Abstract
Hypertensive rats with multiple extra copies of the renin gene (TGR) exert an inverted circadian blood pressure (BP) profile. We investigated whether circadian oscillations in the hypothalamic suprachiasmatic nucleus (SCN), a main circadian oscillator, and the paraventricular nucleus (PVN), involved in BP control, are influenced in TGR rats. The expression of the clock gene per1, a marker of circadian timing, was measured in the SCN and PVN. Moreover, the expression of genes encoding vasopressin (AVP), vasoactive intestinal peptide (VIP) in the SCN, and AVP and oxytocin (OXT) in the PVN were studied by in situ hybridization. Expression of the per1 gene showed a distinct circadian rhythm in both the SCN and PVN with no differences observed between the TGR and control Sprague–Dawley (SD) rats. The expression of avp in the SCN was rhythmic in both strains and moderately higher in TGR than in SD rats while no significant changes were found in the PVN. The expression of vip in the SCN and oxt in the PVN did not differ between both strains. Our results may indicate that changes occurring downstream to the SCN are responsible for the development of the inverted BP rhythm in TGR hypertensive rats.
Keywords: Hypertension, Circadian rhythm, Suprachiasmatic nucleus, Paraventricular nucleus, Vasopressin, Oxytocin
Introduction
Hypertensive TGR(mREN2)27 rats possess an additional mouse renin gene in their genome that elicits a higher activity of the renin–angiotensin system (RAS) accompanied by systemic hypertension. The TGR rats exert an inverted daily blood pressure (BP) profile with higher levels during the resting (light), and lower levels during the active (dark) phases of the day. Other rhythms, such as the heart rate and locomotor activity, display normal circadian pattern (Lemmer et al. 1993). These rats may be acceptable as an animal model of the non-dipping BP rhythm that is frequently observed in hypertensive patients. Although the mechanisms, leading to the development of the inverted BP rhythm, have been extensively studied at different levels (Lemmer et al. 2003, for review), they are still not fully understood. It is assumed that the inverted BP profile emanates from the interaction of at least three systems: i.e., the RAS, autonomous sympathetic nervous system, and circadian system (Monosíková et al. 2007; Zeman et al. 2008).
Hypothalamus is involved in the control of many body functions, including regulation of the circadian rhythmicity and BP. The suprachiasmatic nucleus (SCN) of the hypothalamus, as a central circadian pacemaker, can be implicated in the control of circadian BP rhythm since its lesion in both TGR and normotensive rats leads to an abolition of the daily BP profile (Lemmer et al. 2000, 2003). On the other hand, the destruction of the SCN in TGR rats does not result in normal BP circadian pattern (Witte et al. 1998), which may indicate that other brain structures could be responsible for the inverted BP rhythm observed in TGR rats. Circadian expression of clock genes in different brain areas involved in the BP control differs between TGR and control rats with the most robust difference found in the area postrema (Monosíková et al. 2007), i.e., in one of the circumventricular organs located on the dorsal side of the medulla oblongata at the caudal end of the fourth ventricle.
The circadian system is constituted in a hierarchical mode with the main pacemaker localized in the SCN and subordinated pacemakers distributed in the peripheral organs (Balsalobre 2002). Circadian oscillations emerge based on the cyclic expression of clock genes per1, per2, per3, cry1, and cry2, the transcriptional factors, bmal1 and clock, and transcriptional–translational feedback loops operating between them (Shearman et al. 2000). The arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) belong to important signaling peptides produced by the SCN. VIP-producing cells are localized mainly in the ventrolateral part of the SCN and are primarily involved in the transmitting of the light information (Shinohara and Inouye 1995). AVP is produced in the dorsomedial part of the SCN, and it serves as a local neurotransmitter stimulating a cell electric activity via the internal SCN circuits (Ingram et al. 1998).
The PVN is an important brain structure integrating neuroendocrine, behavioral, and autonomic processes (de Wardener 2001). It is involved directly in the control of cardiovascular system, BP, and fluid retention by producing and releasing oxytocin (OXT) and AVP and indirectly via modulation of the autonomic nervous system (Pyner and Coote 2000). The PVN is interconnected with the SCN via AVP- and VIP-ergic fibers (Abrahamson and Moore 2001). Bilateral inhibition of the PVN in spontaneously hypertensive (Allen 2002) or Dahl’s salt-sensitive hypertensive rats (Ito et al. 2003) results in a decrease of BP.
The aim of this study was to reveal the extent of disturbance of the circadian oscillations in the central oscillator (SCN) and PVN in TGR rats exhibiting inverted BP rhythm. To this purpose, the expression of the clock gene per1 in the SCN and PVN, as a marker of the circadian timing, was studied. Furthermore, the expression of the signaling peptides, AVP and VIP in the SCN, and AVP and OXT in the PVN, was determined in mature control and TGR hypertensive rats.
Materials and Methods
Animals
In our experiment, 36 hypertensive heterozygous TGR[mREN2]27 and 35 normotensive Sprague–Dawley male rats were used. Both strains of the rats were obtained from the Institute of Clinical and Experimental Medicine (Prague, Czech Republic), and breeding pairs came from the Max-Delbrück Center for Molecular Medicine (Berlin, Germany).
Animals were kept in a room with controlled temperature 21 ± 1°C, 12-h:12-h light–dark regimen (lights on from 7:00, that is zeitgeber time 0 = ZT0). Food and drinking water were available ad libitum.
Experiment was performed on 13 weeks old animals. Animals were sacrificed under CO2 anaesthesia in 4-h intervals over the 24-h cycle. During the dark phase of the day, a dim red light was used to avoid the interference with the melatonin production (Zeman et al. 2008). The experimental protocol was approved by the Ethical Committee for the Care and Use of Laboratory Animals at the Comenius University of Bratislava.
In another experiment, daily profiles of systolic blood pressure (BP), heart rate (HR), and locomotor activity (LA) were determined in 10 mature male TGR rats. In accordance with the recently published recommendation for BP measurement in experimental animals, a radiotelemetry system for direct BP measurements was employed (Kurtz et al. 2005). Rats, weighing 261 ± 11 g, were implanted with radiotransmitters TA11PA-C40 (Data Sciences, St. Paul, MN) to continuously monitor all the three parameters by a telemetric device for 3 days to prove the inverted BP profile in TGR rats. The measurement was performed at the Institute for Clinical and Experimental Medicine, Prague, Czech Republic, and was approved by the Ethical Committee at the Institute. The animals were anesthetized with a combination of tiletamine, zolazepam (Zoletil, Virbac SA, Carros Cedex, France; 8 mg/kg), and xylasine (Rometar, Spofa, Czech Republic; 4 mg/kg) intramuscularly. An abdominal midline incision was performed to expose the abdominal aorta that was briefly occluded to allow insertion of the transmitter catheter. The catheter was secured in place with tissue glue. The transmitter body was sutured to the abdominal wall along the incision line as the incision was closed. After 10–12 days of recovery from surgery, data acquisition was initiated, and data were collected daily as described previously (Husková et al. 2010).
In Situ Hybridization
After removing from the skull, the brains were immediately frozen on powdered dry ice and stored at −80°C until used for sectioning. Serial coronal sections (14 μm thick) of the hypothalamus were cut on a cryostat (Microm HM 520, Walldorf, Germany). The sections were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) (pH 7.4) for 5 min, rinsed twice in PBS (pH 7.4), acetylated in 0.25% acetic anhydride, in 0.8% triethanolamine, HCl/0.9% NaCl (10 min), dehydrated through graded ethanol, delipidated in chloroform (5 min), and dipped in 100 and 95% ethanol. Finally, slides were air-dried.
For in situ hybridization, we used oligodeoxynucleotide probes: oxt 41-mer (Patisaul et al. 2003), per1 45-mer (Ohta et al. 2003), vip 48-mer (Duncan 1998), and avp 48-mer (Jác et al. 2000). As controls, sense probes for per1 and vip genes were used. Probes were radiolabeled with 35S-dATP with Terminal Deoxynucletidyl Transferase (Fermentas, Burlington, Canada). Nonincorporated radioactivity was removed with Sephadex G-50 micro columns (GE Healthcare, Little Chalfont, UK).
The slices were incubated with 70 μl of hybridization buffer per slide (50% formamide, 4× SSC, 1× Denhardt’s, 10% dextrane sulfate, 500 μg/ml sheared single stranded DNA, 250 μg/ml yeast tRNA before use mixed with 0.1 M dithiothreitol (DTT) final concentration) with purified radiolabeled oligoprobe (172,000 cpm for avp, vip, oxt and 477,000 cpm for per1 per one slide). Slides were incubated in humid boxes for 16–20 h at 41°C. Post hybridization washes were processed by 1× SSC for 5 min at room temperature, then in 1× SSC for 1 h at 55°C, and in 1× SSC for 1 h at room temperature. Afterward, the slides were dipped in distilled water and 70% ethanol and air-dried.
Slides were exposed to Hyperfilm-Beta-Max (Eastman Kodak Company, Chalon sur Saône, France) (oxt for 9 h, avp for 14 h, vip for 4–5 days, per1 for 4 weeks—times were determined empirically). The film was developed in a developer (LQN) and fixed in FOMAFIX (FOMA, Hradec Králové, Czech Republic) solution. After exposition, the slides were stained with cresyl violet to determine the position and shape of the SCN and PVN areas.
Autoradiographs were analyzed using a Scion Image software program and, on each slide, the relative density of individual structures was measured. Densities of SCN and PVN areas were bilaterally but separately evaluated on each section. As an internal standard, the background density adjacent to each structure was captured and deducted from the SCN and PVN densities (on each side separately). The mean of the left- and right-side values was taken as the final result.
Statistical Analysis
The rhythmic production of the mRNA was evaluated by the cosinor analysis over a 24-h period. When the data significantly matched the cosinor curve at P < 0.05, the acrophase was calculated. Differences between the TGR and SD rats across the 24-h period were analyzed by LSD post hoc tests following the two-way analysis of variance (ANOVA) with grouping factors strain and ZT.
Results
Daily Profile of BP, HR, and LA in TGR Rats
All TGR rats were hypertensive and exhibited an inverted systolic BP profile with higher values measured during their inactive light-time period and lower values during the active darktime period (Fig. 1). The daily rhythms of HR and LA were not inverted, and their peak values were found during the darktime. The HR rhythm followed the one in LA but was out of phase with the BP rhythm.
Fig. 1.
Daily rhythms in systolic blood pressure (Sys BP), heart rate (HR), and locomotor activity (LA) in mature male TGR rats. Each value represents the mean ± SEM (n = 10). The gray fields in the graph represent a dark phase of 24 h cycle (LD 12:12)
Expression of per1 mRNA in the SCN and PVN
Representative autoradiographs from in situ hybridization with Avp and Per1 oligoprobes in TGR and SD rats are shown in Fig. 2. Expression of per1 in the SCN exerted a robust circadian rhythm (P < 0.001) in both rat strains with acrophase at ZT 5:27 and 5:48 in TGR and SD rats, respectively. Low levels of per1 expression were found during the dark phase in both the TGR and SD groups. There were no significant differences in the levels of the per1 mRNA in the SCN between TGR and SD rats (F (1,40) = 0.150, P = 0.70; Fig. 3a).
Fig. 2.
A representative autoradiograph from in situ hybridization with Avp and Per1 oligoprobes in TGR and SD rats at the time of highest and lowest levels of the mRNAs
Fig. 3.
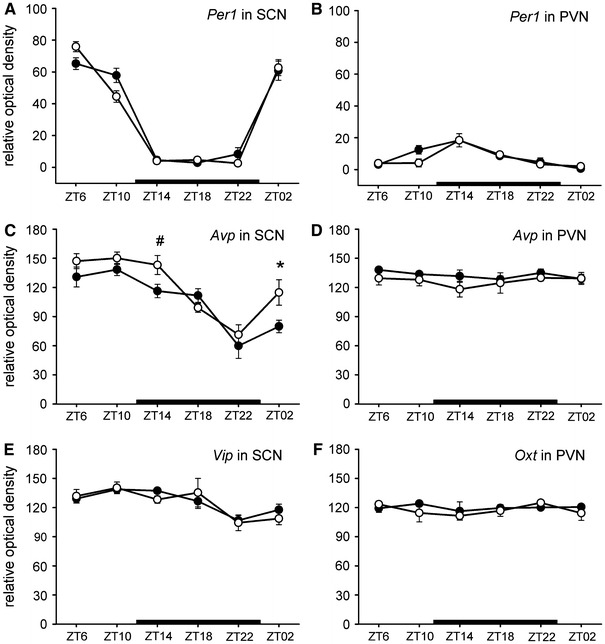
Daily patterns of Per1 (a), Avp (c), and Vip (e) expression in the SCN and Per1 (b), Avp (d), and Oxt (f) expressions in the PVN. Expression of mRNA was examined by in situ hybridization in the hypertensive TGR rats (opened circles) and normotensive SD rats (solid circles). Each value represents the mean ± SEM (n = 4–6). Zeitgeber time (ZT) is shown on the horizontal axis. The black bar on the bottom of each graph represents a dark phase of 24-h cycle (LD 12:12). * P < 0.01, # P = 0.05 (LSD post hoc tests between TGR and SD rats)
In the PVN, per1 mRNA exerted a low-amplitude rhythm with the acrophase at the beginning of the dark phase at ZT 14:14 and 13:35 in TGR and SD rats, respectively. The per1 mRNA expression in the PVN did not differ between TGR and SD rats (F (1,35) = 0.802, P = 0.376; Fig. 3b).
Expression of avp mRNA in the SCN and PVN
The avp expression in the SCN exerted a robust circadian rhythm (P < 0.001) in both TGR and SD rats, with acrophase at ZT 9:21 in TGR and at ZT 10:07 in SD rats. High levels of avp mRNA during the light phase and low levels during the dark phase were found in both groups. The two-way ANOVA revealed a significant effect of strain on the avp expression in the SCN (F (1,34) = 7.620, P < 0.01; Fig. 3c). Levels of the avp mRNA were higher in the SCN of TGR than SD rats at ZT 2 (P < 0.01) and ZT 14 (P = 0.05).
The expression of the avp in the PVN did not exert rhythmic profile in the TGR and SD group (Fig. 3d). Differences between strains were found at the level of significance (F (1,41) = 3.790, P = 0.058) with lower avp mRNA expression in TGR (126.99 ± 2.57; mean ± SEM) in comparison with SD animals (132.7 ± 1.67).
Expression of vip mRNA in the SCN
The expression of the vip in the SCN exerted a low-amplitude rhythm (P < 0.05 in TGR, P < 0.001 in SD rats) with acrophase at ZT 11:46 in TGR rats and at ZT 11:09 in SD rats with a trough at the end of the dark phase. No significant differences were found between TGR and SD rats in the vip mRNA expression (F (1,37) = 0.102, P = 0.751; Fig. 3e).
Expression of oxt mRNA in the PVN
Expression of the oxt mRNA in the PVN was not rhythmic either in the TGR or SD group (cosinor). Statistical analysis did not reveal any significant differences in the oxt mRNA levels between the TGR and SD rats (F (1,29) = 0.655, P = 0.425; Fig. 3f).
Discussion
The TGR rats are characterized by fulminant hypertension and an inverted BP profile (Lemmer et al. 1993) that is detectable under the light/dark conditions and constant darkness (Witte and Lemmer 1995). Hypertension and the inverted BP profile were proven in our present experiment in TGR rats. The reasons why the inverted BP develops are not clear, but the role of interactions between the circadian cycle, RAS, and autonomous nervous system are considered (Lemmer et al. 2003; Monosíková et al. 2007). It is assumed that all the above mentioned systems may interact at the level of different brain “nodal” structures (Monosíková et al. 2007).
Present data demonstrate a robust circadian variation in the per1 mRNA expression in the central circadian oscillator (SCN) and a low-amplitude rhythm in the PVN. The phase difference of rhythms in the per1 mRNA expression between both the SCN and PVN reflects an expected phase difference between the central and peripheral oscillators (Asai et al. 2001). Present results correspond well with recently published data (Herichová et al. 2007; Monosíková et al. 2007) hypothesizing that rhythmic clock gene expression is preserved in the SCN of TGR rats and, therefore, changes in down-stream structures may be responsible for the inverted BP profile in TGR rats.
The avp mRNA levels in the SCN exerted a rhythmic 24-h profile with an acrophase in the second half of the lighttime in both the TGR and SD rats. These results correspond well with the data reported by Yambe and co-workers (2002) in Sprague–Dawley rats. We did not find any differences in the acrophase of the avp mRNA expression in the SCN between the strains studied, although higher levels of avp mRNA occurred in TGR as compared with SD rats, being the most distinct at the beginning of the light (ZT 2) and dark (ZT 14) phases. The rhythmic avp expression in the SCN of TGR rats indicates that the central circadian oscillator is not disturbed in this model of hypertension in spite of the inverted BP profile. In contrast, the higher avp expression in TGR as compared to SD rats suggests that SCN oscillations might be up-regulated like some other endocrine rhythms in TGR rats (Zeman et al. 2008).
In contrast with the SCN, we did not find any increase in the avp expression in the PVN proving a differential control of avp expression in both the above mentioned nuclei (Burbach et al. 1984; Yambe et al. 2002). Moreover, post-transcriptional regulation of AVP concentrations in the lower brain stem, in comparison with the posterior pituitary, has already been demonstrated (Moriguchi et al. 1994). Microinjections of angiotensin II (Ang), a final mediator of the RAS, into the PVN have been shown to elicit dose-dependent AVP secretion (Tsushima et al. 1994), and intracerebroventricular administration of Ang increased avp mRNA expression (Zhang et al. 2009). Vasopressin release is stimulated by Ang also in the TGR rats (Nishioka et al. 1999). Therefore, the authors assume that interactions between the osmotic and angiotensin receptors in the circumventricular regions may be responsible for this stimulation. This notion corresponds to the phase-shifted clock gene expression in the area postrema (Monosíková et al. 2007) and thus strengthens the role of brain structures situated downstream to the SCN in generating the inverted BP profile.
In our study, we found rhythmic expression of vip in the SCN of both TGR and control rats. The daily profile did not differ between these strains. Higher expression of vip mRNA was determined in the SCN of SHR in comparison with normotensive rats (Avidor et al. 1989; Peters et al. 1994). The daily profile of the vip expression seems to reflect predominantly prevailing light–dark conditions (Ban et al. 1997) and is not an integral part of circadian organization. This may explain the differences between the daily profiles of vip mRNA expression in published studies, and suggests an unchanged sensitivity of TGR rats to lighting conditions.
We did not reveal a circadian rhythm in the oxt mRNA in the PVN of TGR or SD rats. This finding is in agreement with the published data (Burbach et al. 1988) where no circadian rhythmicity in the oxt mRNA was observed in the PVN and SON of rats. Only in the PVN of 10-week old SHR, about 25% higher levels of oxt mRNA has been measured (van Tol et al. 1988) in comparison with normotensive Wistar-Kyoto control rats. Since, we did not find any differences in the expression of oxt mRNA in the PVN between SD and TGR rats, we believe that this neurohormone does not play a significant role in the development of hypertension in the TGR rats.
Our study demonstrates the presence of distinct circadian rhythms in the per1 mRNA expression in the SCN and PVN of control and TGR hypertensive rats. These results, in concordance with the previously published data (Herichová et al. 2007; Monosíková et al. 2007), suggest that the transcriptional–translational loop in the SCN of TGR rats is not substantially disturbed in the TGR hypertensive rats exhibiting an inverted BP rhythm. Since none of the investigated signaling peptides displayed deregulation in their gene expressions in the SCN and PVN of the TGR rats, we suggest that structures located downstream from the SCN and PVN are responsible for the development of the inverted BP rhythm.
Acknowledgments
This study was supported by the grants APVV No. 20-022-704, APVV-0214-07, CENDO. L.Č. and L.K. are supported by the institutional financial support of the Institute for Clinical and Experimental Medicine (MZO 00023001). We thank professor D. Ježová (Institute of Experimental Endocrinology, Slovak Academy of Sciences, Bratislava, Slovakia) for providing the avp probe.
References
- Abrahamson EE, Moore RY (2001) The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res 889:1–22 [DOI] [PubMed] [Google Scholar]
- Allen AM (2002) Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39:275–280 [DOI] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S (2001) Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66:1133–1139 [DOI] [PubMed] [Google Scholar]
- Avidor R, Eilam R, Malach R, Gozes I (1989) VIP-mRNA is increased in hypertensive rats. Brain Res 503:304–307 [DOI] [PubMed] [Google Scholar]
- Balsalobre A (2002) Clock genes in mammalian peripheral tissues. Cell Tissue Res 309:193–199 [DOI] [PubMed] [Google Scholar]
- Ban Y, Shigeyoshi Y, Okamura H (1997) Development of vasoactive intestinal peptide mRNA rhythm in the rat suprachiasmatic nucleus. J Neurosci 17:3920–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JP, de Hoop MJ, Schmale H, Richter D, de Kloet ER, ten Haaf JA, de Wied D (1984) Differential responses to osmotic stress of vasopressin-neurophysin mRNA in hypothalamic nuclei. Neuroendocrinology 39:582–584 [DOI] [PubMed] [Google Scholar]
- Burbach JP, Liu B, Voorhuis TA, van Tol HH (1988) Diurnal variation in vasopressin and oxytocin messenger RNAs in hypothalamic nuclei of the rat. Brain Res 464:157–160 [DOI] [PubMed] [Google Scholar]
- de Wardener HE (2001) The hypothalamus and hypertension. Physiol Rev 81:1599–1658 [DOI] [PubMed] [Google Scholar]
- Duncan MJ (1998) Photoperiodic regulation of hypothalamic neuropeptide messenger RNA expression: effect of pinealectomy and neuroanatomical location. Brain Res Mol Brain Res 57:142–148 [DOI] [PubMed] [Google Scholar]
- Herichová I, Mravec B, Stebelová K, Krizanová O, Jurkovicová D, Kvetnanský R, Zeman M (2007) Rhythmic clock gene expression in heart, kidney and some brain nuclei involved in blood pressure control in hypertensive TGR(mREN-2)27 rats. Mol Cell Biochem 296:25–34 [DOI] [PubMed] [Google Scholar]
- Husková Z, Vanourková Z, Erbanová M, Thumová M, Opočenský M, Mullins JJ, Kramer HJ, Bürgelová M, Červenka L (2010) Inappropriately high circulating and intrarenal angiotensin II levels during dietary salt loading exacerbate hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens 28:495–509 [DOI] [PubMed] [Google Scholar]
- Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R (1998) Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res 119:351–364 [DOI] [PubMed] [Google Scholar]
- Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF (2003) Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension 41:744–750 [DOI] [PubMed] [Google Scholar]
- Jác M, Kiss A, Sumová A, Illnerová H, Jezová D (2000) Daily profiles of arginine vasopressin mRNA in the suprachiasmatic, supraoptic and paraventricular nuclei of the rat hypothalamus under various photoperiods. Brain Res 887:472–476 [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE (2005) Recommendations for blood pressure measurements in humans and experimental animals. Part 2: blood pressure measurements in experimental animals. Hypertension 45:299–310 [DOI] [PubMed] [Google Scholar]
- Lemmer B, Mattes A, Böhm M, Ganten D (1993) Circadian blood pressure variation in transgenic hypertensive rats. Hypertension 22:97–101 [DOI] [PubMed] [Google Scholar]
- Lemmer B, Hauptfleisch S, Witte K (2000) Loss of 24 h rhythm and light-induced c-fos mRNA expression in the suprachiasmatic nucleus of the transgenic hypertensive TGR(mREN2)27 rat and effects on cardiovascular rhythms. Brain Res 883:250–257 [DOI] [PubMed] [Google Scholar]
- Lemmer B, Witte K, Enzminger H, Schiffer S, Hauptfleisch S (2003) Transgenic TGR(mRen2)27 rats as a model for disturbed circadian organization at the level of the brain, the heart, and the kidneys. Chronobiol Int 20:711–738 [DOI] [PubMed] [Google Scholar]
- Monosíková J, Herichová I, Mravec B, Kiss A, Zeman M (2007) Effect of upregulated renin–angiotensin system on per2 and bmal1 gene expression in brain structures involved in blood pressure control in TGR(mREN-2)27 rats. Brain Res 1180:29–38 [DOI] [PubMed] [Google Scholar]
- Moriguchi A, Ferrario CM, Brosnihan KB, Ganten D, Morris M (1994) Differential regulation of central vasopressin in transgenic rats harboring the mouse Ren-2 gene. Am J Physiol 267:R786–R791 [DOI] [PubMed] [Google Scholar]
- Nishioka T, Callahan MF, Li P, Ferrario CM, Ganten D, Morris M (1999) Increased central angiotensin and osmotic responses in the Ren-2 transgenic rat. Hypertension 33:385–388 [DOI] [PubMed] [Google Scholar]
- Ohta H, Honma S, Abe H, Honma K (2003) Periodic absence of nursing mothers phase-shifts circadian rhythms of clock genes in the suprachiasmatic nucleus of rat pups. Eur J Neurosci 17:1628–1634 [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Scordalakes EM, Young LJ, Rissman EF (2003) Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol 15:787–793 [DOI] [PubMed] [Google Scholar]
- Peters RV, Zoeller RT, Hennessey AC, Stopa EG, Anderson G, Albers HE (1994) The control of circadian rhythms and the levels of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus are altered in spontaneously hypertensive rats. Brain Res 639:217–227 [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH (2000) Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100:549–556 [DOI] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM (2000) Interacting molecular loops in the mammalian circadian clock. Science 288:1013–1019 [DOI] [PubMed] [Google Scholar]
- Shinohara K, Inouye ST (1995) Photic information coded by vasoactive intestinal polypeptide and neuropeptide Y. Neurosci Biobehav Rev 19:349–352 [DOI] [PubMed] [Google Scholar]
- Tsushima H, Mori M, Matsuda T (1994) Microinjections of angiotensin II into the supraoptic and paraventricular nuclei produce potent antidiureses by vasopressin release mediated through adrenergic and angiotensin receptors. Jpn J Pharmacol 66:241–246 [DOI] [PubMed] [Google Scholar]
- van Tol HH, van den Buuse M, de Jong W, Burbach JP (1988) Vasopressin and oxytocin gene expression in the supraoptic and paraventricular nucleus of the spontaneously hypertensive rat (SHR) during development of hypertension. Brain Res 464:303–311 [DOI] [PubMed] [Google Scholar]
- Witte K, Lemmer B (1995) Free-running rhythms in blood pressure and heart rate normotensive and transgenic hypertensive rats. Chronobiol Int 12:237–247 [DOI] [PubMed] [Google Scholar]
- Witte K, Schnecko A, Buijs RM, van der Vliet J, Scalbert E, Delagrange P, Guardiola-Lemaître B, Lemmer B (1998) Effects of SCN lesions on circadian blood pressure rhythm in normotensive and transgenic hypertensive rats. Chronobiol Int 15:135–145 [DOI] [PubMed] [Google Scholar]
- Yambe Y, Arima H, Kakiya S, Murase T, Oiso Y (2002) Diurnal changes in arginine vasopressin gene transcription in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res 104:132–136 [DOI] [PubMed] [Google Scholar]
- Zeman M, Petrák J, Stebelová K, Nagy G, Krizanová O, Herichová I, Kvetnanský R (2008) Endocrine rhythms and expression of selected genes in the brain, stellate ganglia, and adrenals of hypertensive TGR rats. Ann NY Acad Sci 1148:308–316 [DOI] [PubMed] [Google Scholar]
- Zhang L, Tong M, Xiao M, Li L, Ding J (2009) Nitric oxide mediates feedback inhibition in angiotensin II-induced upregulation of vasopressin mRNA. Peptides 30:913–917 [DOI] [PubMed] [Google Scholar]




