Abstract
The corticotropin-releasing hormone (CRH) and its cognate receptors have been implicated in the pathophysiology of stress-related disorders. Hypersecretion of central CRH and elevated glucocorticoid levels, as a consequence of impaired feedback control, have been shown to accompany mood and anxiety disorders. However, a clear discrimination of direct effects of centrally hypersecreted CRH from those resulting from HPA axis activation has been difficult. Applying a conditional strategy, we have generated two conditional CRH-overexpressing mouse lines: CRH-COE Del mice overexpress CRH throughout the body, while CRH-COE APit mice selectively overexpress CRH in the anterior and intermediate lobe of the pituitary. Both mouse lines show increased basal plasma corticosterone levels and consequently develop signs of Cushing’s syndrome. However, while mice ubiquitously overexpressing CRH exhibited increased anxiety-related behaviour, overexpression of CRH in the pituitary did not produce alterations in emotional behaviour. These results suggest that chronic hypercorticosteroidism alone is not sufficient to alter anxiety-related behaviour but rather that central CRH hyperdrive on its own or in combination with elevated glucocorticoids is responsible for the increase in anxiety-related behaviour. In conclusion, the generated mouse lines represent valuable animal models to study the consequences of chronic CRH overproduction and HPA axis activation.
Keywords: Corticotropin-releasing hormone, Hypothalamic–pituitary–adrenal axis, Mouse model, Overexpression, Anxiety-related behaviour, Stress-coping
Introduction
The corticotropin-releasing hormone (CRH) plays a major role in the adjustment of neuroendocrine, autonomic, and behavioural adaptations to stressors. In this regard, CRH functions as both, a neuroendocrine hormone within the line of the hypothalamic–pituitary–adrenocortical (HPA) axis (Vale et al. 1981) and a neuromodulator via hypothalamic and extrahypothalamic neuronal pathways (Gallagher et al. 2008). Dysregulated and/or hyperactive CRH circuits have been shown to be involved in neuroendocrine disturbances in the context of stress-related disorders such as anxiety and depression (Holsboer 1999; Deussing and Wurst 2005). Elevated levels of CRH in the cerebrospinal fluid, hypersecretion of CRH from the paraventricular nucleus of the hypothalamus, elevated circulating cortisol as well as an impaired glucocorticoid receptor (GR)-mediated negative feedback are consistently replicated findings in patients with major depression (Nemeroff et al. 1984; Lowy et al. 1984; Peeters et al. 2004). Therefore, animal models of CRH excess have attracted major interest as tools to study the consequences of a hyperactive CRH system.
The first CRH overexpressing mouse line was generated via a classical transgenic approach applying the broadly active metallothionine 1 promoter (Stenzel-Poore et al. 1992). These mice (CRF-OE Mt1) showed a strong CRH overexpression in the brain and peripheral organs including lung, adrenal, heart, and testis. CRH overproduction resulted in elevated plasma corticosterone levels and Cushing-like symptoms. Moreover, CRF-OE Mt1 showed increased anxiety-related behaviour, which was reversible by the CRH receptor antagonist α-helical CRH (Stenzel-Poore et al. 1994). Another CRH overexpressing mouse line was developed using the Thy1.2 promoter driving CRH expression in postnatal and adult neurons of the brain (Dirks et al. 2001). However, CRH-OE Thy1.2 did not show an altered stress response or phenotype indicative of increased anxiety- or depression-like behaviour (Dirks et al. 2001; Groenink et al. 2002). Instead, CRH-OE Thy-1.2 mice displayed reduced startle reactivity as well as reduced freezing following fear conditioning (Dirks et al. 2002b; Groenink et al. 2003). With some delay CRH-OE Thy1.2 also developed a mild Cushingoid phenotype (Dirks et al. 2002a). In addition, CRH-overexpressing mouse lines have been established in recent years applying the “tet-ON/tet-OFF” system, which allows for reversible and inducible overexpression of CRH (Vicentini et al. 2009; Kolber et al. 2010). Although both studies applied the Camk2a promoter combined with a tet-operator driven CRH-construct, the behavioural and neuroendocrine consequences of CRH excess were rather specific for each mouse line (Vicentini et al. 2009; Kolber et al. 2010). Taken together, these examples illustrate the difficulties to compare results from different transgenic mouse lines even if they are based on similar constructs.
To circumvent these problems, we have recently developed a mouse model which permits conditional CRH overexpression avoiding common uncertainties of classical transgenesis such as unpredictable influences of the site of transgene insertion and the number of inserted transgene copies (Lu et al. 2008). This was achieved by introducing a CRH expression unit into the ubiquitously expressed ROSA26 (R26) locus. Undesired ubiquitous expression of CRH driven by the R26 promoter is prevented unless a loxP flanked transcriptional terminator is deleted via the site-specific recombinase Cre. The ever growing “zoo” of Cre recombinase transgenic mouse lines offers a plethora of possibilities to induce specific spatial and temporal CRH expression patterns by simple breeding. In this way CRH expression is reproducibly driven by the endogenous R26 promoter, while the utilised Cre recombinase is only determining its expression pattern. This approach allows the meaningful comparison of different CRH-overexpressing mouse lines.
Using this novel mouse model of CRH overexpression we could demonstrate that CNS-restricted CRH overexpression in CRH-COE CNS mice, achieved by breeding with Nestin-Cre mice (Tronche et al. 1999), leads to increased active stress-coping behaviour and altered sleep regulation, whereas forebrain-restricted CRH overexpression via Camk2a-CreERT2 (Erdmann et al. 2007) results in increased anxiety-related behaviour (Lu et al. 2008; Kimura et al. 2010; Kolber et al. 2010; Refojo et al. 2011). Both transgenic lines show normal HPA axis activity demonstrating that the dysregulation of the CRH system can lead to marked behavioural alterations independent of basal HPA axis alterations. However, several lines of evidence also suggest that corticosteroids may cause mood and behaviour changes in depression, but whether they directly contribute to altered mood and anxiety symptoms remains unclear.
Here, we used our conditional mouse model of CRH excess to discriminate between central effects of chronic CRH hyperdrive and effects mediated via the HPA axis and its final effector—corticosterone in mice and cortisol in humans. To this end, we bred conditional CRH overexpressing mice (CRH-COE) to deleter- and Pomc-Cre animals, respectively, thus enabling ubiquitous (CRH-COE Del) and anterior pituitary-specific (CRH-COE APit) CRH overexpression at different dosages. The analyses of neuroendocrine parameters, physiological changes, and emotional behaviour unravelled a predominant effect of central CRH by itself or in combination with excessive corticosterone on anxiety-related behaviour. Moreover, these conditional models may represent very useful tools to study the behavioural and neuroendocrine effects of hypercorticosteriodism in the future.
Materials and Methods
Generation of Mice
Initially R26 flopCrh/flopCrh mice (Lu et al. 2008) were bred to Pomc-Cre mice (Akagi et al. 1997). Subsequently, mice ubiquitously overexpressing CRH (CRH-COE Del) were obtained by breeding female R26 +/flopCrh Pomc-Cre mice to male R26 flopCrh/flopCrh mice. In this combination, Pomc-Cre is transiently expressed during oogenesis and thus acts as a deleter. Early deletion of the floxed stop (flop) cassette results in ubiquitous expression of CRH. R26 Crh/flopCrh mice were intercrossed, however, no viable homozygous R26 Crh/Crh were obtained. Therefore, only R26 flopCrh/flopCrh (Ctrl) and heterozygous ubiquitously overexpressing R26 Crh/flopCrh (COE) mice were used. Anterior pituitary-specific overexpressing mice (CRH–COE APit) were obtained by breeding male R26 +/flopCrh Pomc-Cre mice to female R26 flopCrh/flopCrh mice. The following resulting genotypes were used for further analyses: R26 flopCrh/flopCrh (Ctrl), R26 +/flopCrh Pomc-Cre (COEhet), and R26 flopCrh/flopCrh Pomc-Cre (COEhom) mice. Genotyping was performed by PCR using primers: ROSA-1, 5′-AAA-GTC-GCT-CTG-AGT-TGT-TAT-3′; ROSA-5, 5′-TAG-AGC-TGG-TTC-GTG-GTG-TG-3′; ROSA-6 5′-GCT-GCA-TAA-AAC-CCC-AGA-TG-3′ and ROSA-7, 5′-GGG-GAA-CTT-CCT-GAC-TAG-GG-3′. Standard PCR conditions resulted in a 398-bp wild-type and a 646-bp mutant PCR product, respectively. Animals with a premature deletion of the floxed transcriptional terminator sequence were identified by the occurrence of a 505-bp PCR product. The presence of Cre was evaluated using primers CRE-F, 5′-GAT-CGC-TGC-CAG-GAT-ATA-CG-3′ and CRE-R, 5′-AAT-CGC-CAT-CTT-CCA-GCA-G-3′ resulting in a PCR product of 574 bp. Mice used for this study were kept on a mixed 129S2/Sv × C57BL/6 J background. Pomc-Cre mice had been backcrossed to C57BL/6J for five generations. The animal housing room as well as the experimental room were maintained under standard laboratory conditions (light–dark cycle: 12:12 h, lights on at 8 a.m.; temperature: 22 ± 1°C; relative humidity: 55 ± 10%). Commercial mouse diet (Altromin No. 1324, Altromin GmbH, Lage, Germany) and bottled tap water were available ad libitum.
Assessment of Physiological Parameters
At the age of 10–12 weeks, animals were weighed and then sacrificed via decapitation. Thymus and adrenal glands were extracted and stored in Ringer’s solution. In order to determine the organ weight, additional surrounding tissue was removed.
To assess the neuroendocrine profile of basal and stressed animals, a second batch of 10–12-week-old animals was separated 2 weeks prior to the experiment and singly housed with a 12:12 h light:dark schedule (lights off at 07:00 p.m.). All experiments and data analyses were performed separately for male and female animals. To determine the basal plasma hormone levels, mice were left undisturbed throughout the night before the experiment. Blood sampling was performed in the early morning (08:00–09:00 a.m.) and afternoon (04:30–05:30 p.m.) by collecting trunk blood from animals rapidly decapitated under isoflurane anaesthesia, with the time from first handling of the animal to completion of bleeding not exceeding 45 s. For evaluation of the endocrine response to stress, we collected blood samples immediately after a 10-min restraint stress, for which animals were placed in a 50-ml conical tube with the bottom removed. Stress experiments were performed in the morning (08:00–10:00 a.m.). Plasma corticosterone concentrations were measured in duplicates by a commercially available RIA kit (MP Biomedicals, Irvine, CA, USA) according to the manufactures instructions. Plasma samples from CRH-COE Del mice and from CRH-COE APit mice were measured in two independent RIAs.
Behavioural Phenotyping
At the time of testing, all animals were about 10–12-weeks of age and were single housed in the experimental room for at least 2 weeks. The battery of tests consisted of the open-field test (OF), the elevated plus-maze test (EPM), the dark–light box test (DaLi), and the forced swim test (FST; Touma et al. 2008). All tests were performed in the order listed between 9 a.m. and 12 a.m. The animals’ behaviour during the tests was videotaped (for the FST) and scored by a trained observer blind to the animals’ genotype using ‘Eventlog’ (version 1.0, Emco Software Ltd., Reykjavik, Iceland) or was automatically analysed (for the OF, EPM, and DaLi) by tracking the ‘centre of the animal’ using the ‘ANY-maze’ video-tracking software (Stoelting Co., Wood Dale, Illinois, USA). All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the Government of Upper Bavaria, Germany.
Open-Field Test
The OF was used to measure locomotor activity and explorative behaviour. Testing was performed in an evenly and dimly lit (about 15 lux) OF consisting of a circular arena (60 cm in diameter, surrounded by 40 cm high walls) for 5 min. The total distance travelled, the number of entries and the time the animal spent in the more aversive inner zone (30 cm diameter) or the more protective outer zone near the walls of the OF was measured.
Elevated Plus-Maze Test
Anxiety-related behaviour was measured by means of the EPM. The plus-shaped apparatus was made of grey plastic and consisted of two opposing closed/shielded arms (30 × 5 × 15 cm, dimly lit with about 10–20 lux) and two open/unprotected arms (30 × 5 × 0.5 cm, brightly lit with about 300 lux) connected by a central platform (5 × 5 cm, illuminated with about 140 lux). The maze was elevated 40 cm above the floor and each mouse was tested for 5 min on the apparatus. At the beginning of each trial, the animals were placed on the central platform facing one of the closed arms. Parameters of interest included open arm time and entries.
Dark–Light Box Test
The DaLi, another commonly used paradigm to measure anxiety-related behaviour in mice, was also employed. The apparatus consisted of a rectangular box with two compartments, the relatively protected dark compartment (15 × 20 × 25 cm, dimly lit with <10 lux) and the more aversive light compartment (30 × 20 × 25 cm, brightly lit with about 700 lux). At the beginning of the test, each mouse was placed in the centre of the dark compartment facing the back wall of the apparatus. The time as well as the entries made into the lit compartment were measured for 5 min.
Forced Swim Test
The FST was used to measure stress-coping behaviour. Each animal was placed into a glass beaker (diameter 12 cm, height 24 cm) filled with water (temperature 23°C) for a test period of 6 min. The parameters floating (immobility except small movements to keep balance), swimming and struggling (vigorous attempts to escape) were recorded and scored throughout the 6-min test period by a trained observer.
Sleep Recordings
Nine male CRH-COE APit and nine of the respective male control littermates were implanted with four EEG and two EMG electrodes (for full surgical protocol, please refer to (Romanowski et al. 2010). The animals were allowed to recover from surgery for 2 weeks under standard laboratory conditions (23° ± 1°C, 12 h/12 h light–dark cycle, food and water available ad libitum) before baseline recordings were initiated. After recovery, all mice were connected to an electrical swivel system by a recording cable which allowed EEG and EMG recordings from freely behaving animals. All signals were preamplified (1,000×, custom made) and sent to a main amplifier (10×, custom made) before transformation by an analogue/digital card (64 Hz sampling rate; National Instruments, Austin, TX). The EEG signals were analogue band pass-filtered (0.5–29 Hz, filter frequency roll off 48 dB/octave) and root mean square was applied to the non-filtered EMG signals before its digital conversion (sample rate: 64 Hz). Obtained data were analysed by a LabView-based acquisition program (EGEraVigilanz, SEA, Köln, Germany), and vigilance states were manually defined as WAKE, non-rapid eye movement sleep (NREMS), and rapid eye movement sleep (REMS) in 4-s epochs.
Statistical Analysis
Data and statistical analysis were performed with the computer programs GraphPad Prism 5.0 and SPSS 16.00. All results are shown as means ± standard error of the mean (SEM). Two-group comparisons of independent samples were calculated using the Mann–Whitney–U test. To examine differences between control, heterozygous and homozygous CRH-COE APit mice, the Kruskal–Wallis (KW) H test followed by Dunn’s multiple comparison post hoc test was applied. The effects of time and genotype on corticosterone levels were examined by two-way-multivariate analysis of variance (ANOVA) with Bonferroni post hoc tests. For the assessment of sleep recordings, a two-factorial ANOVA with a repeated measures design was applied and data were further statistically evaluated for significances in each time period (light or dark), if appropriate, by a post hoc test for simple effects (Neumann–Keuls test). Statistical significance was accepted at P < 0.05 and P ≤ 0.1 was considered a trend.
Results
Ubiquitous and Pituitary-Specific Overexpression of CRH
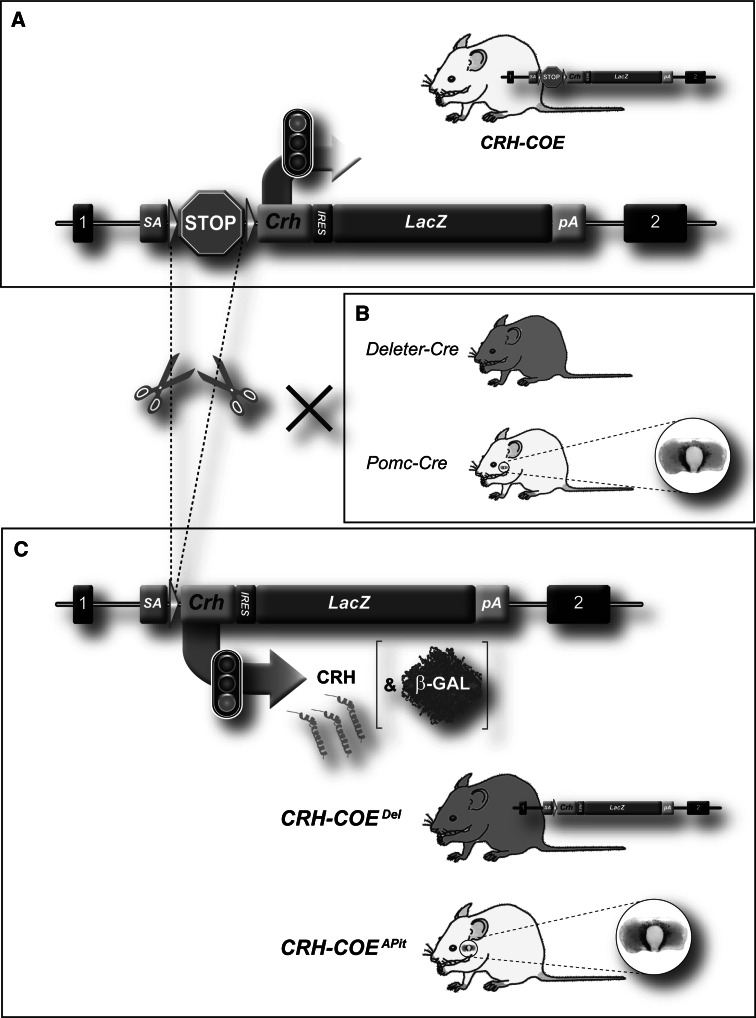
Breeding of CRH-COE mice (Fig. 1a; Lu et al. 2008) to deleter- and Pomc-Cre mice (Fig. 1b; Akagi et al. 1997) resulted in the excision of the transcriptional terminator and expression of exogenous CRH throughout the body or in the pituitary, respectively (Fig. 1c). Cre-mediated deletion of the transcriptional terminator and concomitant expression of CRH mRNA and β-galactosidase were observed in all tissues of CRH-COE Del mice (data not shown). In CRH-COE APit mice CRH mRNA and β-galactosidase expression was selectively observed in the anterior and intermediate lobe of the pituitary as well as in a subset of neurons in the arcuate nucleus (data not shown).
Fig. 1.
Strategy for conditional overexpression of CRH. a Schematic representation of the ROSA26 (R26) locus, which was engineered to harbour a Cre-inducible Crh-LacZ expression unit (R26 flopCRH, flop: floxed stop). b Breeding to deleter-Cre or Pomc-Cre mice to remove the transcriptional terminator sequence (Cre recombinase expression pattern depicted in green). c Cre recombinase induced expression of CRH (depicted in orange) and β-galactosidase throughout the body (CRH-COE Del) or within the anterior pituitary (CRH-COE APit). R26 exons are indicated as black boxes, the transcriptional terminator as a STOP sign and loxP sites as green arrowheads. SA splice acceptor, IRES internal ribosomal entry side, pA polyA signal (Color figure online)
CRH-COEDel Mice Exhibit Endocrine Abnormalities and Increased Anxiety-Related Behaviour
Already at the age of 3-week male and female CRH-COE Del mice showed physical changes reminiscent of Cushing’s syndrome such as hair loss and thin skin (data not shown). Adult mice showed excess fat accumulation as observed by visual inspection of subcutaneous and visceral fat as well as an overall increased body weight (males: Ctrl = 30.73 ± 0.53 g vs. COE = 35.73 ± 2.02 g, U = 37.0, P < 0.05, n = 12; females: Ctrl = 25.00 ± 0.59 g vs. COE = 31.50 ± 1.57 g, U = 15.0, P < 0.01, n = 11–12) (Fig. 2a). Adrenal weights were also significantly increased in male and female CRH-COE Del mice compared to CRH-COE Ctrl littermates (males: Ctrl = 0.124 ± 0.0042 mg/g vs. 0.194 ± 0.024 mg/g, U = 13.0, P < 0.01, n = 8–12; females: Ctrl = 0.247 ± 0.0076 mg/g vs. COE = 0.312 ± 0.0140 mg/g, U = 9, P < 0.05, n = 7–10) (Fig. 2b). In addition, a reduction in thymus weight was observed in male CRH-COE Del mice (Ctrl = 1.06 ± 0.095 mg/g vs. COE = 0.753 ± 0.105 mg/g, U = 8.0, P = 0.1, n = 4–10) (Fig. 2c). In order to evaluate HPA axis rhythmicity, we measured corticosterone levels in the morning (a.m.) and the afternoon (p.m.). Chronic CRH overproduction resulted in drastically elevated levels of circulating plasma corticosterone, in male and female mice compared to control littermates at both times of the day (males: a.m.: Ctrl = 9.35 ± 2.45 ng/ml vs. COE = 162.5 ± 28.16, ng/ml; p.m.: Ctrl = 102.7 ± 14.07 ng/ml vs. COE = 198.5 ± 23.54, two-way ANOVA, time effect F (1,32) = 13.60, P < 0.005, genotype effect F (1,32) = 49.47, P < 0.0001, time × genotype interaction F (1,32) = 0.10; Bonferroni post-test, P < 0.05 n = 8–12; females: a.m.: Ctrl = 60.97 ± 14.20 ng/ml vs. COE = 131.4 ± 30.94 ng/ml, p.m.: Ctrl = 179.6 ± 29.09 ng/ml vs. COE = 283.9 ± 34.01 ng/ml, two-way ANOVA, time effect F (1,31) = 21.33, genotype effect F (1,31) = 8.89, Bonferroni post-test, P < 0.05, n = 8–10) (Fig. 2d). Thus, the regular circadian rhythmicity of corticosterone secretion was virtually absent in male CRH-COE Del mice, as illustrated by similarly elevated corticosterone levels during the diurnal trough and diurnal peak. Interestingly, this effect was much less pronounced in female CRH-COE Del mice where elevated corticosterone levels were still detectable in the afternoon. However, 10 min of acute restraint stress failed to induce a neuroendocrine stress response in CRH-COE Del mice compared to littermate controls, independent of gender (Fig. 2d). Generally, plasma corticosterone concentrations were higher in control females compared to control males (a.m.: m = 9.35 ± 2.45 ng/ml vs. f = 60.97 ± 14.20 ng/ml, U = 3.0, P < 0.001; p.m.: m = 102.7 ± 14.07 ng/ml vs. f = 179.6 ± 29.09 ng/ml, U = 23.0, P = 0.059; stress: m = 174.3 ± 13.59 ng/ml vs. f = 301.0 ± 23.44 ng/ml, U = 4.0, P < 0.001) (Fig. 2d). In the case of CRH-overexpressing mice, gender-specific differences in corticosterone levels were only observed at the circadian peak, and after acute restraint stress (p.m.: m = 198.5 ± 23.54 ng/ml vs. f = 283.9 ± 34.01 ng/ml, U = 10.0, P = 0.075, stress: m = 144.3 ± 24.22 ng/ml vs. f = 284.4 ± 20.94 ng/ml, U = 5.0, P < 0.001).
Fig. 2.
Physiological and neuroendocrine alterations in male and female CRH-COE Del mice. a Body weight and b relative adrenal gland weights were significantly increased in male and female CRH–COE Del mice (black bars) compared to control littermates (white bars). c In addition, a decrease in relative thymus weight was observed in male CRH-COE Del mice. d Corticosterone levels measured in the morning (a.m.) and evening (p.m.) were significantly elevated in male and female COE mice, which also showed an attenuated stress response following 10-min of restraint stress compared to control animals. *Significantly different from control mice (P < 0.05), t trend (P ≤ 0.1), n.d. not determined, COE conditional overexpressing mice, Ctrl control littermates
The OF test was employed to assess novelty-induced locomotor and exploratory activity. CRH-COE Del mice showed no significant differences in locomotion and inner zone time compared to control littermates; however, they made significantly fewer entries into the centre zone (Ctrl = 12.33 ± 2.51 vs. COE = 4.58 ± 1.39, U = 33.0, P < 0.05, n = 12) (Fig. 3a). In the EPM CRH-COE Del mice showed significantly increased anxiety-related behaviour as evidenced by a decreased number of entries (Ctrl = 38.24 ± 5.80 vs. COE = 21.35 ± 3.05%, U = 13.0, P < 0.05, n = 7–11) and time spent (Ctrl = 13.37 ± 3.88 vs. COE = 4.01 ± 1.63%, U = 14, P < 0.05, n = 7–11) in the open arms compared to control littermates (Fig. 3b). Again, general locomotor activity was not altered. An increase in anxiety-related behaviour of CRH-COE Del mice was also detected in the DaLi, indicated by decreased lit compartment time (Ctrl = 10.96 ± 2.51 vs. COE = 3.37 ± 1.44%, U = 24.0, P < 0.05, n = 11–12) and number of entries (Ctrl = 6.25 ± 0.83 vs. COE = 2.73 ± 0.63, U = 18.5, P < 0.05, n = 11–12) as well as an increased latency to enter the lit compartment (Ctrl = 27.35 ± 14.84 vs. COE = 147.9 ± 36.12 s, U = 29.0, P < 0.05, n = 11–12) (Fig. 3c). To examine stress-coping behaviour, CRH-COE Del mice were subjected to the FST. Ubiquitous CRH overexpression resulted in a significantly increased struggling time and a trend towards a decreased floating time (struggling: Ctrl = 11.26 ± 0.97% vs. COE = 14.98 ± 1.49%, P = 0.05; Floating: Ctrl = 67.75 ± 1.8% vs. COE = 62.61 ± 2.6%, P = 0.1, n = 11–12; Fig. 3d).
Fig. 3.
Behavioural characterization of male CRH-COE Del mice. a Locomotor activity in the open-field test was not altered in CRH-COE Del mice (black bars) compared to control littermates (white bars). Anxiety-related behaviour, as assessed in the b elevated plus-maze and c dark–light box test, was significantly increased in CRH-COE Del mice. d A mild increase in active stress-coping behaviour was observed in CRH-COE Del mice compared to control animals. *Significantly different from control mice (P < 0.05), t trend (P ≤ 0.1), COE conditional overexpressing mice, Ctrl control littermates
CRH-COEAPit Mice Exhibit Endocrine Abnormalities and Mild Behavioural Alterations
In contrast to CRH-COE Del mice, pituitary-specific CRH overexpression led to a mild Cushing-like phenotype, which was mainly associated with hair loss and thinning of skin, starting at 5–6 months of age (data not shown). Animals used for the assessment of the neuroendocrine profile and behavioural analysis were between 10 and 12 weeks, and at that time were not distinguishable from controls. We analysed heterozygous as well as homozygous male CRH-COE APit mice in order to assess the dosage-dependent effect of CRH overexpression. Interestingly, heterozygous and homozygous CRH-COE APit mice weight significantly less than control littermates (males: Ctrl = 31.61 ± 0.43 g vs. COEhet = 27.94 ± 0.39 g vs. COEhom 28.99 ± 0.73 g, KW, H = 13.37, P < 0.05, Dunn’s post-test, P < 0.05, n = 10–16; females: Ctrl = 23.0 ± 0.22 g vs. COEhom = 21.58 ± 0.74 g, U = 31.5, P < 0.05, n = 9–11) (Fig. 4a). Furthermore, CRH overexpression in the pituitary resulted in a dose-dependent increase in relative adrenal gland weight (Ctrl = 0.112 ± 0.005 mg/g vs. COEhet = 0.174 ± 0.008 mg/g vs. COEhom = 0.196 ± 0.011 mg/g, KW, H = 20.65, P < 0.0001, Dunn’s post-test, P < 0.05, n = 9–14) and a decrease in relative thymus weight (Ctrl = 1.01 ± 0.046 mg/g vs. COEhet’ = 0.86 ± 0.01 mg/g vs. COEhom = 0.68 ± 0.05 mg/g, KW, H = 12.31, P < 0.05, Dunn’s post-test, P < 0.05) of male mice (Fig. 4b, c). Similarly, female homozygous CRH-COE APit mice also showed enlarged adrenal glands (Ctrl = 0.28 ± 0.0076 mg/g vs. COEhom = 0.38 ± 0.012 mg/g, U = 7, P < 0.05, n = 10) (Fig. 4b). As was the case in CRH-COE Del mice, circulating corticosterone levels were significantly elevated in heterozygous and homozygous CRH-COE APit mice compared to littermate controls in the morning (males: a.m.: Ctrl = 3.9 ± 0.9 ng/ml vs. COEhet = 72.52 ± 9.6 ng/ml vs. COEhom = 104.8 ± 8.4 ng/ml; two-way ANOVA, time effect F (1,94) = 75.44, P < 0.0001, genotype effect F (2,94) = 21.68, P < 0.0001, time × genotype interaction F (2,94) = 16.09, P < 0.001, Bonferroni post-test, P < 0.05, n = 14–23; females: a.m.: Ctrl = 103.9 ± 20.14 ng/ml vs. COEhom = 227.2 ± 18.9 ng/ml; two-way ANOVA, time effect F (1,39) = 6.39, P < 0.05, genotype effect F (1,39) = 6.16, P < 0.05, time × genotype interaction F (1,39) = 10.57, P < 0.05, n = 11–12) (Fig. 3d). Again, a dose-dependent increase in corticosterone could be observed between heterozygous and homozygous CRH-COE APit mice. Female animals showed essentially the same phenotype. However, differences in circulating corticosterone were not observed in the afternoon, neither in male nor in female CRH-COE APit mice compared to control littermates. Thus, similarly to male CRH-COE Del, animals, homozygous male and female CRH-COE APit mice exhibit marked alterations in circadian corticosterone rhythmicity, showing only minimal diurnal changes between morning and afternoon levels. However, morning and afternoon plasma corticosterone levels were generally much lower in male CRH-COE APit compared to male CRH-COE Del mice. Along these lines, HPA axis reactivity was not inhibited in CRH-COE APit mice. Additionally, heterozygous and homozygous CRH-COE APit mice showed the same corticosterone response as control littermates following 10 min of restraint stress (Fig. 4d). In case of the CRH-COE APit line, corticosterone plasma concentrations were not only higher in control but also in CRH-overexpressing females compared to males (Ctrl: a.m.: m = 3.90 ± 0.88 ng/ml vs. f = 103.9 ± 20.14 ng/ml, U = 2.0, P < 0.0001; p.m.: m = 120.3 ± 7.559 ng/ml vs. f = 228.2 ± 24.30 ng/ml, U = 23.0, P < 0.001; stress; m = 261.5 ± 8.29 ng/ml vs. f = 413.3 ± 13.30 ng/ml, U = 0.0, P < 0.0001, n = 12–9/COE hom: a.m.: m = 104.8 ± 8.37 ng/ml vs. f = 227.2 ± 18.94 ng/ml, U = 10.0, P < 0.001; p.m.: m = 121.8 ± 11.91 ng/ml vs. f = 211.6 ± 21.64 ng/ml, U = 33, P < 0.05; stress: m = 260.4 ± 11.56 vs. f = 301.0 ± 16.47 ng/ml, U = 42.0, P < 0.05, n = 11–23) (Fig. 4d).
Fig. 4.
Physiological and neuroendocrine alterations in male and female CRH-COE APit mice. a Body weight in homozygous male and female CRH-COE APit mice (black bars) was significantly decreased compared to control littermates (white bars). b A significant increase in relative adrenal gland weight was observed in male heterozygous (grey bars) and homozygous CRH-COE APit mice compared to control littermates. Relative adrenal gland weights were also significantly increased in homozygous female CRH-COE APit mice compared to their respective controls. c Relative thymus weight, assessed in males only, was significantly decreased in homozygous CRH-COE APit mice compared to control littermates. d A dosage-dependent increase in morning corticosterone levels was observed in heterozygous and homozygous male mice compared to control animals. Elevated coticosterone levels in the morning (a.m.), but not in the evening (p.m.) or in response to 10-min restraint stress, were also observed in female homozygous CRH-COE APit mice compared to control animals. *Significantly different from control mice (P < 0.05), not determined (n.d.). COE het heterozygous conditional overexpressing mice, COE hom homozygous conditional overexpressing mice, Ctrl control littermates
Locomotor activity as well as the number of entries into the inner zone of the OF were not altered in CRH-COE APit mice (Fig. 5a). Homozygous CRH-COE APit mice, however, tended to spent more time in the inner zone (Ctrl = 5.99 ± 0.83% vs. COEhom 9.65 ± 1.38%, U = 28.0, P = 0.062, n = 10–11). Anxiety-related behaviour, as assessed in the EPM and DaLi test, was not changed in homozygous CRH-COE APit mice. In the FST, homozygous CRH-COE APit mice spent more time swimming than control littermates (Ctrl = 19.18 ± 1.54% vs. COEhom = 25.30 ± 1.88%, U = 35.5, P < 0.05, n = 12). However, struggling and floating, which are considered the main readout parameters of stress-coping behaviour in this test, were not significantly altered.
Fig. 5.
Behavioural characterization of male CRH-COE APit mice. a Locomotor activity, measured in the open-field test, and anxiety-related behaviour, as assessed in the b elevated plus-maze and c dark–light box test, were not altered in CRH-COE APit (black bars) mice compared to control littermates (white bars). d An increase in swimming time was observed in homozygous CRH-COE APit mice in the FST. *Significantly different from control mice (P < 0.05), t = trend (P ≤ 0.1). COE hom homozygous conditional overexpressing mice, Ctrl control littermates
CRH-COE APit mice and control littermates displayed a typical sleep/wake distribution of nocturnal rodents with higher levels of NREMS and REMS during the light period than during the dark period (Fig. 6). Under baseline conditions CRH-COE APit mice showed significantly higher WAKE levels at ZT 4 (P = 0.002) and significantly lower WAKE levels from ZT 16 to ZT 22 (P ≤ 0.001; data not shown) as compared to CRH-COE Ctrl animals. Inversely, CRH-COE APit mice showed significantly more NREMS than CRH-COE Ctrl mice from ZT 16 to ZT 22 during baseline recordings (P ≤ 0.011; Fig. 6). As for REMS, no differences could be detected between both genotypes on the baseline day.
Fig. 6.
Baseline non-rapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS) of male CRH-COE APit mice. NREMS was significantly increased in CRH-COE APit mice (filled circles) during the second half of the dark period compared to control littermates (open circles). No differences in REMS could be detected between the two genotypes. White and black bars on the x-axis indicate the light and dark period, respectively. Vigilance states are indicated as percentages of 2 h means (±SEM). *Significantly different from control mice (P < 0.05). COE hom homozygous conditional overexpressing mice, Ctrl control littermates
Discussion
Multiple lines of evidence suggest that a dysregulation of the HPA axis plays an important role in the pathogenesis of mood and anxiety disorders. However, to discriminate between effects of centrally hypersecreted CRH from those resulting from downstream peripheral effects due to HPA axis activation is challenging. To unequivocally dissect central from peripheral effects of CRH on physiology, anxiety-related and stress-coping behaviour, we applied a conditional mouse model that allows CRH overexpression at different levels in a spatially restricted manner. As previously described, this conditional approach provides the opportunity to create different CRH-overexpressing mouse lines, avoiding well-known variables inherent to classical transgenesis such as copy number or site of transgene insertion (Lu et al. 2008). In our model, the pattern of CRH overexpression depends solely on the spatial and/or temporal properties of the introduced Cre recombinase whereas the transcriptional control via the endogenous R26 promoter guarantees stable and fully reproducible expression levels. Moreover, it allows the overexpression of CRH at two different dosages—from a single or both R26 alleles, respectively. It is of note that the homozygous disruption of the R26 locus has no phenotypic consequences. Hence, the R26 locus is the most widely used genomic location for reliable gene expression. Here we generated two mouse lines of HPA axis hyperdrive with and without direct alteration of central CRH expression to distinguish more precisely between the effects of CRH and corticosterone on physiology, anxiety-related and stress-coping behaviour.
As expected, chronic and ubiquitous overexpression of exogenous CRH led to prominent endocrine and physiological changes in CRH-COE Del mice reminiscent of those observed in patients with Cushing’s syndrome and largely identical to those observed in CRF-OE Mt1 mice (Stenzel-Poore et al. 1992). These included excess fat accumulation, thin skin, hair loss and severely elevated plasma corticosterone levels. Chronic stress-like alterations, such as enlarged adrenal glands and decreased thymus weight caused by excessive glucocorticoid production and circulation (van den Brandt et al. 2007; Hartmann et al. 2011; Wagner et al. 2011), were also observed in CRH-COE Del mice. Circadian rhythmicity of corticosterone secretion was virtually absent in male but not female CRH-COE Del mice. Circadian variation in HPA axis activity is known to differ between genders, and could also explain the variations observed between male and female CRH-COE Del animals (Seale et al. 2004; Atkinson et al. 2010). Generally, we found corticosterone levels to be higher in females compared to males, which is most likely attributed to differences in gonadal steroid levels (Rhees et al. 1999; Drossopoulou et al. 2004; Andreano and Cahill 2009; Garcia-Caceres et al. 2010). As displayed by CRH-COE Ctrl mice of both lines, gender-specific HPA axis differences are not only found at baseline but also in response to stress. In contrast, restraint stress was not able to elicit a corticosterone response in neither male nor female CRH-COE Del mice. It has been suggested that chronic HPA axis activation desensitizes the HPA system to further stress-dependent stimulation (Coste et al. 2001). However, the fact that homozygous CRH-COE APit mice, which also show elevated glucocorticoid levels, are still able to respond to a stressor, favours the conclusion that the absence of a stress response in CRH-COE Del mice might rather reflect a ceiling effect caused by sustained HPA axis hyperactivity.
Besides the mentioned endocrine abnormalities, CRH-COE Del mice exhibited increased anxiety-related behaviour in the EPM and DaLi test, which was also observed in CRF-OE Mt1 mice (Stenzel-Poore et al. 1994). We did not see differences in general locomotor activity in the OF and EPM, which might otherwise obscure the interpretation of anxiety-related behaviour. The observation that CRH-COE Del mice made less entries into the inner zone of the OF additionally supports the phenotype of increased anxiety-related behaviour. In the FST, CRH-COE Del showed increased active stress-coping behaviour compared to CRH-COE Ctrl mice. However, these effects were not as strong as previously observed in CRF-OE Mt1 mice, which showed a much more pronounced decrease in immobility (van Gaalen et al. 2002). Similarly, intracerebroventricular application of CRH or cortagine, a potent CRHR1 agonist, decreased immobility in the FST (Garcia-Lecumberri and Ambrosio 2000; Tezval et al. 2004). Along these lines, CNS-restricted CRH overexpression (CRH-COE CNS) also induces a dosage-dependent reduction in immobility, which is not an effect of excessive basal corticosterone secretion since circulating corticosteroids are normal in CRH-COE CNS mice (Lu et al. 2008). In contrast, CRH-OE Thy1.2 mice did not show alterations in FST behaviour (Dirks et al. 2001). These discrepancies might in the first instance be related to the applied promoters, which differ with respect to their spatial and temporal properties driving CRH expression but also with respect to their strength and subsequently triggered compensatory mechanisms. In addition, the behavioural test conditions and genetic background might explain some of the observed behavioural differences. In contrast to CRH-COE Del mice, chronic exposure to exogenous corticosterone has been shown to reduce active stress-coping behaviour and to increase immobility (Murray et al. 2008), suggesting once more that enhanced active stress-coping behaviour in CRH-COE Del and CRF-OE Mt1 mice is a consequence of central CRH hyperdrive. However, a mouse line-specific synergistic effect of hypercorticosteroidism and CRH overproduction on FST behaviour cannot be ruled out. Along these lines, it is also not entirely clear whether the observed anxiogenic phenotype in CRH-COE Del and CRF-OE Mt1 mice is caused by a dysregulation and overproduction of central CRH, secondary effects of glucocorticoids, or a combination of both. Numerous lines of evidence suggest that CRH and CRHR1 regulate behaviour in response to stressors and under basal conditions independent of downstream glucocorticoid action (Muller et al. 2003; Lu et al. 2008; Kolber et al. 2010; Refojo et al. 2011; Flandreau et al. 2011). In addition, application of a CRHR1 antagonist reverted the anxiogenic state observed in CRF-OE Mt1 mice (Stenzel-Poore et al. 1994) as well as the active stress-coping phenotype in CRH-COE CNS mice (Lu et al. 2008). Furthermore, Heinrichs et al. (1997) showed that adrenalectomy, leading to normalisation of plasma corticosterone levels, did not attenuate the anxiogenic effect of CRH overproduction. At the same time, long-term exposure to exogenous corticosterone in rodents has been shown to induce anxiety/depression-like changes in behaviour, neurochemistry, and brain morphology (Ardayfio and Kim 2006; Murray et al. 2008; Gourley et al. 2008; David et al. 2009).
However, chronic application of corticosterone analogues hardly fulfils the criteria of construct validity and is often applied at high and non-physiological concentrations. In order to address the impact of excess glucocorticoids on physiology and behaviour without directly altering central CRH expression, we bred CRH-overexpressing mice to Pomc-Cre mice (Akagi et al. 1997). In this mouse line, CRH overexpression is mainly restricted to the anterior and intermediate lobe of the pituitary as well as to a subset of neurons of the arcuate nucleus. Similarly to CRH-COE Del mice, heterozygous and homozygous CRH-COE APit mice displayed enlarged adrenal glands and an atrophy of the thymus as a result of enhanced corticosterone secretion, which is most likely a consequence of CRH acting in a paracrine fashion directly within the pituitary. Despite elevated plasma corticosterone levels, homozygous CRH-COE APit mice showed only a mild Cushing-like phenotype, which became apparent only after 5-6 months of age. This was associated with hair loss and thinning of skin, but not with excessive fat accumulation. On the contrary, homozygous CRH-COE APit mice were significantly lighter than control littermates. This is probably the result of Pomc-directed CRH overexpression in the arcuate nucleus, which is involved in the regulation of appetite (Schwartz et al. 2000) and where CRH might have elicited its well-known anorectic effects (Heinrichs and Richard 1999). The fact that high glucocorticoid levels have not been associated with a reduction of food intake in experimental animals (Warwick and Romsos 1988; Nieuwenhuizen and Rutters 2008) favours the assumption that CRH overexpression in the arcuate nucleus is responsible for the observed body weight alteration. It has been described that hypothalamic CRH inhibits food intake and orexigenic effects of NPY in the PVN independently of the HPA axis (Menzaghi et al. 1993; Heinrichs et al. 1993; Zorrilla et al. 2003). In addition, CRF-OE Mt1 mice exhibit reduced food intake in response to fasting due to neuronal activation in the arcuate nucleus (Stengel et al. 2009). A possible explanation why CRH-COE Del mice display substantial weight gain may be linked to the heightened constitutive overexpression of brain CRH-signalling pathways that override the NPY signals in the arcuate nucleus, and the general anorexigenic effects of CRH. In addition, CRH-COE Del mice showed constantly elevated corticosterone levels. Thus, corticosterone levels in CRH-COE Del mice are probably high enough to induce hyperphagia, which is also observed after central glucocorticoid administration. However, the exact mechanism by which CRH overexpression in neurons of the arcuate nucleus regulates weight loss/gain is subject of further investigations.
As already mentioned, dosage-dependent differences in corticosterone levels between heterozygous and homozygous CRH-COE APit mice and respective CRH-COE Ctrl mice were only detectable at the circadian trough. Although this led to disrupted circadian corticosterone rhythmicity in male and female homozygous CRH-COE APit mice, these animals still displayed a comparatively normal neuroendocrine stress response.
Similarly to CRH-COE Del mice, gender-specific differences in HPA axis activity were also observed in this mouse line. Interestingly, we observed no alterations in locomotor activity and anxiety-related behaviour in male CRH-COE APit mice, suggesting that chronic hypercorticosteroidism on its own is not sufficient to alter anxiety-related behaviour. In support of this, conditional GR knockout mice, which also display increased basal plasma corticosterone levels and signs of a Cushing-like phenotype, show reduced anxiety (Tronche et al. 1999). Along these lines, FKBP51 knockout mice, which show decreased basal corticosterone levels as well as an enhanced recovery following acute and chronic stress exposure, do not display alterations in anxiety-related behaviour (Hartmann et al. 2011; Touma et al. 2011). This supports the notion that the anxiogenic effects observed in CRH-COE Del and CRH-OE Mt1 mice are not solely caused by elevated glucocorticoids, but rather by central CRH hyperdrive or a synergistic effect of both. However, the process by which central CRH and glucocorticoids may synergistically modulate anxiety-related behaviour is largely unknown. These observations are not in line with studies of chronic corticosterone application, where anxiety-related behaviour is induced upon exogenous glucocorticoid application (Ardayfio and Kim 2006; Murray et al. 2008; David et al. 2009). However, the assessment of hypercorticosteroidism-induced behavioural effects via exogenous glucocorticoid administration faces major drawbacks: differential effects strongly depend on the duration and dose of treatment (Brotto et al. 2001; Gregus et al. 2005); HPA axis activity is down-regulated which bears little resemblence to disease etiology; observed outcomes have not been replicated by many studies and are often contradictory especially concerning effects of corticosterone application on the stress-coping behaviour in the FST (Brotto et al. 2001; Murray et al. 2008; Stone and Lin 2008; David et al. 2009). Moreover, exogenous corticosteroids can have acute antidepressant and anti-stress effects (Reuter 2002; Het and Wolf 2007; Stone and Lin 2008), but have also been shown to induce depression-like behaviour in humans and animals (Brown and Suppes 1998; Celano et al. 2011). These controversies render the interpretation of the mild FST phenotype in homozygous CRH–COE APit mice difficult. Additionally, it should be noted that CRH overexpression in the anterior and intermediate lobes of the pituitary is driven by the Pomc promoter, which is active from early development onwards. Moreover, expression of Pomc in a subset of trophoblast giant cells has been reported (Zhu and Pintar 1998), which could result in a transient overexpression of CRH during gestation. Therefore, expression of Pomc-Cre in the placenta needs to be analysed in the future. In this regard, adaptive processes and compensatory mechanisms in circuitries involved in anxiety-related behaviour and feeding can not be ruled out. Furthermore, expression levels and sensitivity of GRs and mineralocorticoid receptors (MRs) might be altered in homozygous CRH-COE APit mice, partially blunting the effect of elevated corticosterone levels, and thereby sustaining HPA axis reactivity. Hence, the generation of inducible pituitary-specific CRH-overexpressing mice would more precisely assess the role of elevated glucocorticoids during adulthood.
CRH-COE CNS and forebrain-specific CRH overexpressing mice (CRH-COE FB ) exhibit constantly elevated REM sleep levels, suggesting that CRH originating from the forebrain contributes to sleep disturbances in patients with major depression (Kimura et al. 2010). Thus, altered REM sleep architecture is likely to be a consequence of hypersecreted central CRH and may serve as a biomarker predicting an upcoming clinical condition. Under baseline conditions, CRH-COE Apit mice showed a significant decrease in WAKE compared to control littermates mice and significantly increased NREMS, particularly during the latter half of the dark period. The hyperdrive of CRH in the pituitary, through an increase of corticosterone secretion from the adrenal cortex, might entail an increase of negative feedback effects in the CNS, including the PVN, where CRH expression and secretion in turn would be suppressed. CRH is known to be WAKE promoting and NREMS suppressing via its central effects. Consequently, a stronger negative feedback in CRH-COE APit mice might have resulted in a decrease of WAKE and an increase of NREMS during the latter half of the dark period when endogenous CRH levels would be high. Human studies as well have shown that cortisol, injected intravenously, by presumably activating negative feedback pathways, leads to increased NREMS (Friess et al. 1994).
To our knowledge, CRH-COE APit mice represent the first animal model of hypercorticosteroidism independent of direct genetic alterations in the brain. In this regard, CRH-COE APit mice offer valuable additional insights regarding the physiological and behavioural effects of excessive corticosterone production. Further studies will be necessary to investigate, whether endogenous ACTH levels are increased in response to chronic CRH overproduction in CRH-COE Del and CRH-COE APit mice, or whether the effects are attributed to a hyper-responsiveness of the adrenal cortex to ACTH. We only investigated CRH overexpression and HPA axis hyperdrive in the context of anxiety-related and stress-coping behaviour. However, alterations in cognitive, social and reward-seeking behaviour also represent core endophenotypes of depression, and remain to be assessed in CRH-COE Del and CRH-COE APit mice. Moreover, stress in combination with a genetic predisposition can increase the risk to develop psychiatric disorders (de Kloet et al. 2005) and should be examined in both mouse models. In conclusion, the above described mouse lines represent useful tools to address behavioural and neuroendocrine effects of chronic CRH overproduction and HPA axis activation. Nevertheless, the generation of additional, site- and neurotransmitter-specific conditional CRH-overexpressing mouse mutants is mandatory in order to uncover the underlying neuronal circuits and brain regions involved in mediating anxiety-related behaviour via CRH.
Acknowledgments
We would like to thank Sabrina Bauer, Ursula Habersetzer and Cornelia Flachskamm for excellent technical assistance. Moreover, we thank Carola Hetzel for careful reading of the manuscript. This work was partially supported by the Bundesministerium für Bildung und Forschung within the framework of NGFN-Plus (Förderkennzeichen: 01GS08151 and 01GS08155) and by the Initiative and Networking Fund of the Helmholtz Association in the framework of the Helmholtz Alliance for Mental Health in an Ageing Society (HA-215).
Footnotes
Nina Dedic and Chadi Touma contributed equally to this work.
References
- Akagi K, Sandig V, Vooijs M, Van der Valk M, Giovannini M, Strauss M, Berns A (1997) Cre-mediated somatic site-specific recombination in mice. Nucl Acids Res 25:1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L (2009) Sex influences on the neurobiology of learning and memory. Learn Mem 16:248–266 [DOI] [PubMed] [Google Scholar]
- Ardayfio P, Kim KS (2006) Anxiogenic-like effect of chronic corticosterone in the light–dark emergence task in mice. Behav Neurosci 120:249–256 [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Leggett JD, Wood SA, Castrique ES, Kershaw YM, Lightman SL (2010) Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology 151:3720–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotto LA, Gorzalka BB, Barr AM (2001) Paradoxical effects of chronic corticosterone on forced swim behaviours in aged male and female rats. Eur J Pharmacol 424:203–209 [DOI] [PubMed] [Google Scholar]
- Brown ES, Suppes T (1998) Mood symptoms during corticosteroid therapy: a review. Harv Rev Psychiatry 5:239–246 [DOI] [PubMed] [Google Scholar]
- Celano CM, Freudenreich O, Fernandez-Robles C, Stern TA, Caro MA, Huffman JC (2011) Depressogenic effects of medications: a review. Dialogues. Clin Neurosci 13:109–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Murray SE, Stenzel-Poore MP (2001) Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides 22:733–741 [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475 [DOI] [PubMed] [Google Scholar]
- Deussing JM, Wurst W (2005) Dissecting the genetic effect of the CRH system on anxiety and stress-related behaviour. Comptes Rendus Biologies 328:199–212 [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Verdouw MP, Schipholt M, Jvd Gugten, Hijzen T, Olivier B (2001) Behavioural analysis of transgenic mice overexpressing corticotropin-releasing hormone in paradigms emulating aspects of stress, anxiety, and depression. Int J Comp Psychology 16:123–135 [Google Scholar]
- Dirks A, Groenink L, Bouwknecht JA, Hijzen TH, Van Der GJ, Ronken E, Verbeek JS, Veening JG, Dederen PJ, Korosi A, Schoolderman LF, Roubos EW, Olivier B (2002a) Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur J Neurosci 16:1751–1760 [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Schipholt MI, Van Der GJ, Hijzen TH, Geyer MA, Olivier B (2002b) Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol Psychiatry 51:583–590 [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, Papadopoulou-Daifoti Z (2004) Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience 126:849–857 [DOI] [PubMed] [Google Scholar]
- Erdmann G, Schütz G, Berger S (2007) Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci 8:63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB (2011) Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behaviorial anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology [DOI] [PMC free article] [PubMed]
- Friess E, Bardeleben V, Wiedemann K, Lauer CJ, Holsboer F (1994) Effects of pulsatile cortisol infusion on sleep-EEG and nocturnal growth hormone release in healthy men. J Sleep Res 3:73–79 [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P (2008) Synaptic physiology of central CRH system. Eur J Pharmacol 583:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Caceres C, Lagunas N, Calmarza-Font I, Azcoitia I, Diz-Chaves Y, Garcia-Segura LM, Baquedano E, Frago LM, Argente J, Chowen JA (2010) Gender differences in the long-term effects of chronic prenatal stress on the HPA axis and hypothalamic structure in rats. Psychoneuroendocrinology 35:1525–1535 [DOI] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Ambrosio E (2000) Differential effect of low doses of intracerebroventricular corticotropin-releasing factor in forced swimming test. Pharmacol Biochem Behav 67:519–525 [DOI] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR (2008) Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry 63:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregus A, Wintink AJ, Davis AC, Kalynchuk LE (2005) Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res 156:105–114 [DOI] [PubMed] [Google Scholar]
- Groenink L, Dirks A, Verdouw PM, Schipholt M, Veening JG, Van Der GJ, Olivier B (2002) HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry 51:875–881 [DOI] [PubMed] [Google Scholar]
- Groenink L, Pattij T, De JR, Van Der GJ, Oosting RS, Dirks A, Olivier B (2003) 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur J Pharmacol 463:185–197 [DOI] [PubMed] [Google Scholar]
- Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, Cheung-Flynn J, Cox MB, Smith DF, Holsboer F, Muller MB, Schmidt MV (2011) The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 62:332–339 [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Richard D (1999) The role of corticotropin-releasing factor and urocortin in the modulation of ingestive behavior. Neuropeptides 33:350–359 [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF (1993) Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res 611:18–24 [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Min H, Tamraz S, Carmouche M, Boehme SA, Vale WW (1997) Anti-sexual and anxiogenic behavioral consequences of corticotropin-releasing factor overexpression are centrally mediated. Psychoneuroendocrinology 22:215–224 [DOI] [PubMed] [Google Scholar]
- Het S, Wolf OT (2007) Mood changes in response to psychosocial stress in healthy young women: effects of pretreatment with cortisol. Behav Neurosci 121:11–20 [DOI] [PubMed] [Google Scholar]
- Holsboer F (1999) The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res 33:181–214 [DOI] [PubMed] [Google Scholar]
- Kimura M, Muller-Preuss P, Lu A, Wiesner E, Flachskamm C, Wurst W, Holsboer F, Deussing JM (2010) Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry 15:154–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Boyle MP, Wieczorek L, Kelley CL, Onwuzurike CC, Nettles SA, Vogt SK, Muglia LJ (2010) Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J Neurosci 30:2571–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy MT, Reder AT, Antel JP, Meltzer HY (1984) Glucocorticoid resistance in depression: the dexamethasone suppression test and lymphocyte sensitivity to dexamethasone. Am J Psychiatry 141:1365–1370 [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, Refojo D, Ekker M, Rubenstein JL, Stalla GK, Singewald N, Holsboer F, Wotjak CT, Wurst W, Deussing JM (2008) Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry 13:1028–1042 [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Heinrichs SC, Pich EM, Tilders FJ, Koob GF (1993) Functional impairment of hypothalamic corticotropin-releasing factor neurons with immunotargeted toxins enhances food intake induced by neuropeptide Y. Brain Res 618:76–82 [DOI] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W (2003) Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 6:1100–1107 [DOI] [PubMed] [Google Scholar]
- Murray F, Smith DW, Hutson PH (2008) Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol 583:115–127 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen AG, Rutters F (2008) The hypothalamic–pituitary–adrenal-axis in the regulation of energy balance. Physiol Behav 94:169–177 [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J (2004) Levels and variability of daily life cortisol secretion in major depression. Psychiatry Res 126:1–13 [DOI] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM (2011) Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333:1903–1907 [DOI] [PubMed] [Google Scholar]
- Reuter M (2002) Impact of cortisol on emotions under stress and nonstress conditions: a pharmacopsychological approach. Neuropsychobiology 46:41–48 [DOI] [PubMed] [Google Scholar]
- Rhees RW, Al-Saleh HN, Kinghorn EW, Fleming DE, Lephart ED (1999) Relationship between sexual behavior and sexually dimorphic structures in the anterior hypothalamus in control and prenatally stressed male rats. Brain Res Bull 50:193–199 [DOI] [PubMed] [Google Scholar]
- Romanowski CP, Fenzl T, Flachskamm C, Wurst W, Holsboer F, Deussing JM, Kimura M (2010) Central deficiency of corticotropin-releasing hormone receptor type 1 (CRH-R1) abolishes effects of CRH on NREM but not on REM sleep in mice. Sleep 33:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS (2004) Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol 16:516–524 [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Million M, Stenzel-Poore MP, Kobelt P, Monnikes H, Tache Y, Wang L (2009) Corticotropin-releasing factor-overexpressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology 150:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W (1992) Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology 130:3378–3386 [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW (1994) Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci 14:2579–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lin Y (2008) An anti-immobility effect of exogenous corticosterone in mice. Eur J Pharmacol 580:135–142 [DOI] [PubMed] [Google Scholar]
- Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J (2004) Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci USA 101:9468–9473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C, Bunck M, Glasl L, Nussbaumer M, Palme R, Stein H, Wolferstätter M, Zeh R, Zimbelmann M, Holsboer F, Landgraf R (2008) Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology 33:839–862 [DOI] [PubMed] [Google Scholar]
- Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Bull DR, Ionescu IA, Heinzmann JM, Knapman A, Siebertz A, Depping AM, Hartmann J, Hausch F, Schmidt MV, Holsboer F, Ising M, Cox MB, Schmidt U, Rein T (2011) FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry 70:928–936 [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23:99–103 [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397 [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Luhder F, McPherson KG, de Graaf KL, Tischner D, Wiehr S, Herrmann T, Weissert R, Gold R, Reichardt HM (2007) Enhanced glucocorticoid receptor signaling in T cells impacts thymocyte apoptosis and adaptive immune responses. Am J Pathol 170:1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T (2002) Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci 15:2007–2015 [DOI] [PubMed] [Google Scholar]
- Vicentini E, Arban R, Angelici O, Maraia G, Perico M, Mugnaini M, Ugolini A, Large C, Domenici E, Gerrard P, Bortner D, Mansuy IM, Mangiarini L, Merlo-Pich E (2009) Transient forebrain over-expression of CRF induces plasma corticosterone and mild behavioural changes in adult conditional CRF transgenic mice. Pharmacol Biochem Behav 93:17–24 [DOI] [PubMed] [Google Scholar]
- Wagner KV, Wang XD, Liebl C, Scharf SH, Muller MB, Schmidt MV (2011) Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology 36:579–587 [DOI] [PubMed] [Google Scholar]
- Warwick BP, Romsos DR (1988) Energy balance in adrenalectomized ob/ob mice: effects of dietary starch and glucose. Am J Physiol 255:R141–R148 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pintar JE (1998) Expression of opioid receptors and ligands in pregnant mouse uterus and placenta. Biol Reprod 59:925–932 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Tache Y, Koob GF (2003) Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci 24:421–427 [DOI] [PubMed] [Google Scholar]