Abstract
Lipopolysaccharide (LPS) has been used as a reagent for a model of systemic inflammatory response. Ribosomal protein S3 (rpS3) is a multi-functional protein that is involved in transcription, metastasis, DNA repair, and apoptosis. In the present study, we examined the changes of rpS3 immunoreactivity in the mouse hippocampus after systemic administration of 1 mg/kg of LPS. From 6 h after LPS treatment, rpS3 immunoreactivity was decreased in pyramidale cells of the hippocampus proper (CA1–CA3 regions) and in granule cells of the dentate gyrus. At this point in time, rpS3 immunoreactivity began to increase in non-pyramidal cells and non-granule cells. From 1 day after LPS treatment, rpS3 immunoreactivity in pyramidal and granule cells was hardly detected; however, strong rpS3 immunoreactivity was shown in non-pyramidal and non-granule cells. Based on double immunofluorescence staining for rpS3/ionized calcium-binding adapter 1 (Iba-1, a marker for microglia) and glial fibrillary acidic protein (GFAP, a marker for astrocytes), strong rpS3 immunoreactivity was expressed in Iba-1-immunoreactive microglia, not in GFAP-immunoreactive astrocytes, at 1 and 2 days after LPS treatment. These results indicate that rpS3 immunoreactivity changes only in pyramidal and granule cells, and rpS3 is expressed only in activated microglia after LPS treatment: this may be associated with the neuroinflammatory responses in the brain.
Keywords: Multi-functional protein, Lipopolysaccharide, Neuroinflammation, Glia
Introduction
Lipopolysaccharide (LPS) is present in the outer membrane of Gram-negative bacteria, and it causes a strong response from normal animal immune system (Morrison and Ryan 1979; Tough et al. 1997). LPS has been commonly used as a reagent for a model of systemic inflammatory response induced by infections, and its treatment leads to neuroanatomical and neurochemical changes in some regions of the brain (Benicky et al. 2009; Guan and Fang 2006; Hochweller and Anderton 2005; Lee et al. 1993; Linthorst et al. 1995). In addition, LPS has been used as an effectively non-specific stimulator of microglia, which controls neuroinflammatory responses (Block et al. 2007; Kreutzberg 1996; Stoll et al. 1998; Sumi et al. 2010; Zhou et al. 2008; Zielasek and Hartung 1996).
Ribosomes, which are large ribonucleoprotein machines, are made from complexes of RNAs and proteins. Ribosomes consist of two subunits such as small 40S subunit and large 60S subunit (Doudna and Rath 2002). Ribosomal protein S3 (rpS3), which is a component of the 40S small subunit, is a well-known multi-functional protein that is involved in transcription, metastasis, DNA repair, and apoptosis (Jang et al. 2004; Kim et al. 1995; Kim and Kim 2006; Kim et al. 2009; Lee et al. 2010; Wan et al. 2007; Wool 1996). Especially, rpS3 is well known to participate in DNA damage/repair mechanisms (Kim et al. 1995; Wool 1996). Recently, we reported that transduced PEP-1-rpS3 reduced the level of proinflammatory cytokines in a mouse model of 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation (Ahn et al. 2010) and that it ameliorated ischemic damage by reducing DNA fragmentation and lipid peroxidation in the gerbil hippocampal CA1 region (Hwang et al. 2008). We also showed that rpS3 immunoreactivity and its protein level were markedly decreased in the aged hippocampus (Lee et al. 2011).
Although previous studies have focused on effects of LPS on neurodegeneration and immune response in various regions of the brain (Chung et al. 2010; Nishioku et al. 2009; Saavedra and Pavel 2006), there is no study on LPS-induced changes of rpS3 expression in the hippocampus. Therefore, in the present study, we examined the changes of rpS3 immunoreactivity in the mouse hippocampus after a systemic administration of LPS.
Materials and Methods
Experimental Animals
Six-week-old male ICR mice were purchased from the Jackson Laboratory (Maine, ME). The animals were housed in a conventional state under adequate temperature (23 ± 3°C) and relative humidity (55 ± 5%) control with a 12-h light/12-h dark cycle and provided with free access to food and water. The procedures for handling and caring for animals adhered to the guidelines that are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
LPS Treatment
Lipopolysaccharide (Sigma, St. Louis, MO) was dissolved in saline and administered intraperitoneally with 1.0 mg/kg/10 ml dose. The control animals were injected with the same volume of saline.
Production and Purification of Anti-Human rpS3 mAbs
Production and purification of rpS3 monoclonal antibody to the enzyme were performed as previously described (Choi et al. 2006; Hwang et al. 2008). The antibody was produced by cell fusion after immunization of BALB/c mice with purified human rpS3.
Tissue Processing
Mice (n = 7 at each time point) were killed at designated times (3, 6, 12, 24, 48, and 96 h after LPS treatment). For the histological analysis, animals were anesthetized with a mixture of ketamine (60 mg/kg, Yuhan, Seoul, South Korea) and xylazine (8 mg/kg, Bayer Animal Health, Suwon, South Korea) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were removed and postfixed in the same fixative for 4 h. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter, frozen tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30 μm coronal sections, and they were then collected into six-well plates containing 0.1 M PBS.
Immunohistochemistry for rpS3
For rpS3 immunohistochemistry, the sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and 10% normal goat serum in 0.05 M PBS for 30 min. They were next incubated with diluted mouse anti-rpS3 overnight at room temperature and subsequently exposed to biotinylated goat anti-mouse IgG (1:200, Vector, Burlingame, CA) and streptavidin peroxidase complex (1:200, Vector). They were then visualized by staining with 3,3′-diaminobenzidine (Sigma) in 0.1 M Tris–HCl buffer (pH 7.2) and mounted on gelatin-coated slides. After dehydration, the sections were mounted with Canada balsam (Kanto, Tokyo, Japan). In order to establish the specificity of the immunostaining, a negative control test was carried out with pre-immune serum instead of primary antibody. The negative control resulted in the absence of immunoreactivity in any structures (Fig. 1e).
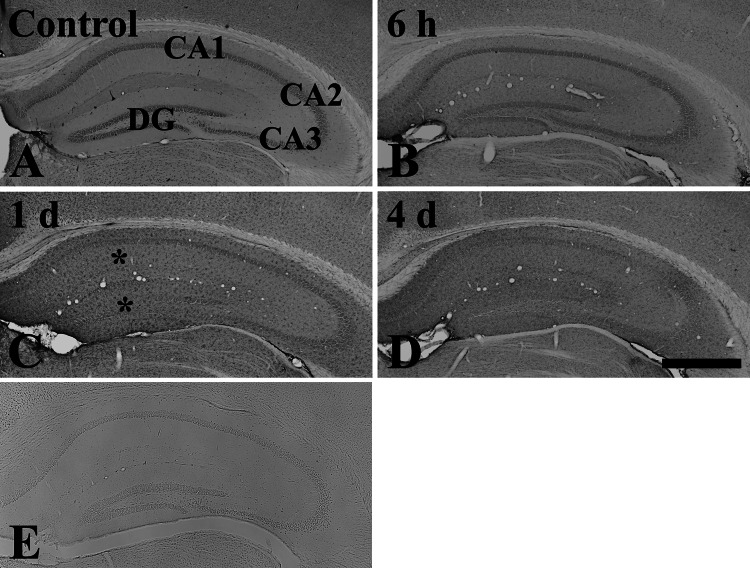
Fig. 1.
Low magnification of immunohistochemical staining for rpS3 in the hippocampus of the control (a) and LPS-treated (b–d) groups. rpS3 immunoreactivity is well observed in the stratum pyramidale of the hippocampus proper and in the granule cell layer of the dentate gyrus of the control-group. At 1 day after LPS treatment, rpS3 immunoreactivity (asterisk) is markedly increased in non-pyramidal and non-granular cells. rpS3 immunoreactivity is decreased at 4 days compared to that at 1 day after LPS treatment. e Negative control test shows the absence of rpS3 immunoreactivity in the hippocampus. CA conus ammonis, DG dentate gyrus. Scale bar 400 μm
Eight sections per animal were selected to quantitatively analyze rpS3 immunoreactivity. rpS3 immunoreactivity was graded. Digital images of the hippocampal subregions were captured with an AxioM1 light microscope (Carl Zeiss, Germany) equipped with a digital camera (Axiocam, Carl Zeiss) connected to a PC monitor. Semi-quantification of the immunostaining intensities was evaluated with digital image analysis software (MetaMorph 4.01, Universal Imaging Corp.). The mean intensity of immunostaining in each immunoreactive structures was measured by a 0–255-gray scale system (white to dark signal corresponded from 255 to 0). Based on this approach, the level of immunoreactivity was scaled as −, ±, +, or ++, representing no staining (gray scale value: ≥200), weakly positive (gray scale value: 150–199), moderate (gray scale value: 100–149), or strong (gray scale value: ≤99), respectively.
Double Immunofluorescence Staining
To confirm the cell type containing rpS3 immunoreactivity, the sections were processed by double immunofluorescence staining. Double immunofluorescence staining was performed using diluted mouse anti-rpS3/rabbit anti-glial fibrillary acidic protein (GFAP) (1:200, Chemicon International) for astrocytes or rabbit anti-ionized calcium-binding adapter 1 (Iba-1) (1:200, Wako, Osaka, Japan) for microglia. The sections were incubated in the mixture of antisera overnight at room temperature. After washing three times for 10 min with PBS, they were then incubated in a mixture of both FITC-conjugated goat anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA) and Cy3-conjugated goat anti-rabbit IgG (1:200; Jackson ImmunoResearch) for 2 h at room temperature. The immunoreactions were observed under the confocal MS (LSM510 META NLO, Carl Zeiss, Germany).
Western Blot Analysis
To confirm change in rpS3 levels in the hippocampus at designated times (6, 48, and 96 h after LPS treatment), animals at each time point (n = 5) were used for western blot analysis. After killing animals, the hippocampus was removed. The tissues were then homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol bis (2-aminoethyl ether)-N,N,N′,N′ tetraacetic acid (pH 8.0), 0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol (DTT). After centrifugation at 10,000g, the protein level in the supernatants was determined using a Micro BCA protein assay kit with bovine serum albumin as a standard (Pierce Chemical, Rockford, IL). Aliquots containing 20 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. The aliquots were then loaded onto a 10% polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Corp., East Hills, NY). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min, followed by incubation with mouse anti-rpS3 antiserum or mouse anti-β-actin (1:5,000, Sigma), peroxidase-conjugated goat anti-mouse IgG (Sigma), and an ECL kit (Pierce Chemical). These two proteins were detected using different lane.
The result of the western blot analysis was scanned, and densitometric analysis for the quantification of the bands was done using Scion Image software (Scion Corp., Frederick, MD), which was used to count relative optical density (ROD): a ratio of the ROD was calibrated as %, with the control-group designated as 100%.
Statistical Analysis
Data are expressed as the mean ± SD. The data were evaluated by SPSS program and the means assessed using two-tailed Student t test or one-way ANOVA test. Statistical significance was considered at P < 0.05.
Results
Change in rpS3 Immunoreactivity
In the control-group, strong rpS3 immunoreactivity was mainly detected in pyramidal cells in the striatum pyramidale of the hippocampus proper (CA1-3 regions), and, in the dentate gyrus, the immunoreactivity was found in granule cells in the granule cell layer and in polymorphic cells in the polymorphic layer (Table 1; Figs. 1a, 2a, 3a, 4a). At 3 h after LPS treatment, the pattern of rpS3 immunoreactivity in the hippocampus proper and dentate gyrus was not changed compared to that in the control-group (Table 1; Figs. 2b, 3b, 4b).
Table 1.
Semi-quantifications of rps3 immunoreactivity in the mouse hippocampus after LPS treatment
| Cell type | Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | LPS treatment | |||||||
| 3 h | 6 h | 12 h | 24 h | 48 h | 96 h | |||
| CA1-3 region | PC | ++ | ++ | + | + | − | − | − |
| NPC | + | + | + | + | ++ | ++ | + | |
| Dentate gyrus | CML | − | − | ± | + | ++ | ++ | + |
| GC | ++ | ++ | + | ± | − | − | − | |
| CPL | ++ | ++ | + | + | ++ | ++ | + | |
Immunoreactivity is scaled as −, ±, +, or ++ representing no staining, weakly positive, moderate, or strong, respectively
CML cells in molecular layer, CPL cells in polymorphic layer, GC granule cells, NPC non-pyramidal cells, PC pyramidal cells
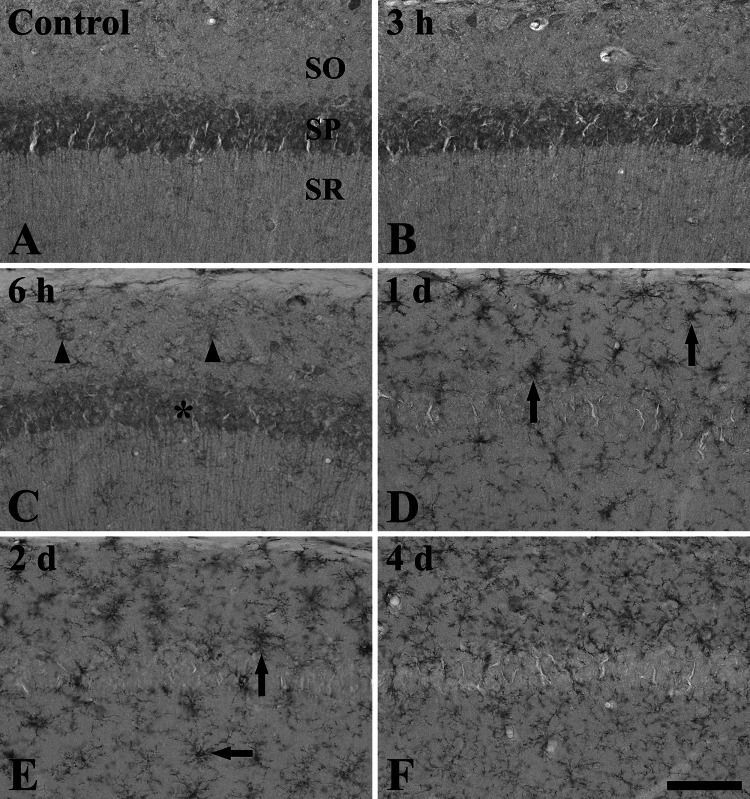
Fig. 2.
Immunohistochemistry for rpS3 in the CA1 region of the control (a) and LPS-treated (b–f) groups. From 6 h after LPS treatment, rpS3 immunoreactivity is decreased in the stratum pyramidale (SP, asterisk) and increased in non-pyramidal cells (arrowheads). Strong rpS3 immunoreactivity is shown in non-pyramidal cells of the stratum oriens (SO) and stratum radiatum (SR) at 1 and 2 days after LPS treatment (arrows). Scale bar 50 μm
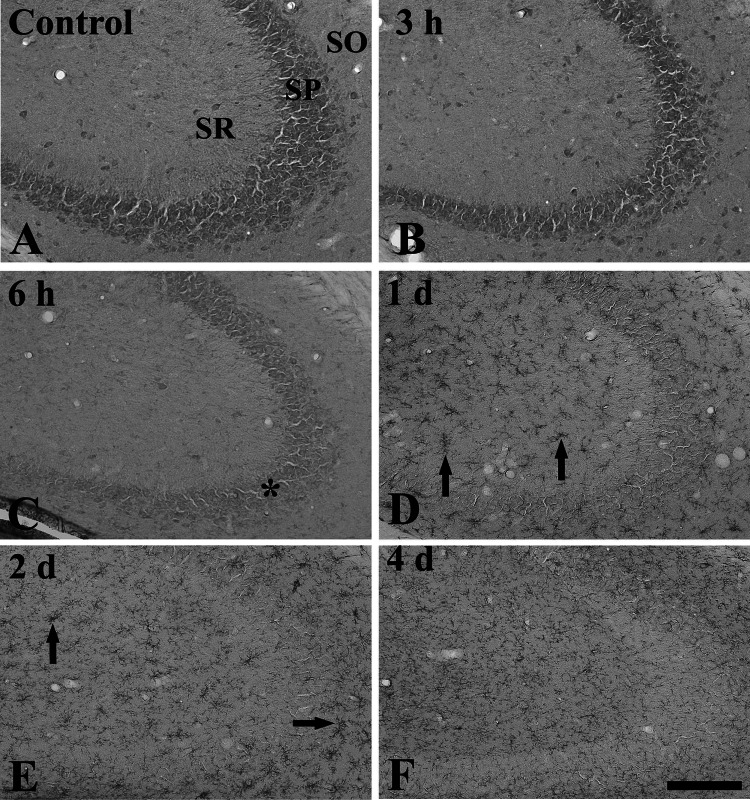
Fig. 3.
Immunohistochemistry for rpS3 in the CA2/3 region of the control (a) and LPS-treated (b–f) groups. From 6 h after LPS treatment, rpS3 immunoreactivity is decreased in the stratum pyramidale (SP, asterisk) and hardly detected in the SP from 1 day after LPS treatment. One and 2 days after LPS treatment, rpS3 immunoreactivity is markedly increased in non-pyramidal cells (arrows). SO stratum oriens, SR stratum radiatum. Scale bar 100 μm
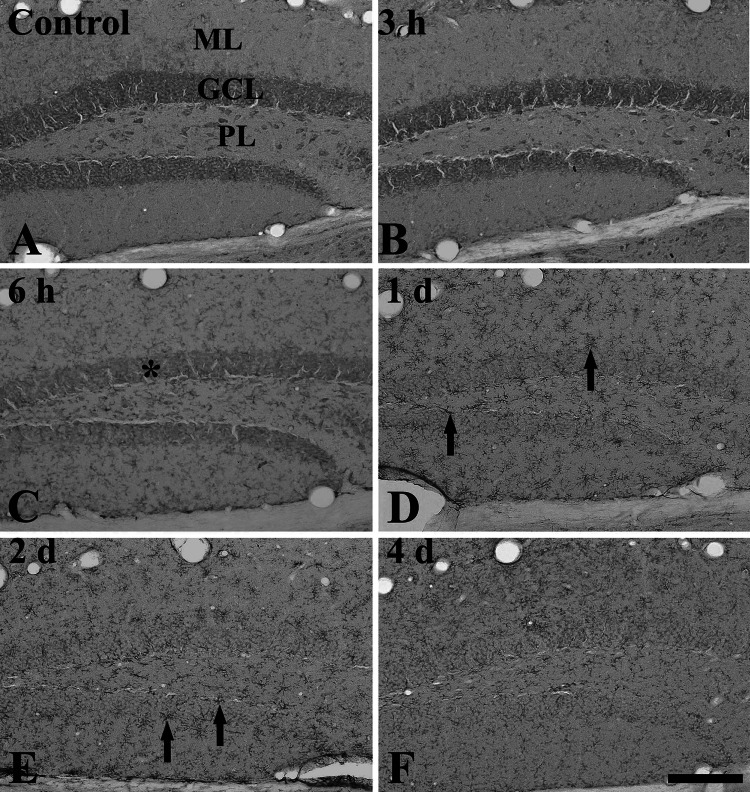
Fig. 4.
Immunohistochemistry for rpS3 in the dentate gyrus of the control (a) and LPS-treated (b–f) groups. From 6 h after LPS treatment, rpS3 immunoreactivity is markedly decreased in the granule cell layer (GCL, asterisk). RpS3 immunoreactivity is increased in non-granule cells at 1 and 2 days after LPS treatment. rpS3 immunoreactivity in non-granule cells is decreased at 4 days after LPS treatment. ML molecular layer, PL polymorphic layer. Scale bar 100 μm
rpS3 immunoreactivity was distinctively decreased 6 h after LPS treatment, and, at 12 h after LPS treatment, the immunoreactivity was similar to that at 6 h after LPS treatment (Table 1; Figs. 1b, 2c, 3c, 4c). One day after LPS treatment, rpS3 immunoreactivity was hardly detected in pyramidal and granule cells; however, non-pyramidal and non-granule cells expressed strong rpS3 immunoreactivity (Table 1; Figs. 1c, 2d, 3d, 4d). Two days after LPS treatment, rpS3 immunoreactivity was similar to that at 1 day after LPS treatment (Table 1; Figs. 2e, 3e, 4e).
Thereafter, rpS3 immunoreactivity in the hippocampus was decreased: 4 days after LPS treatment, the rpS3 immunoreactivity was apparently decreased compared to that at 2 days after LPS treatment, although the distribution pattern of rpS3 immunoreactive structures was not changed (Table 1; Figs. 1d, 2f, 3f, 4f).
rpS3 Expression in Microglia
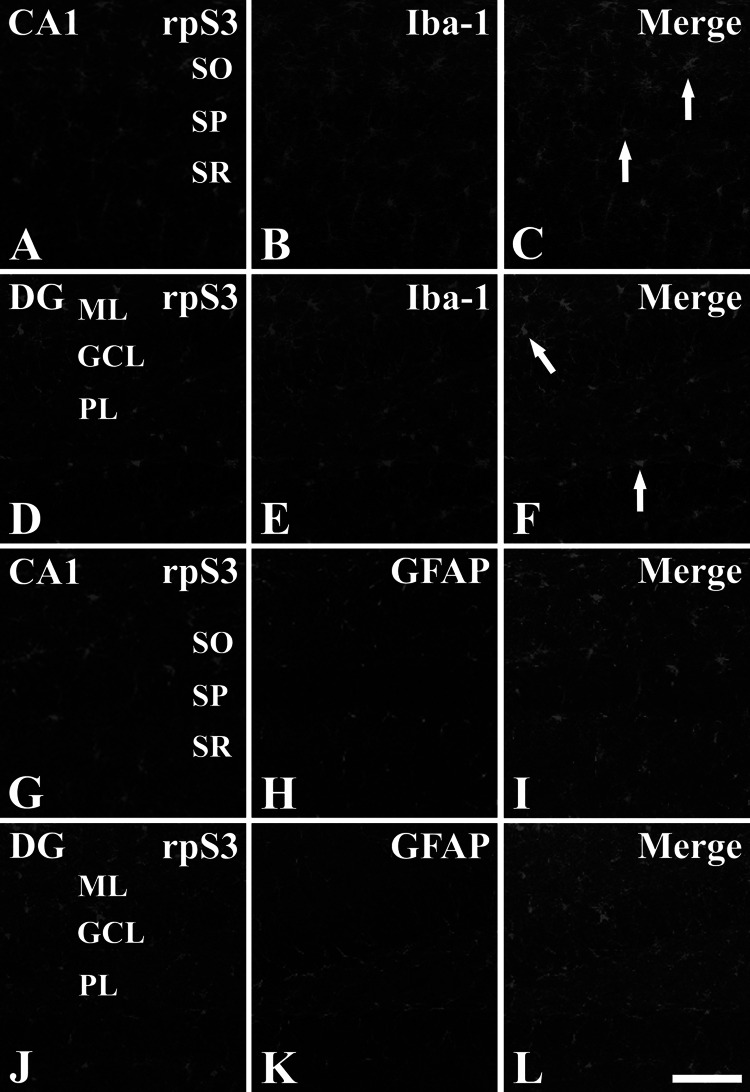
One and two days after LPS treatment, rpS3 immunoreactivity was easily detected in non-pyramidal and non-granule cells in the hippocampus proper and dentate gyrus, respectively. To identify the cell type of rpS3-immunoreactive non-pyramidal and non-granule cells, double immunofluorescence staining was performed for rpS3/Iba-1 (a marker for microglia) or rpS3/GFAP (a marker for astrocytes) in the hippocampus. We found that most of rpS3-immunoreactive non-pyramidal and non-granule cells were identified as Iba-1-immunoreactive microglia, not GFAP-immunoreactive astrocytes (Fig. 5).
Fig. 5.
Double immunofluorescence staining for rpS3 (green, a, d, g, and j), Iba-1 (red, b and e) or GFAP (red, h and k), and merged images (c, f, i, and l) in the CA1 region (a–c and g–i) and dentate gyrus (d–f and j–l) at 1 day after LPS treatment. Most of rpS3-immunoreactive cells are co-localized with Iba-1-immunoreactive microglia (arrows), not with GFAP-immunoreactive astrocytes. SO stratum oriens, SP stratum pyramidale, SR stratum radiatum, ML molecular layer, GCL granule cell layer, PL polymorphic layer. Scale bar 50 μm (Color figure online)
rpS3 Protein Levels
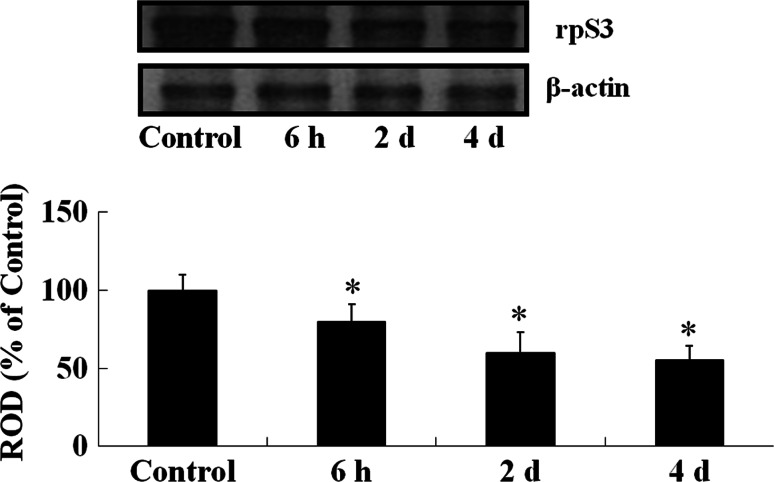
Western blot analysis showed that the levels of rpS3 were changed in the mouse hippocampus after LPS treatment (Fig. 6). rpS3 protein levels were decreased from 6 h after LPS treatment with time. Four days after LPS treatment, the protein level was about 55% of that in the control group.
Fig. 6.
Western blot analysis of rpS3 in the hippocampus of the control- and LPS-treated groups. Relative optical density (ROD) as % values of immunoblot band is also represented (n = 5, *P < 0.05, significantly different from the control group). The bars indicate the means ± SD
Discussion
Neuroinflammation appears to play important roles in neurodegenerative disorders, and it is related to the repair of damaged tissue after some brain injury (Glezer et al. 2007; Martino 2004). In addition, it has been known that chronic or excessive neuroinflammation leads to neuronal damage (Perry et al. 2007; Zipp and Aktas 2006). In the brain, the hippocampus is one of the brain regions most sensitive to LPS (Chung et al. 2010; Gluck and Myers 1997). It was reported that systemic inflammation induced by 10 mg/kg LPS treatment led to cellular apoptosis in the rat hippocampus (Semmler et al. 2005). It was also reported that LPS treatment induced cognitive impairment in mice (Shaw et al. 2001; Sparkman et al. 2005). However, our previous study showed that 1 mg/kg LPS treatment did not induce neuronal damage/death in the mouse hippocampus (Chung et al. 2010).
In the present study, we examined the changes of rpS3 immunoreactivity in the hippocampal CA1-3 regions and dentate gyrus after systemic treatment with 1 mg/kg LPS. It was reported that rpS3-immunoreactive neurons were well observed in the hippocampus, especially, in the pyramidal cells of the CA1-3 regions and in the granule cells of the dentate gyrus (Hwang et al. 2008; Lee et al. 2011). Our present study showed that rpS3 immunoreactivity in pyramidal and granule cells began to decrease from 6 h after LPS treatment and hardly detected from 1 day after LPS treatment. Previously, we reported that 1 mg/kg LPS treatment did not lead to the neuronal death/degeneration in the pyramidal and granule cells of the mouse hippocampus (Chung et al. 2010). rpS3 is known as a signal mediator between neuronal apoptosis and DNA repair (Lee et al. 2010). Therefore, our present result indicates that the decrease of rpS3 immunoreactivity in pyramidal and granule cells might be related to changes in DNA repair ability, although there was no neuronal death/degeneration in the mouse hippocampus after 1 mg/kg LPS treatment.
Microglia, principle immune cells, are quiescent resident macrophage in the brain. Microglia have been known as a possible mediator of inflammation-associated neuronal damage (Ambrosini and Aloisi 2004; Choi et al. 2007). Previously, we reported that 1 mg/kg LPS treatment led to the activation of microglia in the mouse hippocampus (Chung et al. 2010). In addition, there are many reports that showed that LPS-stimulated microglia led to the production of proinflammatory and cytotoxic factors, such as interleukin-1β, tumor necrosis factor-α, and nitric oxide (Aloisi 2001; Raetz et al. 1991; Zielasek and Hartung 1996). It has been well known that pro-inflammatory cytokines and chemokines, such as interleukin-1β, are induced in microglia after systemic LPS administration (Buttini and Boddeke 1995; Chakravarty and Herkenham 2005; Lacroix et al. 1998). In addition, Tanaka et al. (2006) showed that LPS injection into the hippocampal CA1 region increased expressions of interleukin-1β and TNF-α, and that interleukin-1β immunopositive cells were LPS-induced activated microglia, not astrocytes. Recently, some studies also showed that LPS-induced pro-inflammatory responses, such as activations of NF-kB and MAPKs as well as increases in interleukin-1β and TNF-α, were well observed in LPS-stimulated BV-2 microglia (Cheong et al. 2011; Wang et al. 2011).
In the present study, we observed that strong rpS3 immunoreactivity was shown in microglia, not astrocytes, in the hippocampus at 1 and 2 days after LPS treatment. This is the first finding to demonstrate the rpS3 expression in activated microglia, and this result is associated with a previous study (Bae et al. 2010). They showed that rpS3 interacted with hematopoietic cell kinase, which is a non-receptor protein tyrosine kinase that is expressed in myeloid cells and participated in phagocytosis and myeloid cell differentiation (Guiet et al. 2008). Moreover, NF-κB, one of the important transcription factors in activated microglia by LPS (Cheong et al. 2011; Wang et al. 2011), is thought to be an important regulator of microglial responses to diverse stimuli (O’Neill and Kaltschmidt 1997). Recently, it has been suggested that rpS3 play a role as an integral subunit conferring NF-κB regulatory specificity (Wan et al. 2007). They have urged that the role of rpS3 in certainly key physiological processes is closely related to the regulation of NF-κB transcription. Although we cannot clearly explain why rpS3 was expressed in the activated microglia, it can be postulated that rpS3 expression in the activated microglia after LPS treatment may be related to LPS-induced inflammatory changes as well as the function of the activated microglia in the mouse hippocampus, such as changes in the production of pro-inflammatory cytokines by microglia.
In conclusion, rpS3 immunoreactivity was apparently decreased in the pyramidal and granule cells of the mouse hippocampus, and the immunoreactivity was expressed in activated microglia after the systemic administration of 1 mg/kg LPS. These results indicate that changes in rpS3 expression may be associated with microglial functions related to neuroinflammatory responses in the brain.
Acknowledgment
The authors would like to thank Mr. Seung Uk Lee for their technical help in this study. This study was supported by Mid-career Researcher Program from the NRF Grant funded by the MEST (2009-0086319) and by 2011 Research Grant from Kangwon National University.
Footnotes
Hui Young Lee and Joon Ha Park contributed equally to this study.
Contributor Information
Choong Hyun Lee, Phone: +82-41-550-1441, Email: vet9536@snu.ac.kr.
Moo-Ho Won, Phone: +82-33-250-8891, FAX: +82-33-256-1614, Email: mhwon@kangwon.ac.kr.
References
- Ahn EH, Kim DW, Kang HW, Shin MJ, Won MH, Kim J, Kim DJ, Kwon OS, Kang TC, Han KH, Park J, Eum WS, Choi SY (2010) Transduced PEP-1-ribosomal protein S3 (rpS3) ameliorates 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice. Toxicology 276:192–197 [DOI] [PubMed] [Google Scholar]
- Aloisi F (2001) Immune function of microglia. Glia 36:165–179 [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Aloisi F (2004) Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res 29:1017–1038 [DOI] [PubMed] [Google Scholar]
- Bae H, Gray JS, Li M, Vines L, Kim J, Pestka JJ (2010) Hematopoietic cell kinase associates with the 40S ribosomal subunit and mediates the ribotoxic stress response to deoxynivalenol in mononuclear phagocytes. Toxicol Sci 115:444–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicky J, Sanchez-Lemus E, Pavel J, Saavedra JM (2009) Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol 29:781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69 [DOI] [PubMed] [Google Scholar]
- Buttini M, Boddeke H (1995) Perippheral lipoppolysaccharide stimulation induces interleukin-1β messenger RNA in rat brain microglial cells. Neuroscience 65:523–530 [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M (2005) Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 25:1788–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong MH, Lee SR, Yoo HS, Jeong JW, Kim GY, Kim WJ, Jung IC, Choi YH (2011) Anti-inflammatory effects of polygala tenuifolia root through inhibition of NF-κB activation in lipopolysaccharide-induced BV2 microglial cells. J Ethnopharmacol 137:1402–1408 [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim SY, An JJ, Lee SH, Kim DW, Won MH, Kang TC, Park J, Eum WS, Kim J, Choi SY (2006) Immunohistochemical studies of human ribosomal protein S3 (rpS3). J Biochem Mol Biol 39:208–215 [DOI] [PubMed] [Google Scholar]
- Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, Lee HS, Lee IS (2007) Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J Vet Med Sci 69:1131–1136 [DOI] [PubMed] [Google Scholar]
- Chung DW, Yoo KY, Hwang IK, Kim DW, Chung JY, Lee CH, Choi JH, Choi SY, Youn HY, Lee IS, Won MH (2010) Systemic administration of lipopolysaccharide induces cyclooxygenase-2 immunoreactivity in endothelium and increases microglia in the mouse hippocampus. Cell Mol Neurobiol 30:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Rath VL (2002) Structure and function of the eukaryotic ribosome: the next frontier. Cell 109:153–156 [DOI] [PubMed] [Google Scholar]
- Glezer I, Simard AR, Rivest S (2007) Neuroprotective role of the innate immune system by microglia. Neuroscience 147:867–883 [DOI] [PubMed] [Google Scholar]
- Gluck MA, Myers CE (1997) Psychobiological models of hippocampal function in learning and memory. Annu Rev Psychol 48:481–514 [DOI] [PubMed] [Google Scholar]
- Guan Z, Fang J (2006) Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun 20:64–71 [DOI] [PubMed] [Google Scholar]
- Guiet R, Poincloux R, Castandet J, Marois L, Labrousse A, Le Cabec V, Maridonneau-Parini I (2008) Hematopoietic cell kinase (Hck) isoforms and phagocyte duties—from signaling and actin reorganization to migration and phagocytosis. Eur J Cell Biol 87:527–542 [DOI] [PubMed] [Google Scholar]
- Hochweller K, Anderton SM (2005) Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur J Immunol 35:1086–1096 [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Kim DW, Kim SY, Park JH, Ryoo ZY, Kim J, Choi SY, Won MH (2008) Ischemia-induced ribosomal protein S3 expressional changes and the neuroprotective effect against experimental cerebral ischemic damage. J Neurosci Res 86:1823–1835 [DOI] [PubMed] [Google Scholar]
- Jang CY, Lee JY, Kim J (2004) RpS3, a DNA repair endonuclease and ribosomal protein, is involved in apoptosis. FEBS Lett 560:81–85 [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim J (2006) Reduction of invasion in human fibrosarcoma cells by ribosomal protein S3 in conjunction with Nm23-H1 and ERK. Biochim Biophys Acta 1763:823–832 [DOI] [PubMed] [Google Scholar]
- Kim J, Chubatsu LS, Admon A, Stahl J, Fellous R, Linn S (1995) Implication of mammalian ribosomal protein S3 in the processing of DNA damage. J Biol Chem 270:13620–13629 [DOI] [PubMed] [Google Scholar]
- Kim TS, Kim HD, Kim J (2009) PKCdelta-dependent functional switch of rpS3 between translation and DNA repair. Biochim Biophys Acta 1793:395–405 [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318 [DOI] [PubMed] [Google Scholar]
- Lacroix S, Feinstein D, Rivest S (1998) The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol 8:625–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Liu W, Roth P, Dickson DW, Berman JW, Brosnan CF (1993) Macrophage colony-stimulating factor in human fetal astrocytes and microglia. Differential regulation by cytokines and lipopolysaccharide, and modulation of class II MHC on microglia. J Immunol 150:594–604 [PubMed] [Google Scholar]
- Lee SB, Kwon IS, Park J, Lee KH, Ahn Y, Lee C, Kim J, Choi SY, Cho SW, Ahn JY (2010) Ribosomal protein S3, a new substrate of Akt, serves as a signal mediator between neuronal apoptosis and DNA repair. J Biol Chem 285:29457–29468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Yoo KY, Choi JH, Hwang IK, Choi SY, Won MH (2011) Ribosomal protein s3 immunoreactivity in the young, adult and aged gerbil hippocampus. J Vet Med Sci 73:361–365 [DOI] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Holsboer F, Reul JM (1995) Intraperitoneal administration of bacterial endotoxin enhances noradrenergic neurotransmission in the rat preoptic area: relationship with body temperature and hypothalamic–pituitary–adrenocortical axis activity. Eur J Neurosci 7:2418–2430 [DOI] [PubMed] [Google Scholar]
- Martino G (2004) How the brain repairs itself: new therapeutic strategies in inflammatory and degenerative CNS disorders. Lancet Neurol 3:372–378 [DOI] [PubMed] [Google Scholar]
- Morrison DC, Ryan JL (1979) Bacterial endotoxins and host immune responses. Adv Immunol 28:293–450 [DOI] [PubMed] [Google Scholar]
- Nishioku T, Dohgu S, Takata F, Eto T, Ishikawa N, Kodama KB, Nakagawa S, Yamauchi A, Kataoka Y (2009) Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 29:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Kaltschmidt C (1997) NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 20:252–258 [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7:161–167 [DOI] [PubMed] [Google Scholar]
- Raetz CR, Ulevitch RJ, Wright SD, Sibley CH, Ding A, Nathan CF (1991) Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 5:2652–2660 [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Pavel J (2006) The discovery of a novel macrophage binding site. Cell Mol Neurobiol 26:509–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT (2005) Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat 30:144–157 [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM (2001) Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res 124:47–54 [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW (2005) Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res 159:145–151 [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M (1998) Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol 56:149–171 [DOI] [PubMed] [Google Scholar]
- Sumi N, Nishioku T, Takata F, Matsumoto J, Watanabe T, Shuto H, Yamauchi A, Dohgu S, Kataoka Y (2010) Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol Neurobiol 30:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, Yoshida T (2006) Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res 83:557–566 [DOI] [PubMed] [Google Scholar]
- Tough DF, Sun S, Sprent J (1997) T cell stimulation in vivo by lipopolysaccharide (LPS). J Exp Med 185:2089–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F, Anderson DE, Barnitz RA, Snow A, Bidere N, Zheng L, Hegde V, Lam LT, Staudt LM, Levens D, Deutsch WA, Lenardo MJ (2007) Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell 131:927–939 [DOI] [PubMed] [Google Scholar]
- Wang YP, Wu Y, Li LY, Zheng J, Liu RG, Zhou JP, Yuan SY, Shang Y, Yao SL (2011) Aspirin-triggered lipoxin A4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-κB and MAPKs in BV-2 microglial cells. J Neuroinflammation 8:95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool IG (1996) Extraribosomal functions of ribosomal proteins. Trends Biochem Sci 21:164–165 [PubMed] [Google Scholar]
- Zhou D, Fei M, Shen Q, Cheng C, Wang Y, Zhao J, Liu HO, Sun L, Liu Y, Yu X, Shen A (2008) Phosphorylation of extracellular signal-regulated kinases 1/2 predominantly enhanced in the microglia of the rat spinal cord following lipopolysaccharide injection. Cell Mol Neurobiol 28:867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielasek J, Hartung HP (1996) Molecular mechanisms of microglial activation. Adv Neuroimmunol 6:191–222 [DOI] [PubMed] [Google Scholar]
- Zipp F, Aktas O (2006) The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci 29:518–527 [DOI] [PubMed] [Google Scholar]