Abstract
We have previously shown that chronic treatment with angiotensin-(1–7) [Ang-(1–7)] can prevent diabetes-induced cardiovascular dysfunction. However, effect of Ang-(1–7) treatment on diabetes-induced alterations in the CNS is unknown. The aim of this study was to test the hypothesis that treatment with Ang-(1–7) can produce protection against diabetes-induced CNS changes. We examined the effect of Ang-(1–7) on the number of cyclooxygenase-2 (COX-2) immunoreactive neurons and the glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes and assessed the changes in the neuronal growth-associated protein-43 (GAP-43) of the hippocampal formation in streptozotocin-induced diabetes in rats. Animals were sacrificed 30 days after induction of diabetes and/or treatment with Ang-(1–7). Ang-(1–7) treatment significantly prevented diabetes-induced decrease in the number of GFAP immunoreactive astrocytes and GAP-43 positive neurons in all hippocampal regions. Co-administration of A779, a selective Ang-(1–7) receptor antagonist, inhibited Ang-(1–7)-mediated protective effects indicating that Ang-(1–7) produces its effects through activation of receptor Mas. Further, Ang-(1–7) treatment through activation of Mas significantly prevented diabetes-induced increase in the number of the COX-2 immunolabeled neurons in all sub-regions of the hippocampus examined. These results show that Ang-(1–7) has a protective role against diabetes-induced changes in the CNS.
Keywords: Hippocampus, Angiotensin, ACE2, ACE, Memory, Diabetes, Cyclooxygenase-2, Glial fibrillary acidic protein
Introduction
Diabetes mellitus is associated with neurodegenerative and cerebral functional disorders of the central nervous system (CNS). It increases the risk of stroke, seizures, memory loss, and mental impairment (Gispen and Biessels 2000). The cellular mechanisms responsible for neurodegenerative and functional impairments of CNS disorders in diabetes are still not clearly understood. The hippocampal formation, which is known to play a major role in learning and memory, exhibits severe neurochemical, neurophysiological and structural changes in diabetic animals with consequent decrease in learning capability and alteration in hippocampal synaptic plasticity (Artola et al. 2005; Kamal et al. 2006). It has been reported that both hippocampal neurons and astroglia are vulnerable to diabetes which enhances apoptosis (Li et al. 2004) and causes hippocampal dendritic and synaptic reorganization (Magarinos and McEwen 2000). Diabetes induces neuronal death in the hippocampus and causes damage of dendrites, pre- and post-synaptic processes in CA3 neurons, and decreases the expression of insulin growth factors and their receptors in the hippocampus (Magarinos and McEwen 2000). Diabetes also decreases cell proliferation and produces neuronal and astroglial alterations in the dentate gyrus of the hippocampus (Revsin et al. 2005). Furthermore, diabetes reduces hippocampal cell differentiation in the sub-granular zone of the dentate gyrus (Hwang et al. 2008) and impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons (Stranahan et al. 2008).
Astrocytes play a more active role in neuronal activity, including regulation ion flux currents, energy production, neurotransmitter release and synaptogenesis. Astrocytes are proving critical for normal CNS function, and alterations in their activity could contribute to diabetes-related disturbances in the brain (Coleman et al. 2010). Diabetes affects nervous glial cells, especially the astrocytes. Astrocytes are prevalent site of glucose uptake, metabolism and coupling to synaptic activity due to their intimate connection to intraparenchymal capillaries and neurons (Saravia et al. 2002). Diabetes-induced decrease in the level of intermediate filament glial fibrillary acidic protein (GFAP) and number of GFAP immunoreactive astrocytes has been previously reported (Barber et al. 2000; Lechuga-Sancho et al. 2006; Afsari et al. 2008; Coleman et al. 2010). Recent studies have shown that diabetic neurocognitive dysfunction in streptozotocin (STZ)-induced diabetic animals is associated with a decrease in hippocampal and cerebrospinal fluid GFAP immunoreactive levels (de Senna et al. 2011) and down-regulation of GFAP in the hippocampus at protein and mRNA levels (Zuo et al. 2011). This decrease has been shown to produce poor white matter vascularization, disturbances in the blood–brain barrier (Huber 2008), and alterations in LTP (Artola et al. 2005; Kamal et al. 2006). The suggestion that there is a link between decreases in GFAP and diabetes-induced CNS complications is supported by observations in GFAP knock-out mice and in diabetic patients. Growth-associated protein-43 (GAP-43) is a protein found in growth cones or actively remodeling terminals and is thus used as a neuronal marker of regions undergoing neurite outgrowth or capable of plasticity in adult animals (Rekart et al. 2004). GAP-43 expression has been shown to be decreased in the hippocampal CA1 area and dentate gyrus in diabetic rats (Zhou et al. 2007). Abnormal GAP-43 expression has also been linked with several neurological disorders such as temporal lobe epilepsy (Proper et al. 2000) and Alzheimer’s disease (Rekart et al. 2004). Cyclooxygenase-2 (COX-2) is an inflammatory mediator that has been shown to play a role in free radical mediated cellular damage, vascular dysfunction, and alteration in cellular metabolism promoting neuropathology and worsening behavioral outcome (Ellis et al. 1989). Normally, COX-2 is not detectable, except at low levels in the brain. However, COX-2 expression increases in the CNS in response to numerous insults and chronic neurodegenerative diseases such as stroke, seizures and epilepsy, Parkinson’s and Alzheimer’s diseases and diabetes mellitus (Takemiya et al. 2006; Ho et al. 2006; Zhang et al. 2008).
Angiotensin-(1–7) [Ang-(1–7)] is formed from angiotensin II by angiotensin-converting enzyme-2 (ACE2) and exhibits anti-inflammatory, anti-oxidant, anti-thrombotic and anti-proliferative properties (Benter et al. 1993; Chappell 2007). The effects of Ang-(1–7) are mediated through the G protein-coupled receptor Mas which is highly expressed in several tissues including the CNS, heart, kidney, and the vasculature (Chappell 2007). Ang-(1–7) produces relaxation of several vascular beds including coronary, cerebral, renal, pulmonary, femoral, and mesenteric arteries (Chappell 2007). In the human, Ang-(1–7) attenuates Ang II-induced vasoconstriction in resistant vessels (Ueda et al. 2001). Our recent studies have shown that treatment with Ang-(1–7) can attenuate end-organ damage in models of diabetes, hypertension and endothelial dysfunction (Benter et al. 2006, 2007, 2008). Increasing evidences indicate that Ang-(1–7) system has important biological activities in the CNS (Gallagher et al. 2006; von Bohlen et al. 2006; Xu et al. 2011). Ang-(1–7) acts as an important neuromodulator in the dorsomedial and ventrolateral medulla and in the hypothalamus and high levels of Mas receptor mRNA was described predominantly in the neurons of forebrain areas, including hippocampus, cortex, and olfactory bulb of the rat and mouse (Santos et al. 2000). We have shown that Ang-(1–7) administration inhibits diabetes-induced cardiovascular dysfunction, formation of inflammatory mediators and elevation in the activities of NADPH oxidase and NFκB (Benter et al. 2008; Al-Maghrebi et al. 2009; El-Hashim et al. 2012). The aim of this study was to determine whether treatment with Ang-(1–7) can prevent diabetes-induced changes in the CNS. Thus, we examined the effect of chronic treatment with Ang-(1–7) on the expression of GFAP, GAP-43 and COX-2 in the CNS of rats treated with STZ—a model of type-1 diabetes.
Materials and Methods
Animals
All animals were maintained and cared for as outlined in the Guide for the Care and Use of Laboratory Animals (Kuwait University, Faculty of Health Publication). Animals were housed in a well-ventilated and air-conditioned area provided with independently adjustable light–dark cycle (12 h light/12 h dark cycle) and temperature regulation systems. Temperature was maintained at 22–24 °C and humidity was kept at 40–70 %. The rooms and animal cages were cleaned daily, and the animals were provided with fresh food and water ad libitum on a daily basis. 14-week-old male Wistar rats (Kuwait Animal Laboratory Center colony) weighing about 300 g were used in this study. Animals were housed individually throughout the experiment.
Experimental Protocols
Diabetes was induced by a single 0.3-ml i.p. injection of 55 mg/kg body weight STZ (Sigma, St. Louis, MO) dissolved in citrate buffer (pH 4.5). Age-matched control rats were injected with the citrate buffer vehicle. Basal glucose levels were determined using blood collected from a tail artery and an automated blood glucose analyzer (glucometer Elite XL). Blood glucose concentrations were determined before and 48 h after STZ injection. Rats with a blood glucose concentration above 250 mg/dl were declared diabetic. Diabetic animals were studied after 4 weeks of diabetes. Group 1 was vehicle-treated control rats [control, C]. Group 2 was STZ-treated rats [diabetic, D]. Groups 3 was diabetic animals treated with treated daily with Ang-(1–7) (576 μg/kg/day i.p.) [D + Ang-(1–7)]. Group 4 was diabetic animals treated with treated daily with Ang-(1–7) (576 μg/kg/day i.p.) + Ang-(1–7) antagonist [D-Ala7]-Ang-(1–7) (A779) (0.8 mg/kg/day i.p.) [D + Ang-(1–7) + A779]. A779 is a Mas receptor antagonist. Treatment with Ang-(1–7) or Ang-(1–7) + A779 was started at the same time as STZ injection and continued for 30 days until the animals were sacrificed.
Specimen Collection
Animals were sacrificed using anesthesia (mixture of Ketallar® 62.5 mg/kg and Rompun® 3.2 mg/kg i.p.) 30 days after induction of diabetes and/or treatment with drugs and the brains were dissected out from the skull. The fresh brain tissues were gently excised and immediately immersion-fixed in 10 % (v/v) buffered formalin and kept overnight. The brains were washed with saline and kept in 70 % ethanol (Riedel-de Haen AG) at 48 °C overnight. Next day, tissues were gradually dehydrated in ethanol then treated with xylene (Univar Ajax Chemicals, Australia) followed by paraffin embedding (Jung Histoembedder, Leica). Twenty coronal sections of 4-μm thickness (Jung Histocut, Leica) were obtained from each brain starting from rostral coordinates (interaural 6.48 mm, Bregma −2.52) to caudal coordinates (interaural 3.36 mm, Bregma −5.64 mm) (Paxinos and Watson 1998) for further immunostaining processing.
GFAP Immunohistochemistry
Tissues were processed for GFAP immunohistochemical staining as described previously (Renno et al. 2008). Briefly, sections of 4 μm were cut and fixed on positively charged super frost slides. The tissue sections were deparaffinized, dipped in phosphate buffer saline (PBS, pH 7.4) (Sigma Chemical Co., USA), and then kept in cold methanol (BDH Laboratory Supplies, UK) at −22 °C for 4 min and they were washed in PBS for several times. Tissues were treated for 30 min with 3 % (dilution from 30 %) H2O2 (Fluka chemika, Switzerland) and washed with PBS before applying the primary antibody. The primary antibody rabbit anti-GFAP was diluted 1:20 in PBS (Sigma Chemical Co., USA. cat # G9269-1ML) and added and incubated for 1 h. Then, the slides were washed three times in PBS before adding the extra avidin–peroxidase (Sigma Chemical Co., USA) diluted 1:20 in PBS containing 1 % BSA for 30 min. Slides were then washed thrice in PBS and treated with diaminobenzidene tetrahydrochloride (Bio-Rad Laboratories, Canada) for 30 s. Finally, the slides were washed in distilled water, dried, and placed in xylene for 5 min and mounted with DPX (Fluka Chemika, Switzerland).
GAP-43 Immunohistochemistry
For GAP-43 immunohistochemistry, the brain samples were processed as described previously by Zhou et al. (2007) with slight modifications. Briefly, the mounted sections were washed in 0.01 M PBS containing 0.3 % Triton X-100 (pH 7.4, PBS-T), then immersed in 2 % normal horse serum in PBS for 120 min at 37 °C, incubated overnight at 4 °C with rabbit polyclonal anti-GAP-43 (1:500, Abcam, Cambridge) in PBS containing 1 % bovine serum albumin (BSA), washed in PBS (3 × 5 min), incubated in biotinylated horse-anti-rabbit IgG (Vector Labs, USA) in PBS for 2 h at room temperature, washed in PBS-T (3 × 5 min). Staining was done according to the manufacturer’s protocol using avidin–biotin–peroxidase complex solution (ABC Kit, 1:100, Vector Labs, USA) for 2 h at room temperature, then rinsed again in PBS. Visualization was made by incubating the tissue for 10 min in 0.04 % 3,3-diaminobenzidine as a chromogen (DAB kit, Vector Labs, USA) containing 0.01 % H2O2.
Cyclooxygenase-2 immunohistochemistry
The brain sections for COX-2 immunolabeling were processed as described previously (Takemiya et al. 2006). In brief, tissue sections were deparaffinated, rehydrated and treated for 30 min with 1 % (v/v) H2O2 in distilled water, washed thrice in cold PBS (pH 7.4) and incubated with blocking solution for 30 min, and then incubated with rabbit anti-COX-2 antibody (Chemicon International Inc., USA) diluted 1:50 in PBS for overnight at 4 °C. Serial brain sections were then washed in PBS and incubated for 2 h at room temperature in biotinylated anti-rabbit IgG secondary antibody (Vector Laboratories, USA) diluted 1:100 in PBS, followed by several rinses in PBS. Sections were then incubated for 1 h at room temperature in avidin–biotin complex detection solution (ABC Elite kit, Vector Laboratories, USA), rinsed 3 times in 0.1 M Tris–HCl buffer (pH 7.4), Visualization was made as described above for the GAP-43 staining. Finally, sections were stained with haematoxylin before dehydration to determine the number of COX-2 immunopositive cells in each subfield of the hippocampus. Controls staining for the GFAP, GAP-43 and COX-2 immunoreactions were done in parallel and as follows: (a) the use of normal rabbit or goat serum to replace the specific primary antibodies, and (b) the primary antibody was omitted before adding the secondary antibody biotin anti-rabbit, which was diluted 1:20 in PBS containing 1 % BSA (Sigma Chemical Co., USA) for 30 min. No immunoreactivity was found in these sections. Sections of treated and control rats were processed simultaneously in order to reduce technical errors.
Quantitation of GFAP, GAP-43, and COX-2 Positive Immunostained Neuronal and Astrocytic Cells
All measurements were carried out by two independent investigators under the same settings in order to insure objectivity in blind conditions. For quantitative analysis of GFAP, GAP-43 and COX-2 immunopositive cells were analyzed using Olympus Bx51 light microscope Olympus PM-C35DX camera mounted to a microscope. Using a light intensity meter lock, light intensity was maintained at a constant level for all images. Sections were examined and counted by investigators blinded to the protocol used, the identity of the slides or the experimental groups. Images were processed and analyzed using ImageJ program software (Image processing and analysis in Java, USA). The numbers of GFAP, GAP-43 and COX-2 immunostained cells in the hippocampus were counted using a 100× objective lens and a rectangular field within a 300 × 220 μm frame. Immunopositive cells were counted and analyzed in a total of 5 fields sampled randomly from three randomly selected sections from each hippocampal area from each animal examined (N = 10), and compared to an equal number of fields from control group, ensuring that fields did not overlap and ensuring that each represented the anatomical area of interest. The percentage increase in immunopositive cells in every area compared to that seen in the control rats was also calculated. GAP-43 immunostaining was further subjected to a second quantitative analysis which involved measuring the intensity of the immunoreactions seen in the pyramidal and granule cells of the hippocampus. Ten images from 5 sections per rat from each experimental group were captured and the optical density analysis by two blind observers was performed using ImageJ Sigma Gel program (Jandel Scientific Co., USA). For this study, the areas inspected were CA3 hippocampal subfield and granular dentate gyrus. Within the selected areas, optical densities were measured within three circumscribed areas evenly spaced. The optical light density of the immunoprecipitants in the hippocampal pyramidal and granule neurons was calculated for all rats in all treatment groups (Table 1), and the percentage decrease was compared to that seen in the controls. Group means and standard deviation calculated from the two independent investigators for all measurements were first statistically compared using the Student’s t test for two independent samples. No statistical difference was found between the two independent observes. Therefore, a final mean value was calculated for each group from the two different investigators’ data and used in the statistical analysis for all the different groups and parameters reported in this study.
Table 1.
Comparison of GAP-43 positive neurons and GAP-43 optical density in dentate gyrus and CA3 area of the hippocampus among control, diabetic, and diabetic treated with Ang(1–7) or Ang-(1–7) + A779 rats (mean ± SEM)
| Group | GAP-43 positive neurons | |||
|---|---|---|---|---|
| Dentate gyrus | CA3 area | |||
| Number (mm2) | Light density | Number (mm2) | Light density | |
| Control | 15.5 ± 2.4 | 156.6 ± 9.4 | 12.1 ± 2.9 | 115.5 ± 5.4 |
| Diabetes | 9.4 ± 1.2** | 102.3 ± 6.6* | 5.5 ± 2.4** | 70.9 ± 4.2* |
| Diabetic + Ang-(1–7) | 13.3 ± 1.9 | 149.8 ± 8.1 | 10.5 ± 2.1 | 105.5 ± 6.3 |
| Diabetic + Ang-(1–7) + A799 | 8.2 ± 1.1* | 89.4 ± 6.9* | 6.1 ± 1.1* | 76.5 ± 3.8* |
* P < 0.01 compared with the control and diabetes + Ang-(1–7)
** P < 0.05 compared with the diabetes + Ang-(1–7)
Statistical Analysis
A one-way analysis of variance (ANOVA) was applied to assess significant differences between the mean values of number of immunostained cells quantified in control, diabetic and Ang-(1–7) and Ang-(1–7) + A779 treated diabetic rat groups. The Bonferroni’s F and Fisher LSD Post Hoc comparison tests were performed to determine individual probability values for multiple comparisons using SPSS software (v. 17.0; SPSS Inc., USA). All data was calculated and presented as mean ± SEM. A difference among the means of a P value <0.05 was considered statistically significant. The graphs were drawn using Microsoft Excel and Publisher software.
Results
Hyperglycemia in Diabetic Animals
Induction of diabetes by STZ resulted in a significant increase in blood glucose concentration. Hyperglycemia persisted in the diabetic animals and was 504 ± 18 mg/dl after 4 weeks of diabetes as compared with 86 ± 8 mg/dl in the non-diabetic control animals. None of the treatments with drugs had any significant effects on lowering blood glucose levels (data not shown).
Immunohistochemistry for GFAP and Astrocyte Cell Counts
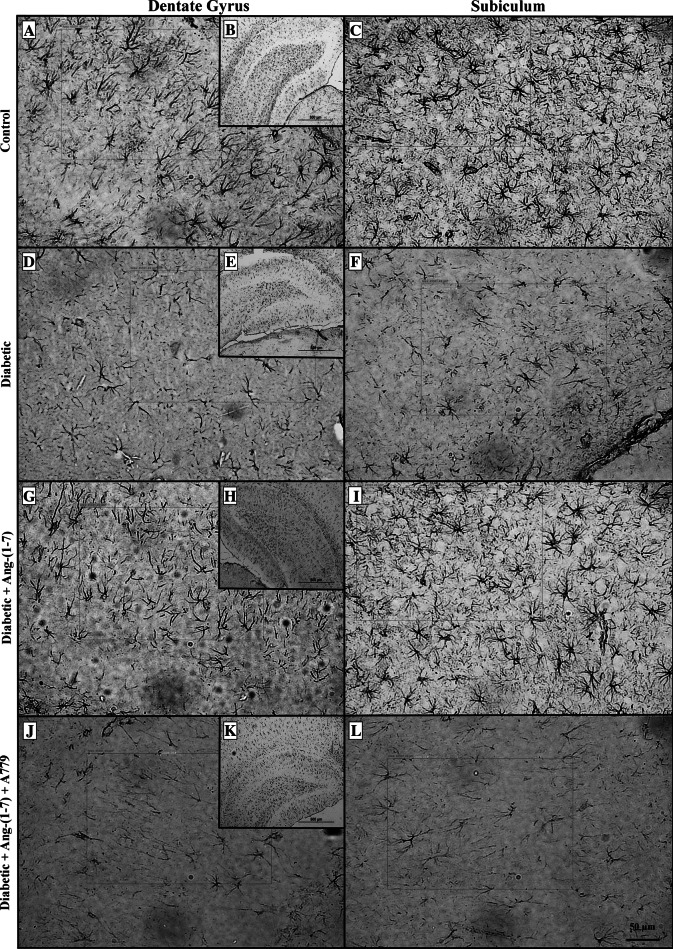
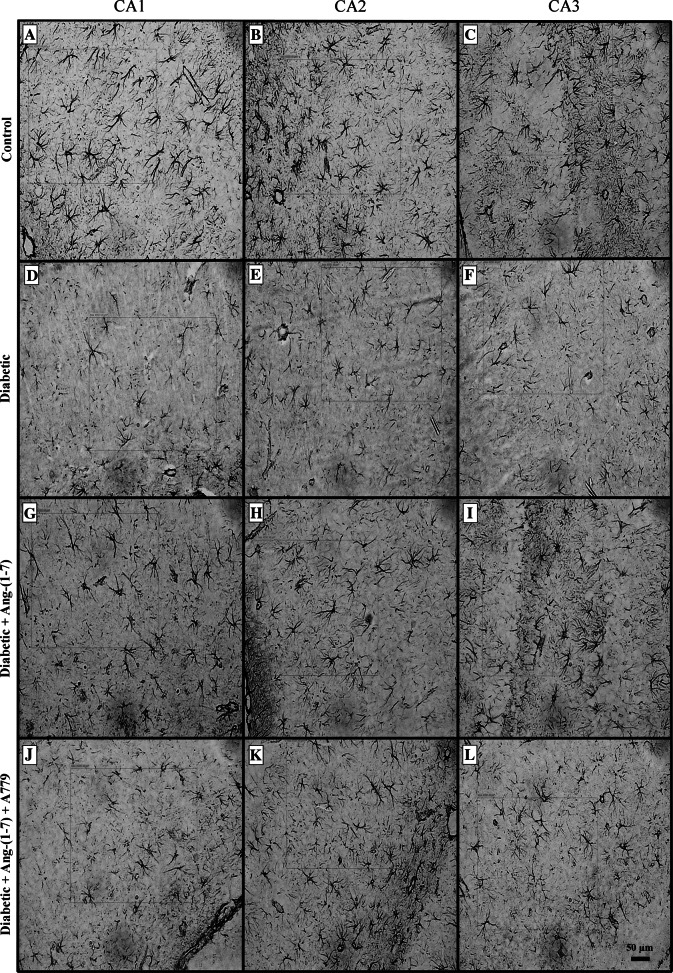
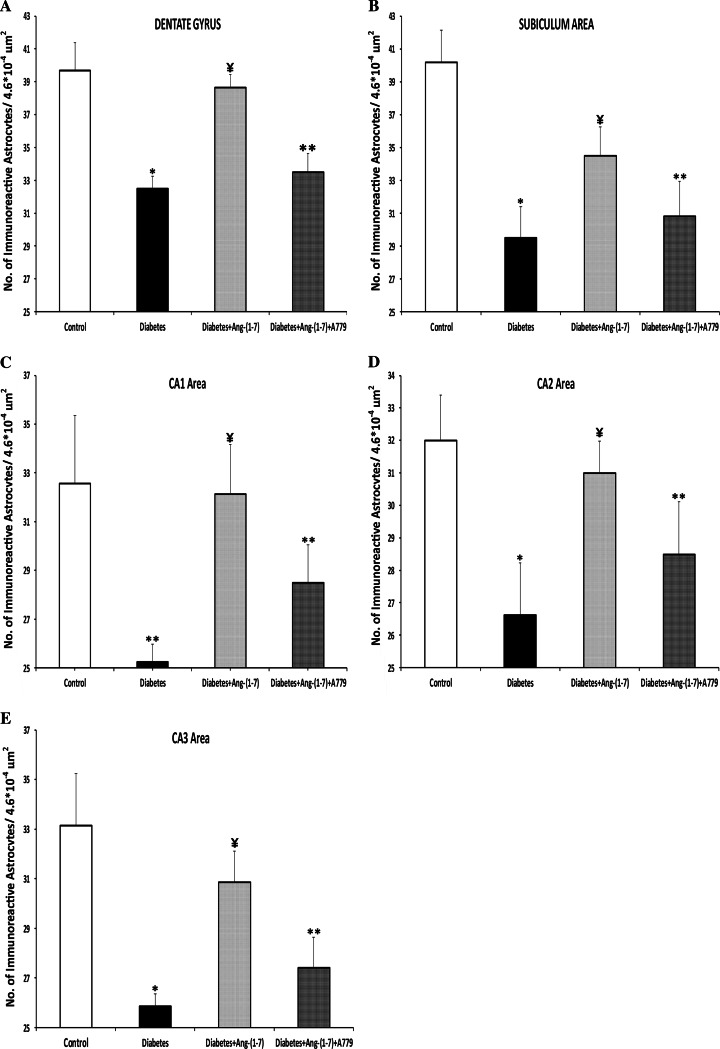
There was no significant difference in the size of hippocampus of animals from different groups studied. Induction of diabetes resulted in a significant decrease in GFAP immunoreactive astrocytes in the dentate gyrus (Fig. 1d, e) and subiculum (Fig. 1f) sub-regions of the hippocampal formation (Fig. 1a–c). Treatment with Ang-(1–7) prevented diabetes-induced decrease in GFAP immunoreactive astrocytes in both dentate gyrus (Fig. 1g, h) and subiculum (Fig. 1i) sub-regions of the hippocampus. Ang-(1–7) effect was blocked when diabetic animals that were treated with a combination of Ang-(1–7) and A779 (Fig. 1j–l). Induction of diabetes also resulted in a significant decrease in GFAP immunoreactive astrocytes in CA1 (Fig. 2d), CA2 (Fig. 2e), and CA3 (Fig. 2f) areas of the rat hippocampus (Fig. 2a–c, respectively). Treatment of diabetic rats with Ang-(1–7) prevented the decrease in the number of GFAP immunoreactive astrocytes in the three CA areas (Fig. 2g–i). Ang-(1–7) effect in all of the CA areas (Fig. 2j–l) was blocked when diabetic animals that were treated with a combination of Ang-(1–7) and A779. Quantitative analysis of the different areas of the hippocampus examined confirmed the observation that Ang-(1–7) through activation of Mas receptor prevented diabetes-induced decrease in the number of GFAP immunoreactive astrocytes in the dentate gyrus (Fig. 3a), Subiculum (Fig. 3b), CA1 (Fig. 3c), CA2 (Fig. 3d), and CA3 (Fig. 3e).
Fig. 1.
Representative photomicrographs of glial fibrillary acidic protein (GFAP) immunoreactive astrocytes from dentate gyrus (a, d, g, j) and subiculum (c, f, i, l) areas of the hippocampus in control, diabetic, and diabetic + Ang-(1–7) rats and diabetic + Ang-(1–7) + A779. a, b, c Sections showing GFAP immunoreactive astrocytes from control animals; d–f examples from diabetic group showing visible decrease in the number of GFAP immunoreactive astrocytes, while g–i sections from diabetic rats treated with Ang-(1–7) showing a significant increase in the number of GFAP immunoreactive astrocytes. This increase was blocked in diabetic + Ang-(1–7) group when treated with A779 (j–l)
Fig. 2.
Representative light photomicrographs of GFAP immunoreactive astrocytes from CA1 (a, d, g, j), CA2 (b, e, h, k), and CA3 (c, f, i, l) areas of the hippocampus in control, diabetic, and diabetic + Ang-(1–7) rats and diabetic + Ang-(1–7) + A779. a–c Sections showing GFAP immunoreactive astrocytes from control animals; d–f examples from diabetic group showing visible decrease in the number of GFAP immunoreactive astrocytes, while g–i sections from diabetic rats treated with Ang-(1–7) showing a substantial increase in the number of GFAP immunoreactive astrocytes. This increase was reduced in diabetic + Ang-(1–7) group treated with A779 (j–l)
Fig. 3.
Quantitative analysis of the number of GFAP immunoreactive astrocytes in the different areas of the hippocampus: a dentate gyrus, b subiculum, c CA1, d CA2, and e CA3. Diabetic animals showed significant decrease in the number of GFAP immunoreactive astrocytes compared to control group in all the areas of the hippocampus investigated. Treatment of diabetic rats with Ang-(1–7) significantly increased the number of GFAP immunostained astrocytes in all hippocampal areas. This increase was attenuated with in the diabetic animals treated with Ang-(1–7) and A779. Statistical analysis was carried out by one-way ANOVA followed by Bonferroni’s test. Results are expressed are mean ± SEM. *P < 0.001; compared to control and diabetic + Ang-(1–7) groups. **P < 0.001; compared to control group. ¥ P < 0.02; compared to diabetic and diabetic + Ang-(1–7) + A779 groups
Immunohistochemistry for GAP-43 and Immunolabeled Cell Counts
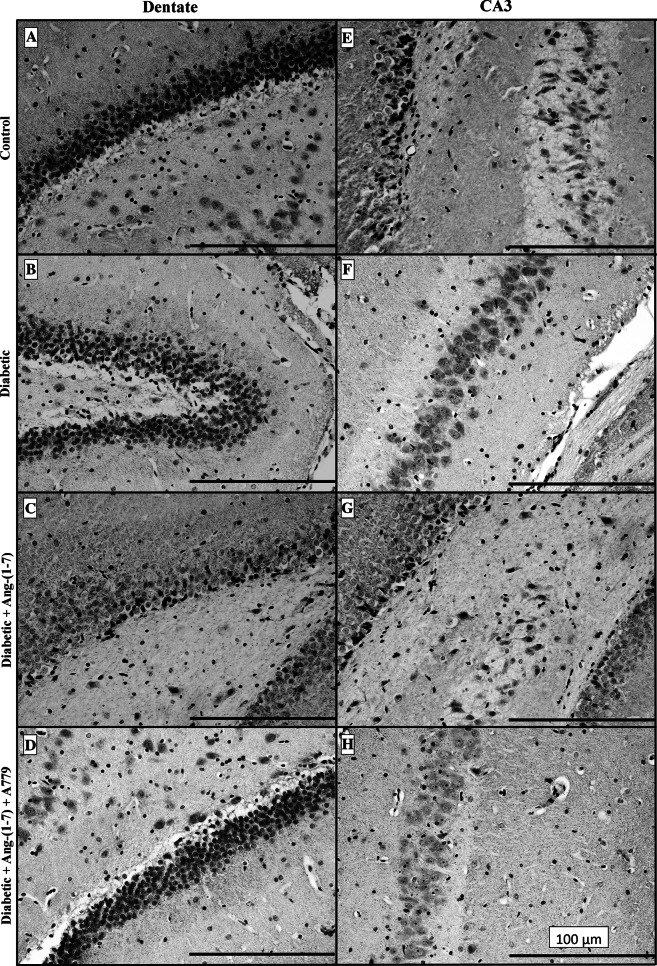
Immunostaining for the axonal remodeling marker GAP-43 revealed that it is ubiquitously expressed in rat hippocampus of the control group. Closer examination of GAP-43 signals in the dentate gyrus and CA3 area showed remarkable and diffuse GAP-43 immunostaining (Fig. 4a, e). Neuronal terminals and dendritic layers showed more intense GAP-43 labeling compared to neuronal cell bodies (cytoplasm). The extent and density of the GAP-43 immunostaining varied in pyramidal neurons of hippocampus. These neurons exhibited intense GAP-43 immunoreactivity in control animals. However, GAP-43 staining signals in the dentate gyrus (Fig. 4b) and CA3 (Fig. 4f) of the diabetic group were markedly reduced compared to control animals. Similarly, GAP-43 immunoreactivities in the inner molecular layer and the granule cells of the dentate gyrus were lower in diabetics compared to controls. Treatment of the diabetic animals with Ang-(1–7) prevented diabetes-induced decrease in GAP-43 immunolabeled fibers and neurons in both dentate (Fig. 4c) and CA3 (Fig. 4g) sub-regions of the hippocampus. Ang-(1–7) effect was blocked when diabetic animals that were treated with a combination of Ang-(1–7) and A779 (Fig. 4d, h). Similar results were seen in the other sub-fields (subiculum, CA1, and CA3) of the hippocampus (data not shown). Quantitative analysis results of the number and optical density, which represents immunoreactive density, of GAP-43 immunoreactive neurons for the dentate gyrus and CA3 hippocampal subfield are given in Table 1. The number of GAP-43 positive neurons in the dentate gyrus and CA3 area of the diabetic group decreased (P < 0.01) ~40 and 54 %, respectively, compared to control group. However, the number of GAP-43 immunopositive neurons in the dentate gyrus and CA3 area in the diabetic group treated with Ang-(1–7) was not significantly different from non-diabetic control values. Co-administration of A779 with Ang-(1–7) inhibited Ang-(1–7) effect on the number of GAP-43 positive cells (Table 1).
Fig. 4.
Representative photomicrographs of GAP-43 immunoreactivity in dentate (a–d) and CA3 (e–h) areas of the hippocampus from control, diabetic, and diabetic + Ang-(1–7) and diabetic + Ang-(1–7) + A779 rat groups. Dentate and CA3 hippocampal sections from control animals (A and E) showed visible GAP-43 immunoreactive density. The GAP-43 dense labeling was mainly confined to terminals and dendritic layers but few neuronal cell bodies. Hippocampal sections from diabetic rats (b, f) showed prominent GAP-43 immunolabeling density decrease. In contrast, sections from diabetic rats treated with Ang-(1–7) (c, g) showed visible increase of GAP-43 immunolabeling which was blocked in diabetic animals treated with Ang-(1–7) and A779 (d, h)
Immunohistochemistry for COX-2 and Cell Counts
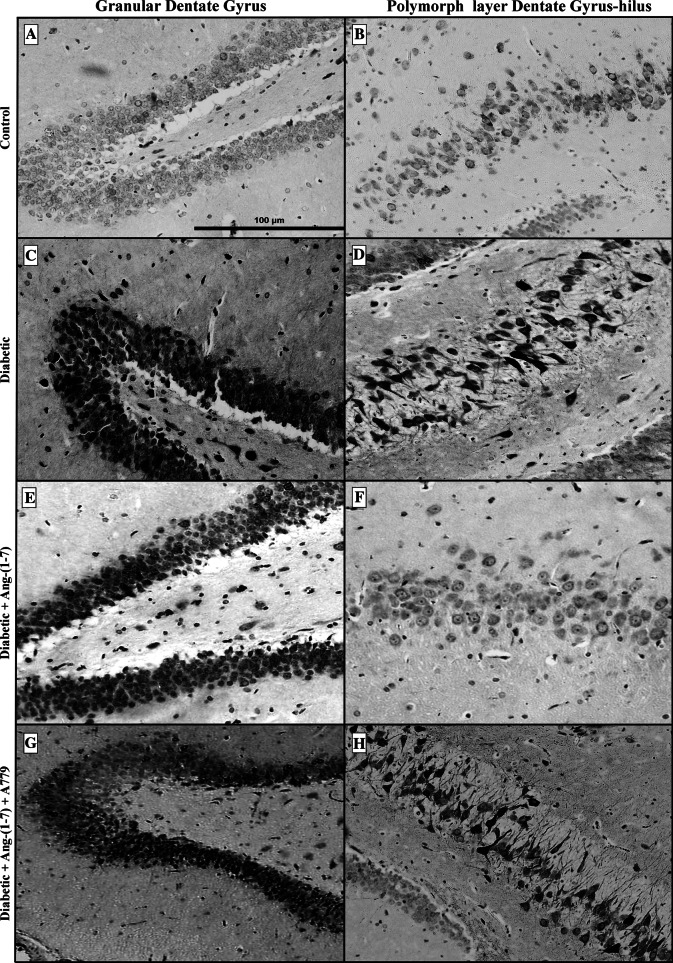
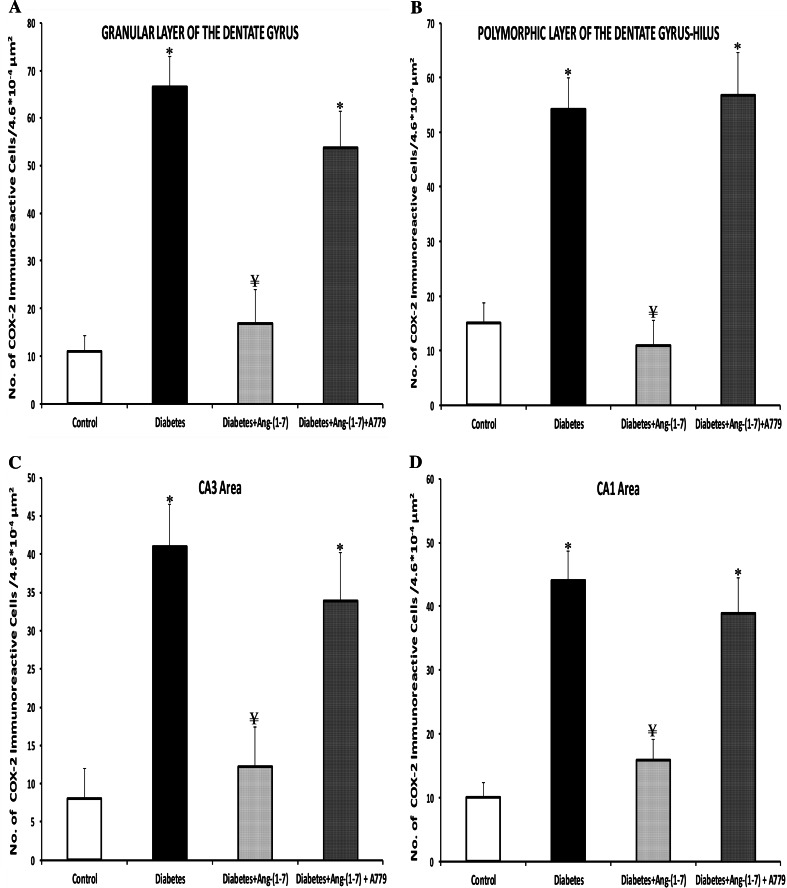
In control animals, neuronal localization of COX-2 immunoreactivity was very low and sporadic throughout the different sub-regions of the hippocampus (Fig. 5a, b). There were a few large and small COX-2 immunoreactive cells (generally neurons) in the control granular dentate gyrus (Fig. 5a), and polymorphic layer of dentate gyrus-hilus (Fig. 5b) of the hippocampus. This faint COX-2 immunostaining appeared to be confined to the cytoplasm and in cellular processes; no nuclear staining was detected. In contrast, COX-2 staining was dramatically increased in the whole hippocampus of the diabetic animals. The neuronal COX-2 immunoreactivity localization was evident and was relatively strong in the granular (Fig. 5c) and polymorphic (hilus) layers of dentate gyrus (Fig. 5d) of the hippocampus in diabetic rats. The immunoreactivity showed a characteristically perinuclear localization, as reported earlier (Nakayama et al. 1998). In the granule cell layer of the dentate gyrus (Fig. 5c), a large number of cells showed COX-2 immunoreactivity of moderate to high intensity. In the polymorphic layer of the dentate gyrus (Fig. 5d), a number of large neurons showed the most intense reactions. COX-2 immunoreactivity in CA3 sectors was morphologically pyknotic and condensed to a perinuclear location (data not shown). The densest and most intensely COX-2-immunostained neurons were localized in the stratum pyramidale of CA3 and CA1. The cytoplasmic immunoreaction involved fine grains, mainly surrounding the nuclei. Ang-(1–7) treatment of diabetic rats remarkably attenuated the neuronal COX-2 immunoreactivity throughout the dentate (Fig. 5e, f), CA3 and CA1 (data not shown) hippocampal areas. Few and variable numbers of immunoreactive neurons were seen throughout hippocampus of the Ang-(1–7)-treated diabetic rats. However, diabetic animals treated with a combination of Ang-(1–7) and A779 showed a degree of COX-2 immunoreactivity in the granular (Fig. 5g) and polymorphic (Fig. 5h) layers of the dentate gyrus not different from untreated diabetic animals. The cytoplasmic immunopositivity was confined mostly to neurons and no well-defined glial cells were observed. CA1, CA2 and CA3 hippocampal areas showed similar pattern of COX-2 immunopositivity (data not shown). Quantitative analysis showed that diabetes induced a significant (P < 0.001) increase in the number of the COX-2 immunolabeled neurons in all sub-regions of the hippocampus (Fig. 6) examined. The number of the COX-2 positive neurons in the granular (Fig. 6a) and polymorphic (Fig. 6b) cell layers of the dentate gyrus increased ~5.1- and 2.6-fold, respectively, compared to control animals. Likewise, COX-2 positive pyramidal neurons in the CA3 (Fig. 6c) and CA1 (Fig. 6d) sub-regions of the rat hippocampus in diabetic animals increased ~4.16- and 3.40-fold, respectively, compared to control rats. In contrast, Ang-(1–7) treatment of diabetic rats attenuated significantly the neuronal COX-2 positive cell counts in all hippocampus sub-regions. The number of COX-2 immunoreactive cells was comparable to the controls in dentate gyrus (Fig. 6a, b), CA3 (Fig. 6c) and CA1 (Fig. 6d). Combination treatment of diabetic animals with Ang-(1–7) + A779 significantly (P < 0.001) blocked the Ang-(1–7)-mediated decrease in the number of COX-2 immunolabeled cells. There was no significant difference in the COX-2 immunopositive cell count between untreated diabetic and Ang-(1–7) + A779-treated diabetic groups in the granular or polymeric layers of the dentate gyrus, CA3 or CA1 areas of the hippocampus.
Fig. 5.
Distribution of COX-2 immunoreactivity in granular dentate gyrus (a, c, e, g) and polymorphic layer of dentate gyrus-hilus (b, d, f, h) areas of the hippocampus in control, diabetic, and diabetic + Ang-(1–7) rats and diabetic + Ang-(1–7) + A779. Diabetes resulted in a substantial increase in COX-2 immunoreactivity in both sub-regions of the dentate hippocampal sector (c, d) compared to faintly labeled control group (a, b). The levels and density of COX-2 immunoreactivity returned to basal levels throughout the dentate hippocampal areas after Ang-(1–7) treatment (e, f). However, marked increase in COX-2 immunoreactivity was observed in diabetic rats treated with Ang-(1–7) and A779 (g, h). Moreover, this marked COX-2 staining was morphologically pyknotic and condensed to a perinuclear location
Fig. 6.
Effect of Ang-(1–7) and Ang-(1–7) + A779 on the COX-2 immunoreactive neurons in the hippocampus of diabetic rats. Quantitative analysis of the number of the COX-2 positive neurons in the granular (a) and polymorphic (b) layers of the dentate gyrus, CA3 (c), and CA1 (d) sub-regions of the rat hippocampus showed a significant increase in all areas of the diabetic animals. Treatment of diabetic rats with Ang-(1–7) resulted in significant reduction in the number of COX-2 immunopositive neurons in all hippocampal sub-regions; while Ang-(1–7) + A779 treatment significantly blocked the decrease in number of the COX-2-immunolabeled cells. Note that there is no significant difference between diabetic-untreated and diabetic Ang-(1–7) + A779-treated groups in the granular and polymorphic layers of the dentate gyrus, CA3 or CA1 areas of the hippocampus. Results are expressed are mean ± SEM. *P < 0.001; compared to control and diabetic + Ang-(1–7) groups. ¥ P < 0.001; compared to diabetic and diabetic + Ang-(1–7) + A779 groups
Discussion
Induction of diabetes for 30 days resulted in decreased expression of GFAP and GAP-43 and elevated levels of COX-2 in various CNS regions and cells. Chronic administration of Ang-(1–7) starting at the same time as induction of diabetes prevented diabetes-induced changes in the CNS through activation of receptor Mas without correcting STZ-induced hyperglycemia. This observation is consistent with previous reports where activation of ACE-2/Ang-(1–7)/Mas signaling has been shown to play a protective role against diabetes-induced vascular, renal and cardiac dysfunction. The present findings also suggest that anti-oxidant and anti-inflammatory effects of Ang-(1–7) that were previously shown in the periphery may also contribute to the observed beneficial effects of Ang-(1–7) in the CNS (Benter et al. 2008; El-Hashim et al. 2012).
Recent studies have shown that diabetic neurocognitive dysfunction in diabetic animals is associated with a decrease in hippocampal and cerebrospinal fluid GFAP immunoreactive levels and down-regulation of GFAP in the hippocampus at protein and mRNA levels (de Senna et al. 2011; Zuo et al. 2011). Our data show that diabetes induction decreased the number of GFAP immunoreactive astrocytes. All components of the renin-angiotensin system are present in the astrocytes including angiotensinogen, Ang II, Ang-(1–7), angiotensin receptors as well as both forms of angiotensin-converting enzymes (ACE and ACE-2) (Gallagher et al. 2006). Recent data from Guo et al. (2010) have implicated astroglia as a possible cellular target of Ang-(1–7) in rostral ventrolateral medulla and showed a decrease in astrocytic responsiveness to Ang-(1–7) in spontaneously hypertensive rats. Furthermore, the competitive Ang (1–7) receptor antagonist A779 suppressed Ang-(1–7)-induced [Ca2+] elevations in the astroglia (Guo et al. 2010). Ang-(1–7) was also shown to prevent the Ang II-mediated reduction in ACE-2 mRNA in rat astrocytes through activation of Mas (Gallagher et al. 2006). Our present data showing that Ang-(1–7) treatment significantly attenuated diabetes-induced decrease in the GFAP immunoreactivity in the hippocampus suggest that activation of ACE-2/Ang-(1–7)/Mas signaling may have a therapeutic role preventing the CNS disorders associated with decreased GFAP levels.
This study showed significantly less GAP-43 staining in the diabetic animals compared to non-diabetic controls suggesting that synaptic remodeling has been negatively affected. Our data is similar to a previous study showing a significant decrease in the GAP-43 immunopositive neurons for the whole CA1 and dentate gyrus of diabetic animals (Zhou et al. 2007). The reduction in the GAP-43 staining is consistent with a decrease in the density of neurons in the CA areas of the hippocampus and the neurodegenerative changes that occur in the hippocampus after diabetes induction (Lebed et al. 2008). Using immunohistochemistry techniques, it was also shown in brain-ischemic rats that decreased expression of GAP-43 in the hippocampus indicates increased neuronal cell death and impairment of learning and memory (Li et al. 2011). In addition, diabetes has been shown to impair hippocampal neurogenesis associated with significant functional impairment of learning and memory in diabetic animals (Zhang et al. 2008; Lang et al. 2009). Zhou et al. (2007) showed that the ultrastructure of diabetic hippocampus neurons including areas CA1 and dentate gyrus are characterized by swollen mitochondria, increased heterochromatin accumulation and decreased GAP-43 optical density. Our present observations showed that Ang-(1–7) treatment through activation of Mas significantly prevented diabetes-induced decrease in the GAP-43 immunostaining in different areas of the hippocampal formation.
Diabetes is associated with a low-grade inflammation leading to vascular and neuronal dysfunction (Bagi et al. 2006). In line with our current data, it was previously shown that COX-2 is up regulated in the peripheral nerves and dorsal root ganglia neurons in diabetic rats leading to downstream inflammatory reactions and COX-2 gene inactivation and selective COX-2 inhibition protects against diabetic peripheral neuropathy (Kellogg et al. 2008). COX-2 has a NF-κB binding site in its promoter region and NF-κB pathway directly modulates cellular inflammatory processes with the CNS. The NF-κB derived TNF-α is elevated by diabetes in the peripheral nerve and COX-2 gene inactivation or pharmacological COX-2 blockers prevents this increase (Kellogg et al. 2008). A recent study by Benicky et al. (2011) has demonstrated that systemic administration of angiotensin type-1 receptor (AT1) blocker (ARB) candesartan to normotensive rats decreased acute brain inflammatory response to injection of LPS throughout different brain areas including the hippocampus. Direct anti-inflammatory effects of ARBs were also shown in culture rat microglia, cerebellar granule cells, and cerebral microvascular endothelial cells (Benicky et al. 2011). Several recent experiments in animal models have demonstrated the neuroprotective effect of systemic administration of ARBs which reduce exaggerated stress responses and anxiety, prevent stress-induced gastric ulcerations, decrease vulnerability to ischemia and stroke, reverse chronic cerebrovascular inflammation, and reduce acute inflammatory responses produced by bacterial endotoxin (Saavedra et al. 2011). Several studies have shown that administration of ARBs results in increased expression of ACE-2 and increased formation of Ang-(1–7) (Chappell 2007). Further, Ang-(1–7) has been shown to contribute to the established beneficial effects of ARBs since co-administration of Mas antagonists with ARBs results in decreased ARB-mediated protective effects (Chappell 2007; Benter et al. 2011). The present results give further direct evidence to the hypothesis that Ang-(1–7) is a protective agent in the periphery and the CNS by showing that administration of Ang-(1–7) prevented diabetes-induced increase in ACE-2 expression in the CNS. This finding is in line with our previous studies showing in a model of combined hypertension and diabetes that chronic treatment with Ang-(1–7), using the same doses as the present study, can inhibit cardiac NF-kB and the expression of pro-inflammatory agents like C3, IL-6, IL-1β, Na 1p12, Casp 1, Tlr2, and Irak1 in the heart and attenuate I/R-induced cardiac dysfunction (Benter et al. 2008; Al-Maghrebi et al. 2009).
In conclusion, our study shows that Ang-(1–7) treatment through activation of receptor Mas significantly prevented diabetes-induced decrease in the number of GFAP immunoreactive astrocytes and GAP-43 positive neurons in all hippocampal regions. Further, Ang-(1–7) treatment prevented diabetes-induced increase in the number of the COX-2 expressing neurons in all sub-regions of the hippocampus examined. These results suggest that treatment with Ang-(1–7) is an effective way to prevent diabetes-induced pathologies in the CNS.
Acknowledgments
This work was financially supported by Department of Anatomy, Faculty of Medicine, Kuwait University, Kuwait.
References
- Afsari ZH, Renno WM, Abd-El-Basset E (2008) Alteration of glial fibrillary acidic proteins immunoreactivity in astrocytes of the spinal cord diabetic rats. Anat Rec 291:390–399 [DOI] [PubMed] [Google Scholar]
- Al-Maghrebi M, Benter IF, Diz DI (2009) Endogenous angiotensin-(1–7) reduces cardiac ischemia-induced dysfunction in diabetic hypertensive rats. Pharmacol Res 59:263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Kamal A, Ramakers GM, Biessels GJ, Gispen WH (2005) Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Euro J Neurosci 22:169–178 [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Papp Z, Edes I, Koller A (2006) Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep 58:52–56 [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Gardner TW (2000) Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Investig Ophthalmol Vis Sci 41:3561–3568 [PubMed] [Google Scholar]
- Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM (2011) Angiotensin II AT(1) receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36:857–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benter IF, Diz DI, Ferrario CM (1993) Cardiovascular actions of angiotensin-(1–7). Peptides 14:679–684 [DOI] [PubMed] [Google Scholar]
- Benter IF, Yousif MHM, Anim JT, Cojocel C, Diz DI (2006) Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol 290:H684–H691 [DOI] [PubMed] [Google Scholar]
- Benter IF, Yousif MHM, Cojocel C, Al-Maghrebi M, Diz DI (2007) Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 292:H666–H672 [DOI] [PubMed] [Google Scholar]
- Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI (2008) Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28:25–33 [DOI] [PubMed] [Google Scholar]
- Benter IF, Yousif MH, Al-Saleh FM, Raghupathy R, Chappell MC, Diz DI (2011) Angiotensin-(1–7) blockade attenuates captopril- or hydralazine-induced cardiovascular protection in spontaneously hypertensive rats treated with NG-nitro-l-arginine methyl ester. J Cardiovasc Pharmacol 57:559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen von, Halbach O, Albrecht D (2006) The CNS renin–angiotensin system. Cell Tissue Res 326:599–616 [DOI] [PubMed] [Google Scholar]
- Chappell MC (2007) Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS receptor axis: more than regulation of blood pressure? Hypertension 50:596–599 [DOI] [PubMed] [Google Scholar]
- Coleman ES, Dennis JC, Braden TD, Judd RL, Posner P (2010) Insulin treatment prevents diabetes-induced alterations in astrocyte glutamate uptake and GFAP content in rats at 4 and 8 weeks of diabetes duration. Brain Res 1306:131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Senna PN, Ilha J, Baptista PP, do Nascimento PS, Leite MC, Paim MF, Gonçalves CA, Achaval M, Xavier LL (2011) Effects of physical exercise on spatial memory and astroglial alterations in the hippocampus of diabetic rats. Metab Brain Dis 26:269–279 [DOI] [PubMed] [Google Scholar]
- El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF (2012) Angiotensin 1–7 inhibits allergic inflammation, via the Mas receptor, through suppression of ERK1/2 and NF-κB dependent pathways. Br J Pharmacol. doi:10.1111/j.1476-5381.2012.01905.x [DOI] [PMC free article] [PubMed]
- Ellis EF, Police RJ, Rice LY, Grabeel M, Holt S (1989) Increased plasma PGE2, 6-keto-PGF1 alpha, and 12-HETE levels following experimental concussive brain injury. J Neurotrauma 6:31–37 [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Chappell MC, Ferrario CM, Tallant EA (2006) Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 290:C420–C426 [DOI] [PubMed] [Google Scholar]
- Gispen WH, Biessels GJ (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 11:542–549 [DOI] [PubMed] [Google Scholar]
- Guo F, Liu B, Tang F, Lane S, Souslova EA, Chudakov DM, Paton JF, Kasparov S (2010) Astroglia are a possible cellular substrate of angiotensin(1–7) effects in the rostral ventrolateral medulla. Cardiovasc Res 87:578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Qin W, Stetka BS, Pasinetti GM (2006) Is there a future for cyclo-oxygenase inhibitors in Alzheimer’s disease? CNS Drugs 20:85–98 [DOI] [PubMed] [Google Scholar]
- Huber JD (2008) Diabetes, cognitive function, and the blood–brain barrier. Curr Pharm Des 14:1594–1600 [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yi SS, Kim YN, Kim IY, Lee IS, Yoon YS, Seong JK (2008) Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res 33:394–400 [DOI] [PubMed] [Google Scholar]
- Kamal A, Biessels GJ, Gispen WH, Ramakers GM (2006) Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res 1073–1074:276–280 [DOI] [PubMed] [Google Scholar]
- Kellogg AP, Cheng HT, Pop-Busui R (2008) Cyclooxygenase-2 pathway as a potential therapeutic target in diabetic peripheral neuropathy. Curr Drug Targets 9:68–76 [DOI] [PubMed] [Google Scholar]
- Lang BT, Yan Y, Dempsey RJ, Vemuganti R (2009) Impaired neurogenesis in adult type-2 diabetic rats. Brain Res 1258:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebed YV, Orlovsky MA, Lushnikova IV, Skibo GG (2008) Neurodegenerative changes in the hippocampus with the early period of experiment diabetes mellitus. Neurophysiology 40:26–31 [Google Scholar]
- Lechuga-Sancho AM, Arroba AI, Frago LM, García-Cáceres C, de Célix AD, Argente J, Chowen JA (2006) Reduction in the number of astrocytes and their projections in associated with increased synaptic protein density in the hypothalamus of poorly controlled diabetic rats. Endocrinology 147:5314–5324 [DOI] [PubMed] [Google Scholar]
- Li ZG, Britton M, Sima AA, Dunbar JC (2004) Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci 76:249–262 [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Xie Y, Yang Z, Zhang T (2011) Protective effects of exogenous hydrogen sulfide on neurons of hippocampus in a rat model of brain ischemia. Neurochem Res 36:1840–1849 [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS (2000) Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increase glucocorticoid reactivity to stress. Proc Natl Acad Sci USA 97:11056–11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH (1998) Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA 95:10954–10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G, Watson, C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, New York [Google Scholar]
- Proper EA, Oestreicher AB, Jansen GH, Veelen CW, van Rijen PC, Gispen WH, de Graan PN (2000) Immunohistochemical characterization of mossy fibre sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 123:19–30 [DOI] [PubMed] [Google Scholar]
- Rekart JL, Quinn B, Mesulam MM, Routtenberg A (2004) Subfield-specific increase in brain growth protein in postmortem hippocampus of Alzheimer’s patients. Neuroscience 126:579–584 [DOI] [PubMed] [Google Scholar]
- Renno WM, Alkhalaf M, Afsari Z, Abd-El-Basset E, Mousa A (2008) Consumption of green tea alters glial fibriliary acidic protein immunoreactivity in the spinal cord astrocytes of STZ-diabetic rats. Nutr Neurosci 11:32–40 [DOI] [PubMed] [Google Scholar]
- Revsin Y, Saravia F, Roig P et al (2005) Neuronal and astroglial alterations in the hippocampus of a mouse model for type 1 diabetes. Brain Res 1038:22–31 [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Sánchez-Lemus E, Benicky J (2011) Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology 36:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RAS, Campagnole-Santos MJ, Andrade S (2000) Angiotensin-(1–7): an update. Regul Pept 91:45–62 [DOI] [PubMed] [Google Scholar]
- Saravia FE, Revsin Y, Gonzalez Deniselle MC, Gonzalez SL, Roig P, Lima A, Homo-Delarche F, De Nicola AF (2002) Increased astrocyte reactivity in the hippocampus of murine models of type 1 diabetes: the nonobese diabetic (NOD) and streptozotocin-treated mice. Brain Res 957:345–353 [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP (2008) Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 11:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K (2006) Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res 56:103–110 [DOI] [PubMed] [Google Scholar]
- Ueda S, Masumori-Maemoto S, Wada A, Ishii M, Brosnihan KB, Umemura S (2001) Angiotensin(1–7) potentiates bradykinin-induced vasodilatation in man. J Hypertens 19:2001–2009 [DOI] [PubMed] [Google Scholar]
- Xu P, Sriramula S, Lazartigues E (2011) ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol 300:R804–R817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM (2008) Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand 117:205–210 [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang L, Ling S, Shang X (2007) Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of Streptozotocin-inducted diabetic cognitive impairment rats. Exp Neurol 206:201–208 [DOI] [PubMed] [Google Scholar]
- Zuo ZF, Wang W, Niu L, Kou ZZ, Zhu C, Wang W, Zhao XH, Luo DS, Zhang T, Zhang FX, Liu XZ, Wu SX, Li YQ (2011) RU486 (mifepristone) ameliorates cognitive dysfunction and reverses the down-regulation of astrocytic N-myc downstream-regulated gene 2 in streptozotocin-induced type-1 diabetic rats. Neuroscience 190:156–165 [DOI] [PubMed] [Google Scholar]