Abstract
It has been reported that young animals are less vulnerable to brain ischemia. In the present study, we compared gliosis in the hippocampal CA1 region of the young gerbil with those in the adult gerbil induced by 5 min of transient cerebral ischemia by immunohistochemistry and western blot for glial cells. We used male gerbils of postnatal month 1 (PM 1) as the young and PM 6 as the adult. Neuronal death in CA1 pyramidal neurons in the adult gerbil occurred at 4 days posti-schemia; the neuronal death in the young gerbil occurred at 7 days post-ischemia. The findings of glial changes in the young gerbil after ischemic damage were distinctively different from those in the adult gerbil. Glial fibrillary acidic protein-immunoreactive astrocytes, ionized calcium-binding adapter molecule (Iba-1), and isolectin B4-immunoreactive microglia in the ischemic CA1 region were activated much later in the young gerbil than in the adult gerbil. In brief, very less gliosis occurred in the hippocampal CA1 region of the young gerbil than in the adult gerbil after transient cerebral ischemia.
Keywords: Ischemia/reperfusion, Pyramidal neurons, Delayed neuronal death, Astrocytes, Microglia
Introduction
Stroke, which is induced by a lacking of cerebral blood flow, is the third leading cause of death. Recently, stroke has been increased in young people. Childhood stroke is one of the top ten causes of death in the U.S., and it has a high risk of serious morbidity for survivors (Ganesan et al. 2000). In India, 10–15 % of strokes occur in young people (Tripathi and Vibha 2010). Stroke or ischemic damage varies according to age (Blanco et al. 2007; Montori et al. 2010a; Nagayama et al. 1999; Saucier et al. 2007; Schaller 2007; Won et al. 2006).
Transient cerebral ischemia induced by the deprivation of blood flow via both carotid arteries occlusion to the brain causes delayed neuronal death in some specific vulnerable regions such as hippocampus and neocortex (Buiatti de Araujo et al. 2008; Himeda et al. 2007; Hu et al. 2007; Lorrio et al. 2009; Satoh et al. 2011). It has been reported that young animals appear less vulnerable to ischemia (Chen et al. 2009; Oguro et al. 2004; Tortosa and Ferrer 1994). Many studies have focused on neuronal death using adult gerbils (Brahma et al. 2009; Shiraishi et al. 2011; Yamashita et al. 2010). In addition, in aging studies, neuronal death induced by ischemia occurs much later in the aged than in the adult (Crain et al. 1988; Lee et al. 2010; Tamagaki et al. 2000). The gerbil is one of useful animal models of transient cerebral ischemia for the assessment of potential therapeutic strategy for human stroke (Canistro et al. 2010; Crain et al. 1988; Lin et al. 1990; Szilagyi et al. 2009; Zhang et al. 2009). Some studies have shown that developing gerbil is resistant to ischemic damage (Kusumoto et al. 1995; Oguro et al. 2004; Tortosa and Ferrer 1994). Kusumoto et al. (1995) has reported that less neuronal death occurred in young gerbils after 5 min of ischemia/reperfusion (I/R). Recently, we compared neuronal damage in the ischemic CA1 region between the young and adult gerbils at various times after I/R (Yan et al. 2011).
On the other hand, astrocytes play important roles during several processes, such as neurotransmission and neuronal functions, in the central nervous system (Horner and Palmer 2003). Microglia, as principle immune cells in the brain, defend the brain against pathological events in the central nervous system. They participate in the repair and regeneration of damaged neurons and cause neuronal death or dysfunction (Boje and Arora 1992; Nagayama et al. 1999). The gliosis of astrocytes and microglia are easily induced in the hippocampus after transient cerebral ischemia in the adult gerbil (Bonnekoh et al. 1990; Hwang et al. 2006). In the present study, we compared gliosis in the hippocampal CA 1 region at various time points after 5 min of ischemia/reperfusion between the young gerbil at postnatal month (PM) 1 and the adult gerbil at PM 6.
Materials and Methods
Experimental Animals
We used the progeny of male Mongolian gerbils (Meriones unguiculatus) obtained from the Experimental Animal Center, Hallym University, Chunchon, South Korea. Mongolian gerbils were used at 1 month (young, B.W. 25–30 g) of age for the young group and 6 months (adult, B.W. 65–75 g) of age for the adult group. The animals were housed in a conventional state under adequate temperature (23 °C) and humidity (60 %) control with a 12-h light/12-h dark cycle, and were provided with free access to water and food. Procedures involving animals and their care conformed to the guidelines, which are in compliance with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). All experiments were conducted to minimize the number of animals used and suffering caused.
Induction of Transient Cerebral Ischemia
The animals were anesthetized with a mixture of 2.5 % isoflurane in 33 % oxygen and 67 % nitrous oxide. A midline ventral incision was then made in the neck, and bilateral common carotid arteries were isolated, freed of nerve fibers, and occluded using non-traumatic aneurysm clips. The complete interruption of blood flow was confirmed by observing the central artery in retinae using an ophthalmoscope. After 5 min of occlusion, the aneurysm clips were removed from the common carotid arteries. The restoration of blood flow (reperfusion) was observed directly using the ophthalmoscope. Body (rectal) temperature was maintained under free-regulating or normothermic (37 ± 0.5 °C) conditions with a rectal temperature probe (TR-100; Fine Science Tools, Foster City, CA) and a thermometric blanket before, during and after the surgery until the animals completely recovered from anesthesia. Thereafter, animals were kept in the thermal incubator (Mirae Medical Industry, Seoul, South Korea) to maintain the body temperature until the animals were euthanized. Sham-operated animals were subjected to the same surgical procedures except that the common carotid arteries were not occluded.
Tissue Processing for Histology
For histology, sham- and ischemia-operated young and adult gerbils (n = 7 at each time point) at designated times (1, 4, 7, 10, and 15 days after reperfusion) were euthanized. The animals were anesthetized with pentobarbital sodium and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4 % paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were removed and postfixed in the same fixative for 6 h. The brain tissues were cryoprotected by infiltration with 30 % sucrose overnight. Thereafter, frozen tissues were serially sectioned on a cryostat (Leica, Germany) into 30-μm coronal sections, and they were then collected into six-well plates containing PBS.
Hematoxylin-Eosin (H-E) Staining
To investigate the morphological changes and neuronal changes in the CA1 region after I/R, H-E staining was performed. In brief, the sections were mounted on gelatin-coated microscopy slides and stained with H-E according to the general method. After they were stained, they were dehydrated and mounted with Canada balsam (Kanto, Tokyo, Japan).
NeuN Immunohistochemistry
To investigate the neuronal changes in the CA1 region after I/R, NeuN was used in this study. The sections were sequentially treated with 0.3 % hydrogen peroxide (H2O2) in PBS for 30 min and 10 % normal goat serum in 0.05 M PBS for 30 min. The sections were next incubated with diluted mouse anti-NeuN (1:1000, Chemicon, Temecula, CA) overnight at 4 °C. Thereafter, the tissues were exposed to biotinylated goat anti-mouse IgG (Vector, Burlingame, CA) and streptavidin peroxidase complex (1:200, Vector); they were visualized by staining using 3,3′-diaminobenzidine tetrahydrochloride in 0.1 M Tris–HCl buffer (pH 7.2) and mounted on gelatin-coated slides. After dehydration, the sections were mounted with Canada balsam (Kanto, Tokyo, Japan).
Cell Counts
All measurements were performed to insure objectivity in blind conditions, by two observers for each experiment, carrying out the measures of control and experimental samples under the same conditions. The studied tissue sections were selected according to anatomical landmarks corresponding to AP from −1.4 to −1.8 mm of gerbil brain atlas (Loskota et al. 1974). The number of NeuN-immunoreactive neurons and H-E positive cells were counted in a 250 × 250-μm square, applied approximately at the center of the CA1 region in the stratum pyramidale. Cell counts were obtained by averaging the total cell numbers from each animal per group: A ratio of the count was calibrated as %.
Immunohistochemistry for GFAP, Iba-1, and Isolectin B4 (IB4)
In order to examine the change of astrocytes and microglia in the CA1 region after I/R, we carried out immunohistochemical staining with rabbit anti-glial fibrillary acidic protein (GFAP, 1:800, Chemicon, Temecular) for astrocyte, rabbit anti-ionized calcium-binding adapter molecule (Iba-1, 1:500, Wako, Japan) and anti-peroxidase-conjugated isolectin B4 (1:50, Sigma) for microglia, and biotinylated goat anti-rabbit IgG (Vector, Burlingame, CA) for secondary antibody according to the above mentioned-method (see the NeuN immunohistochemistry). In order to quantitatively analyze GFAP, Iba-1, and IB4 immunoreactivity, the corresponding areas of the hippocampal CA1 region were measured from 15 sections per animal. Images of all GFAP- and Iba-1-immunoreactive structures were taken from three layers (strata oriens, pyramidale, and radiatum in the hippocampus proper) through an AxioM1 light microscope (Carl Zeiss, Germany) equipped with a digital camera (Axiocam, Carl Zeiss) connected to a PC monitor. Images were calibrated into an array of 512 × 512 pixels corresponding to a tissue area of 140 × 140 μm (40 × primary magnification). The densities of all GFAP- and Iba-1-immunoreactive structures were evaluated on the basis of optical density (OD), which was obtained after the transformation of the mean gray level using the formula: OD = log (256/mean gray level). The OD of background was taken from areas adjacent to the measured area. After the background density was subtracted, a ratio of the optical density of image file was calibrated as % (relative optical density, ROD) using Adobe Photoshop version 8.0 and then analyzed using NIH Image 1.59 software.
Western Blot Analysis
In order to examine the protein levels of glia in the ischemic CA1 region, the animals (n = 7 in each group) were used for western blot analysis sham 4 and 7 days after the ischemic surgery in the young and adult group. After euthanizing them and removing the hippocampus, it was serially and transversely cut into a thickness of 400 μm on a vibratome (Leica, Germany), and the hippocampal CA1 region was then dissected using a surgical blade. The tissues were homogenized in 50 mM PBS (pH 7.4) containing EGTA (pH 8.0), 0.2 % NP-40, 10 mM EDTA (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM PMSF, and 1 mM DTT. After centrifugation, and the protein level in the supernatants was determined using a Micro BCA protein assay kit using bovine serum albumin as a standard (Pierce Chemical, USA). Aliquots containing 50 μg of total protein were boiled in loading buffer containing 250 mM Tris (pH 6.8), 10 mM DTT, 10 % SDS, 0.5 % bromophenol blue, and 50 % glycerol. The aliquots were then loaded onto a suitable polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY, USA). In order to incubate antibodies, the same nitrocellulose membrane strips were used. To reduce background staining, the membranes were incubated with 5 % non-fat dry milk in TBS containing 0.1 % Tween 20 for 45 min, followed by incubation with GFAP (diluted 1:400, Chemicon, Temecular) and Iba-1 (diluted 1:200, Wako) overnight at 4 °C and subsequently exposed to peroxidase-conjugated goat anti-mouse IgG (Santa Cruz, USA), goat anti-rabbit IgG (Santa Cruz, USA), and an ECL kit (Amersham, UK).
Statistical Analysis
Data are expressed as the mean ± SEM. The data were evaluated by an one-way ANOVA SPSS program, and the means were assessed by Duncan’s multiple-range test. Statistical significance was considered at P < 0.05.
Results
Neuronal Damage
H-E Staining
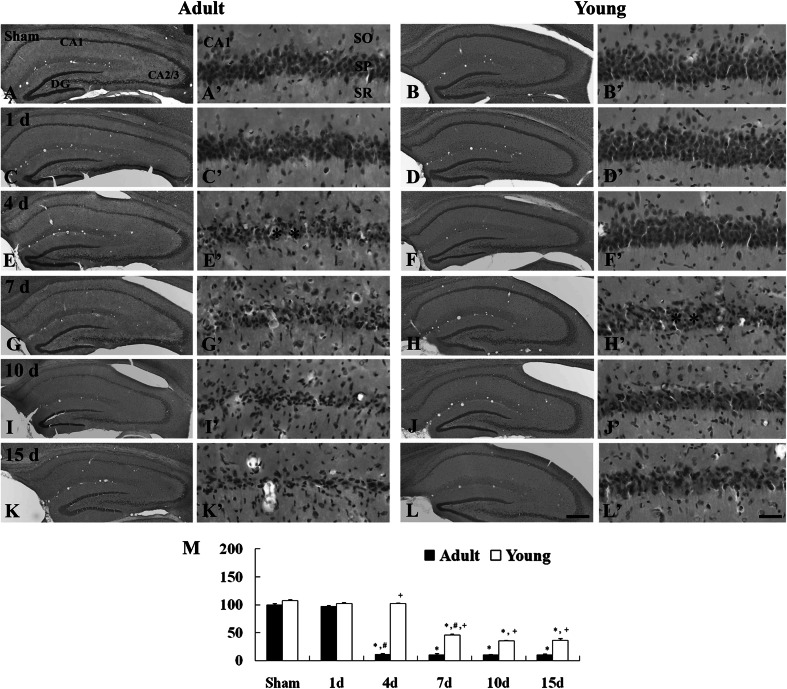
Morphological changes in the ischemic CA1 region of the adult and young and gerbils were examined by H-E staining. One day after I/R, cells in the CA1 region were similar to those in the adult-ischemia group (Fig. 1c, c′, m). However, 4 days after I/R, H-E positive cells were apparently damaged, and the mean percentage of H-E positive cells was significantly decreased compared to that of the adult-sham group (Fig. 1e, e′, m). Thereafter, the findings and the number of H-E positive cells were similar to those in the 4 days post-ischemia group until 15 days after I/R (Fig. 1g, g′, i, i′, k, k′, m).
Fig. 1.
H-E staining in the CA1 region of the adult (left two columns) and young (right two columns) of the sham- (a–b′) and ischemia- (c-l′) groups. In the adult group, significantly decreased H-E positive cells are shown in the stratum pyramidale (SP) (asterisks) 4 day after I/R. In the young group, many H-E positive cells are lost in the SP (asterisks) 7 days after I/R. SO stratum oriens, SR stratum radiatum. Scale bar = 200 μm (low magnification photos, b–l), 50 μm (high magnification photos, a′–l′). M Relative analysis as percent in the number of H-E positive cells in the CA1 region (n = 7 per group; * P < 0.05, significantly different from the corresponding sham group, # P < 0.05, significantly different from the respective preceding group; + P < 0.05, significantly different from the corresponding adult-group). The bars indicate the means ± SEM
In the young-ischemia groups, the morphology and the number of H-E positive cells was not changed 1 to 4 days after I/R (Fig. 1d, d′, f, f′, m). However, 7 days after I/R, H-E positive cells in the ischemic CA1 region were damaged and the number of the cells was significantly decreased; the number was much more than that of the corresponding adult-ischemia group (Fig. 1h, h′, m). Ten and 15 days after I/R, the number of H-E positive cells was similar to that at 7 days post-ischemia (Fig. 1j, j′, l, l′, m).
NeuN-Immunoreactive Cells
Numerous NeuN-immunoreactive cells were well distributed in the CA1 region of the adult- and young-sham groups (Fig. 2a, a′, b, b′). One day after I/R, no change in NeuN-immunoreactive neuronal numbers was found in the CA1 region of the adult-ischemia group (Fig. 2c, c′, m). At 4 days after I/R, a significant loss of NeuN-immunoreactive neurons was observed in the CA1 region in the adult group: The mean percentage of NeuN-immunoreactive neurons 12 % of that in the adult-sham group (Fig. 2e, e′, m). Thereafter, the number of NeuN-immunoreactive neurons was not changed in the adult-ischemia group (Fig. 2h, h′, j, j′, l, l′, m).
Fig. 2.
Immunohistochemistry for NeuN in the CA1 region of the adult (left 2 columns) and young (right 2 columns) of the sham- (a–b′) and ischemia- (c–l′) groups. In the adult group, a few NeuN immunoreactive cells are shown in the stratum pyramidale (SP) 4 days after I/R (asterisk), However, in the young group, many NeuN immunoreactive cells are lost in the SP 7 days after I/R (asterisks). SO stratum oriens, SR stratum radiatum. Scale bar = 200 μm (low magnification photos, a–l), 50 μm (high magnification photos, a′–l′). M Relative analysis as percent in the number of NeuN positive cells in the CA1 region (n = 7 per group; * P < 0.05, significantly different from the corresponding sham group, # P < 0.05, significantly different from the respective preceding group; + P < 0.05, significantly different from the corresponding adult-group). The bars indicate the means ± SEM
In the young-ischemia group at 1 and 4 days post-ischemia, NeuN-immunoreactive neurons in the CA1 region was similar to those in the young-sham group (Fig. 1d, d′, f, f′, m). However, at 7 days after I/R, the number of NeuN-immunoreactive neurons in the CA1 region was significantly decreased (mean percentage, 41 % of the young-sham group) (Fig. 2h, h′ m). Thereafter, in the young-ischemia groups, the number of NeuN-immunoreactive neurons was similar to that in the 7 days post-ischemia group (Fig. 2j, j′, l, l′, m).
Glial Activation
GFAP-Immunoreactive Astrocytes
In the adult- and young-sham group, GFAP-immunoreactive astrocytes, which showed a resting form (a small body with thread like thin process), were distributed throughout the CA1 region (Fig. 3a–b′).
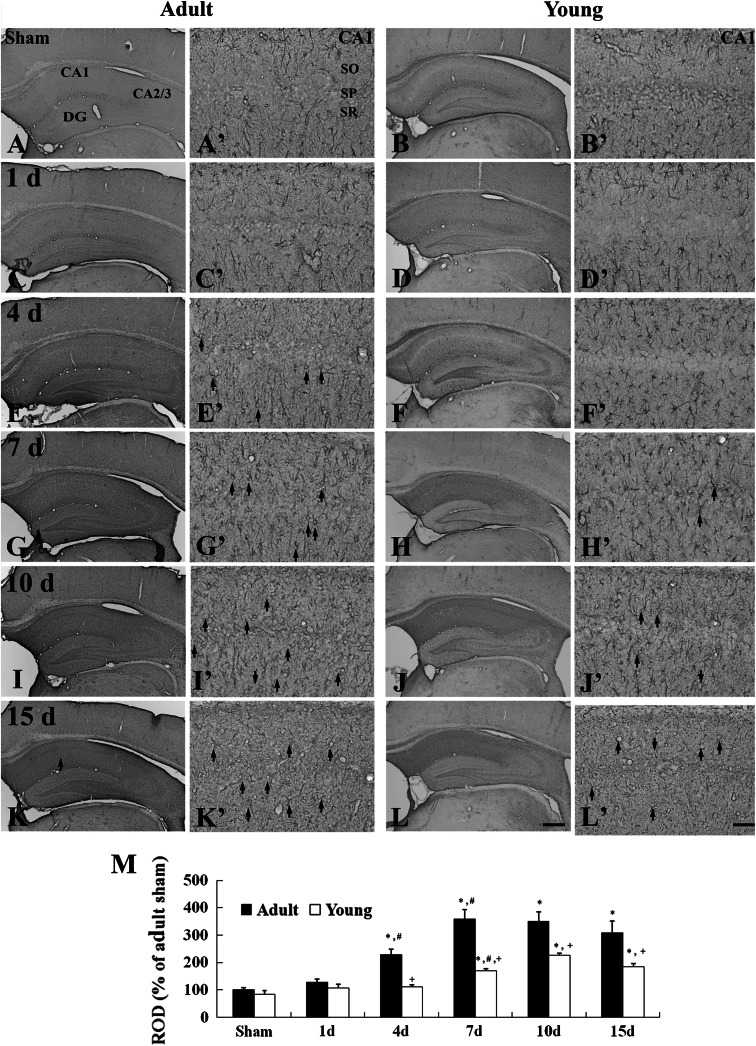
Fig. 3.
Immunohistochemistry for GFAP in the CA1 region of the adult (left 2 columns) and young (right 2 columns) of the sham- (a–b′) and ischemia- (c–l′) groups. Many reactive astrocytes (arrows) are detected from 4 and 7 days after I/R, respectively, in the adult- and young-group. SO stratum oriens, SP stratum pyramidale, SR stratum radiatum. Scale bar = 200 μm (low magnification photos, a–l), 50 μm (high magnification photos, a′–l′). M: Relative optical density as % of GFAP-immunoreactive structures in the adult- and young-group (n = 7 per group; * P < 0.05, significantly different from the young-sham group, # P < 0.05, significantly different from the respective preceding group; + P < 0.05, significantly different from the corresponding adult-group). The bars indicate the means ± SEM
In the adult-ischemia group, 1 day after I/R, the morphology of GFAP-immunoreactive astrocytes and their GFAP immunoreactivity were similar to those in the adult-sham group (Fig. 3c, c′, m). However, at 4 days post-ischemia, GFAP-immunoreactive astrocytes with reactive form were markedly increased, and their GFAP immunoreactivity was higher than that in the adult-sham group (Fig. 3e, e′, m). Thereafter, GFAP immunoreactivity in the CA1 region was still increased until 10 days after I/R (Fig. 3g, g′, i, i′, m). GFAP immunoreactivity, then, was slightly decreased 15 days after I/R (Fig. 3k, k′, m).
GFAP-immunoreactive astrocytes in the young-ischemia group were not markedly changed until 4 days after I/R (Fig. 3d, d′, f, f′). Seven days after I/R, many of the GFAP-immunoreactive astrocytes showed reactive form: A punctuated cytosol with thick processes was observed in the CA1 region, and their GFAP immunoreactivity was increased at this time point (Fig. 3h, h′, m). Thereafter, reactive GFAP-immunoreactive astrocytes were increased until 10 days after I/R, and GFAP immunoreactivity and reactive processes in astrocytes were decreased 15 days after I/R in the young-ischemia group (Fig. 3j, j′, l, l′, m).
Iba-1-Immunoreactive Microglia
In the adult- and young-sham groups, Iba-1-immunoreactive microglia cells were ubiquitously distributed in all the layers of the CA1 region, and they had fine processes with web-like network characteristics of ramified or resting microglia (Fig. 4a–b′).
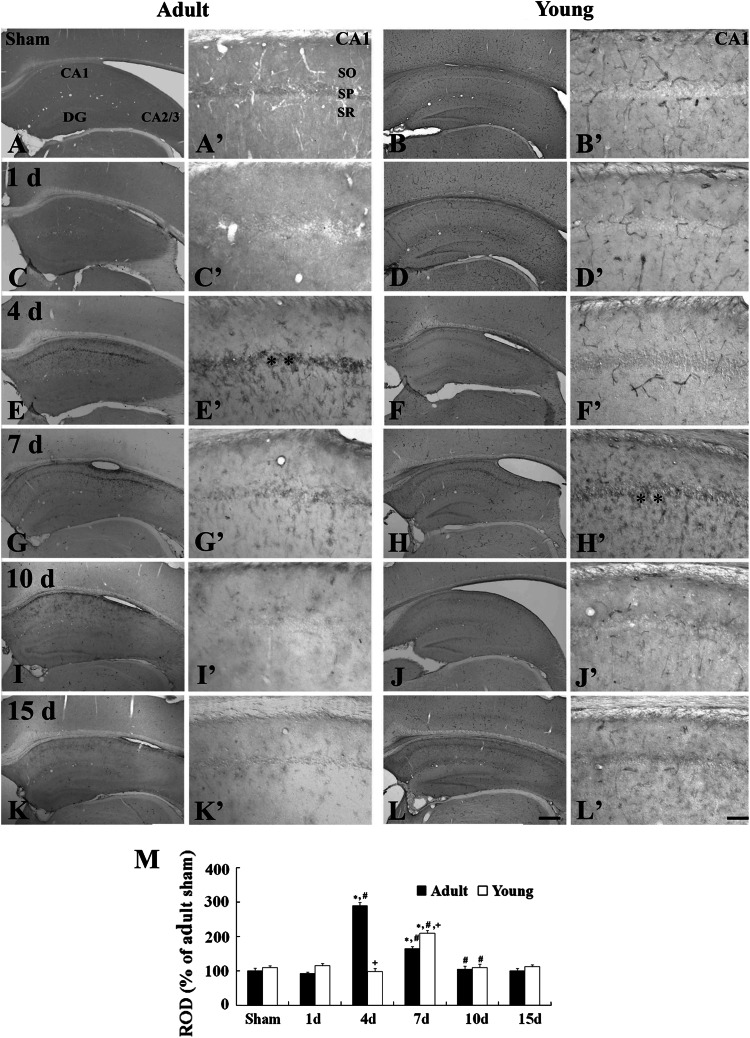
Fig. 4.
Immunohistochemistry for Iba-1 in the CA1 region of the adult (left 2 columns) and young (right 2 columns) of the sham- (a–b′) and ischemia- (c–l′) groups. Iba-1-immunoreactive microglia activation (arrows) is observed from 1 day after I/R in both the groups. The aggregation (asterisks) of Iba-1-immunoreactive microglia in the stratum pyramidale (SP) is observed 4 and 7 days after I/R, respectively, in the adult and young group. SO stratum oriens, SR stratum radiatum. Scale bar = 200 μm (low magnification photos, a–l), 50 μm (high magnification photos, a′–l′). M Relative optical density as % of Iba-1-immunoreactive structures in the adult- and young-groups (n = 7 per group; * P < 0.05, significantly different from the corresponding sham group, # P < 0.05, significantly different from the respective preceding group; + P < 0.05, significantly different from the corresponding adult-group). The bars indicate the means ± SEM
In the adult-ischemia group, morphological change in microglia was detected 1 day after I/R: Iba-1-immunoreactive microglia had an enlarged body with short and thicken processes, which was an activated form of microglia (Fig. 4c, c′); however, the immunoreactivity of Iba-1-immunoreactive structures was not markedly changed in this group (Fig. 4m). At 4 days post-ischemia, Iba-1-immunoreactive microglia were aggregated near the stratum pyramidale, and their Iba-1 immunoreactivity was significantly increased: This pattern continued until 10 days after I/R (Fig. 4e, e′, g, g′, i, i′, m). At 15 days post-ischemia, activated Iba-1 immunoreactive microglia in the stratum pyramidale were decreased and their Iba-1 immunoreactivity was slightly decreased (Fig. 4k, k′, m).
In the young-ischemia group, the morphological changes of Iba-1-immunoreactive microglia were also observed in the CA1 region from 1 day to 4 days after I/R; however, the immunoreactivity of Iba-1-immunoreactive structures was not markedly changed until 4 days post-ischemia compared to that in the young-sham group (Fig. 4f, f′, m). At 7 days post-ischemia, activated Iba-1-immunoreactive microglia were markedly aggregated near the stratum pyramidale, where neuronal degeneration occurred in the young-ischemia group, and their Iba-1 immunoreactivity was significantly increased (Fig. 4h, h′, m). Ten days after I/R, the immunoreactivity of Iba-1-immunoreactive microglia was more increased (Fig. 4j, j′, m). At 15 days post-ischemia, the number of activated Iba-1-immunoreactive microglia was reduced in the stratum pyramidale, and its immunoreactivity was slightly decreased compared to that in the 10 days young-ischemia group (Fig. 4l, l′, m).
IB4-Immunoreactive Microglia
In the adult- and young-sham groups, IB4-immunoreactive microglia was not found in any layers of the CA1 region (Fig. 5a–b′).
Fig. 5.
Immunohistochemistry for IB4 in the CA1 region of the adult (left 2 columns) and young (right 2 columns) of the sham- (a–b′) and ischemia- (c–l′) groups. IB4-immunoreactive microglia activation and aggregation (asterisks) in the stratum pyramidale (SP) are observed 4 and 7 days after I/R, respectively, in the adult and young group. SO stratum oriens, SR stratum radiatum. Scale bar = 200 μm (low magnification photos, a–l), 50 μm (high magnification photos, a′–l′). M Relative optical density as % of IB4-immunoreactive structures in the adult- and young-groups (n = 7 per group; * P < 0.05, significantly different from the corresponding sham group, # P < 0.05, significantly different from the respective preceding group; + P < 0.05, significantly different from the corresponding adult-group). The bars indicate the means ± SEM
In the adult-ischemia group, morphological change in microglia was detected 4 day after I/R: IB4-immunoreactive microglia were aggregated near the stratum pyramidale and their immunoreactivity was significantly increased (Fig. 5e, e′, m). However, IB4-immunoreactive microglia in the stratum pyramidale were significantly decreased from 7 days after I/R, and their immunoreactivity was dramatically decreased (Fig. 5g, g′, i, i′, k, k′, m).
In the young-ischemia group, IB4-immunoreactive microglia were hardly observed in the CA1 region 4 days after I/R (Fig. 5f, f′, m). Seven days after I/R, IB4-immunoreactive microglia were markedly increased and they were aggregated in the stratum pyramidale where neuronal degeneration occurred (Fig. 5h, h′, m). Thereafter, the immunoreactivity of IB4-immunoreactive microglia was dramatically decreased (Fig. 5j, j′, l, l′, m).
Levels of GFAP and Iba-1
In this study, we examined the levels of GFAP and Iba-1 proteins in the CA1 region after I/R (Fig. 6). The levels of GFAP and Iba-1 in the young-sham group were similar to those in the adult-sham group.
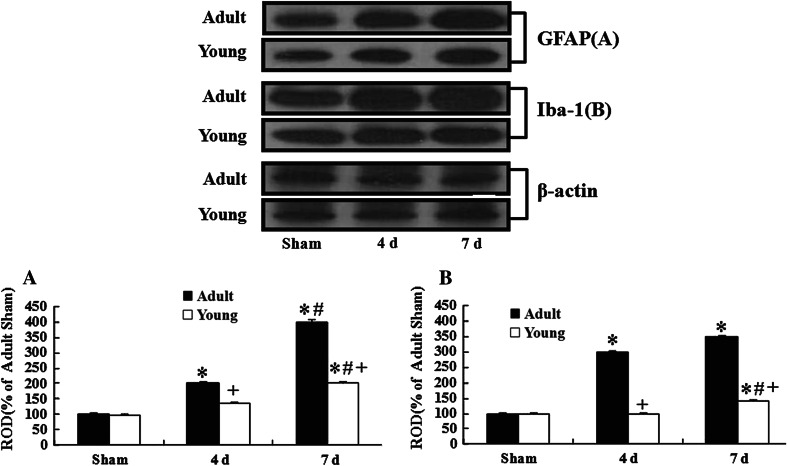
Fig. 6.
Western blot analysis of GFAP and Iba-1 in the CA1 of the young and adult groups after I/R. At 4 and 7 days post-ischemia, the levels of GFAP and Iba-1 are much lower in the young-ischemia group than those in the adult-ischemia group (n = 7 per group; * P < 0.05, significantly different from the corresponding sham-group, # P < 0.05, significantly different from the respective preceding group; + P < 0.05, significantly different from the corresponding adult-group). The bars indicate the means ± SEM
In the ischemia groups, the levels of all the proteins were changed with time. At 4 days post-ischemia, all the levels of the proteins in the adult-ischemia group were apparently increased in the ischemic CA1 region; however, in the young-ischemia group, their levels were not distinctively changed. Seven days after I/R, GFAP levels in both the groups were increased: The level in the adult-ischemia group was much higher than that in the young-ischemia group. At this time after I/R, Iba-1 levels in both the groups were slightly increased compared to those in the 4 days post-ischemia groups.
Discussion
In the present study, we compared neuronal damage in the CA1 region of the young gerbil with that in the adult gerbil after 5 min of I/R by H-E staining and NeuN immunohistochemistry. The delayed neuronal death of the CA1 pyramidal neurons was found in the adult gerbil 4 days after I/R: This finding is coincident with previous studies (Kirino 1982; Lee et al., 2011). Recently, we reported that the pattern of neuronal damage in the ischemic CA1 region of the young gerbil was very different from that in the adult gerbil at various times after I/R (Yan et al. 2011). In the present study, we also observed that the delayed neuronal death in the CA1 region of the young gerbil after I/R occurred much later than in the adult gerbil: Neuronal death was not observed in the CA1 region of the young group until 4 days after I/R; however, distinctive neuronal death was observed in many CA1 pyramidal neurons at 7 days post-ischemia. In addition, the number of the survived CA1 neurons in the young-ischemia group was much higher than that in the adult-ischemia group: About 35 % of CA1 pyramidal neurons remained in the CA 1 region until 15 days after I/R. This result indicates that young gerbils may be more resistant to ischemic insult compared with adult gerbils.
In the present study, we compared the changes of astrocytes and microglia in the ischemic CA1 region between the young and adult gerbils after I/R. The hallmark of astrocytes’ response to a brain injury is an increase in the level of expression of GFAP (Kato et al. 1994; Petito and Halaby 1993). It was reported that changes in GFAP immunoreactivity in the ischemic CA1 region was associated with neuronal death (Ordy et al. 1993; Petito and Halaby 1993; Steward et al. 1992; Stoll et al. 1998). In the present study, marked increases of GFAP immunoreactivity and reactive GFAP-immunoreactive astrocytes after I/R were observed in the young and adult groups. However, time points of the increases were different (7 and 4 days after I/R in the young- and adult-ischemia group, respectively). Some researchers reported that the down-regulation of astrocyte level, as a marker decreasing the cerebral ischemic inflammation, offered a neuroprotective mechanism (Montori et al. 2010b). In addition, our previous studies showed that decreased astrocyte activation, which was produced by some extracts from plants, could protect against neuronal damage induced by cerebral ischemia (Park et al. 2011; Yoo et al. 2011). Therefore, we can postulate that more delayed astrocyte activation in the young-ischemia group must be related to the time of neuronal death that occurred in the ischemic CA1 region of the young gerbil.
Morphological and functional changes in microglia are involved in the response to various neural environments (Hailer et al. 1996; Schwartz et al. 2006). It was reported that microglia activation was significantly increased in the infarct region following focal ischemia and in the CA1 region following global ischemia (Mabuchi et al. 2000; Stoll et al. 1998; Sugawara et al. 2002). Iba-1 and IB4 have been widely used as reliable markers for microglia (Gehrmann et al. 1992; Lee et al. 2010; Yu et al. 2010; Zemtsova et al. 2011). We previously reported that Iba-1 and IB4 microglia were apparently aggregated in the stratum pyramidale of the ischemic CA1 region and their immunoreactivity was significantly increased from 4 days post-ischemia (Lee et al. 2010; Yu et al. 2010). In the present study, many activated Iba-1 and IB4-immunoreactive microglia were aggregated in the stratum pyramidale of the CA1 region in both the young and adult groups. However, the peak time of microglia activation and their aggregation in the CA1 region was 7 days post-ischemia and 4 days post-ischemia, respectively, in the young- and adult-ischemia group. In addition, the number of activated microglia and their immunoreactivity in the young-ischemia group were much lower than those in the adult-ischemia group. It was reported that the inhibition of microglia activation was beneficial for the treatment of brain disorder such as Parkinson’s disease and cerebral ischemia (Chung et al. 2011; Lee et al. 2003). In addition, Yu et al. (2010) showed that decreased microglia activation contributed to neuronal survival. Therefore, our present results, including more delayed, lower microglia activation and aggregation, must be associated with more delayed and lower neuronal death in the young gerbil.
In conclusion, we found that the activation of astrocytes and microglia in the young gerbil was much lower and occurred much later than those in the adult gerbil. These results indicate that the young gerbil must be much more resistant to an ischemic insult than the adult gerbil.
Acknowledgments
The authors would like to thank Mr. Seung Uk Lee and Ms. Hyun Sook Kim for their technical help in this study. This work was supported by a Grant (2010K000823) from the Brain Research Center of the twentyfirst Century Frontier Research Program funded by the Ministry of Education, Science and Technology, the Republic of Korea, and by the Technology Innovation Program funded by the Ministry of Knowledge Economy (MKE, Korea).
Footnotes
Bing Chun Yan and Joon Ha Park contributed equally to this article.
Contributor Information
Hyung-Cheul Shin, Phone: +82-33-248-2580, Email: hcshin@hallym.ac.kr.
Moo-Ho Won, Phone: +82-33-250-8891, FAX: +82-33-256-1614, Email: mhwon@kangwon.ac.kr.
References
- Blanco S, Castro L, Hernandez R, Del Moral ML, Pedrosa JA, Martinez-Lara E, Siles E, Peinado MA (2007) Age modulates the nitric oxide system response in the ischemic cerebellum. Brain Res 1157:66–73 [DOI] [PubMed] [Google Scholar]
- Boje KM, Arora PK (1992) Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res 587(2):250–256 [DOI] [PubMed] [Google Scholar]
- Bonnekoh P, Barbier A, Oschlies U, Hossmann KA (1990) Selective vulnerability in the gerbil hippocampus: morphological changes after 5-min ischemia and long survival times. Acta Neuropathol 80(1):18–25 [DOI] [PubMed] [Google Scholar]
- Brahma MK, Dohare P, Varma S, Rath SK, Garg P, Biswal PK, Chowdhury PD, Ray M (2009) The neuronal apoptotic death in global cerebral ischemia in gerbil: important role for sodium channel modulator. J Neurosci Res 87(6):1400–1411 [DOI] [PubMed] [Google Scholar]
- Buiatti de Araujo FL, Bertolino G, Rodrigues Funayama CA, Coimbra NC, Eduardo de Araujo J (2008) Influence of treadmill training on motor performance and organization of exploratory behavior in Meriones unguiculatus with unilateral ischemic stroke: histological correlates in hippocampal CA1 region and the neostriatum. Neurosci Lett 431(2):179–183 [DOI] [PubMed] [Google Scholar]
- Canistro D, Affatato AA, Soleti A, Mollace V, Muscoli C, Sculco F, Sacco I, Visalli V, Bonamassa B, Martano M, Iannone M, Sapone A, Paolini M (2010) The novel radical scavenger IAC is effective in preventing and protecting against post-ischemic brain damage in Mongolian gerbils. J Neurol Sci 290(1–2):90–95 [DOI] [PubMed] [Google Scholar]
- Chen W, Ostrowski RP, Obenaus A, Zhang JH (2009) Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury. Exp Neurol 216(1):7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Bok E, Huh SH, Park JY, Yoon SH, Kim SR, Kim YS, Maeng S, Park SH, Jin BK (2011) Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation. J Immunol 187(12):6508–6517 [DOI] [PubMed] [Google Scholar]
- Crain BJ, Westerkam WD, Harrison AH, Nadler JV (1988) Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience 27(2):387–402 [DOI] [PubMed] [Google Scholar]
- Ganesan V, Hogan A, Shack N, Gordon A, Isaacs E, Kirkham FJ (2000) Outcome after ischaemic stroke in childhood. Dev Med Child Neurol 42(7):455–461 [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Bonnekoh P, Miyazawa T, Hossmann KA, Kreutzberg GW (1992) Immunocytochemical study of an early microglial activation in ischemia. J Cereb Blood Flow Metab 12(2):257–269 [DOI] [PubMed] [Google Scholar]
- Hailer NP, Jarhult JD, Nitsch R (1996) Resting microglial cells in vitro: analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia 18(4):319–331 [DOI] [PubMed] [Google Scholar]
- Himeda T, Tounai H, Hayakawa N, Araki T (2007) Postischemic alterations of BDNF, NGF, HSP 70 and ubiquitin immunoreactivity in the gerbil hippocampus: pharmacological approach. Cell Mol Neurobiol 27(2):229–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Palmer TD (2003) New roles for astrocytes: the nightlife of an “astrocyte”. La vida loca! Trends Neurosci 26(11):597–603 [DOI] [PubMed] [Google Scholar]
- Hu Z, Zeng L, Xie L, Lu W, Zhang J, Li T, Wang X (2007) Morphological alteration of Golgi apparatus and subcellular compartmentalization of TGF-beta1 in Golgi apparatus in gerbils following transient forebrain ischemia. Neurochem Res 32(11):1927–1931 [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Kim DW, Choi SY, Kang TC, Kim YS, Won MH (2006) Ionized calcium-binding adapter molecule 1 immunoreactive cells change in the gerbil hippocampal CA1 region after ischemia/reperfusion. Neurochem Res 31(7):957–965 [DOI] [PubMed] [Google Scholar]
- Kato H, Kogure K, Araki T, Itoyama Y (1994) Astroglial and microglial reactions in the gerbil hippocampus with induced ischemic tolerance. Brain Res 664(1–2):69–76 [DOI] [PubMed] [Google Scholar]
- Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239(1):57–69 [DOI] [PubMed] [Google Scholar]
- Kusumoto M, Arai H, Mori K, Sato K (1995) Resistance to cerebral ischemia in developing gerbils. J Cereb Blood Flow Metab 15(5):886–891 [DOI] [PubMed] [Google Scholar]
- Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ, Choi WS, Suk K (2003) Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J 17(13):1943–1944 [DOI] [PubMed] [Google Scholar]
- Lee CH, Yoo KY, Choi JH, Park OK, Hwang IK, Kim SK, Kang IJ, Kim YM, Won MH (2010) Neuronal damage is much delayed and microgliosis is more severe in the aged hippocampus induced by transient cerebral ischemia compared to the adult hippocampus. J Neurol Sci 294(1–2):1–6 [DOI] [PubMed] [Google Scholar]
- Lee CH, Park OK, Yoo KY, Byun K, Lee B, Choi JH, Hwang IK, Kim YM, Won MH (2011) The role of peroxisome proliferator-activated receptor gamma, and effects of its agonist, rosiglitazone, on transient cerebral ischemic damage. J Neurol Sci 300(1–2):120–129 [DOI] [PubMed] [Google Scholar]
- Lin CS, Polsky K, Nadler JV, Crain BJ (1990) Selective neocortical and thalamic cell death in the gerbil after transient ischemia. Neuroscience 35(2):289–299 [DOI] [PubMed] [Google Scholar]
- Lorrio S, Negredo P, Roda JM, Garcia AG, Lopez MG (2009) Effects of memantine and galantamine given separately or in association, on memory and hippocampal neuronal loss after transient global cerebral ischemia in gerbils. Brain Res 1254:128–137 [DOI] [PubMed] [Google Scholar]
- Loskota WJ, Lomax P, Verity MA (1974). A stereotaxic atlas of the Mongolian gerbil brain (Meriones unguiculatus). In: Loskota WJ, Lomax P, Anthony Verity M (eds). Ann Arbor Science, Ann Arbor
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M (2000) Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 31(7):1735–1743 [DOI] [PubMed] [Google Scholar]
- Montori S, Dos-Anjos S, Martinez-Villayandre B, Regueiro-Purrinos MM, Gonzalo-Orden JM, Ruano D, Fernandez-Lopez A (2010a) Age and meloxicam attenuate the ischemia/reperfusion-induced down-regulation in the NMDA receptor genes. Neurochem Int 56(8):878–885 [DOI] [PubMed] [Google Scholar]
- Montori S, Martinez-Villayandre B, Dos-Anjos S, Llorente IL, Burgin TC, Fernandez-Lopez A (2010b) Age-dependent modifications in the mRNA levels of the rat excitatory amino acid transporters (EAATs) at 48 hour reperfusion following global ischemia. Brain Res 1358:11–19 [DOI] [PubMed] [Google Scholar]
- Nagayama M, Aber T, Nagayama T, Ross ME, Iadecola C (1999) Age-dependent increase in ischemic brain injury in wild-type mice and in mice lacking the inducible nitric oxide synthase gene. J Cereb Blood Flow Metab 19(6):661–666 [DOI] [PubMed] [Google Scholar]
- Oguro K, Miyawaki T, Yokota H, Kato K, Kamiya T, Katayama Y, Fukaya M, Watanabe M, Shimazaki K (2004) Upregulation of GluR2 decreases intracellular Ca2+ following ischemia in developing gerbils. Neurosci Lett 364(2):101–105 [DOI] [PubMed] [Google Scholar]
- Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, Dunlap WP, Kates B (1993) Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol 119(1):128–139 [DOI] [PubMed] [Google Scholar]
- Park JH, Joo HS, Yoo KY, Shin BN, Kim IH, Lee CH, Choi JH, Byun K, Lee B, Lim SS, Kim MJ, Won MH (2011) Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem Res 36(11):2043–2050 [DOI] [PubMed] [Google Scholar]
- Petito CK, Halaby IA (1993) Relationship between ischemia and ischemic neuronal necrosis to astrocyte expression of glial fibrillary acidic protein. Int J Dev Neurosci 11(2):239–247 [DOI] [PubMed] [Google Scholar]
- Satoh K, Niwa M, Binh NH, Nakashima M, Kobayashi K, Takamatsu M, Hara A (2011) Increase of galectin-3 expression in microglia by hyperthermia in delayed neuronal death of hippocampal CA1 following transient forebrain ischemia. Neurosci Lett 504(3):199–203 [DOI] [PubMed] [Google Scholar]
- Saucier DM, Yager JY, Armstrong EA, Keller A, Shultz S (2007) Enriched environment and the effect of age on ischemic brain damage. Brain Res 1170:31–38 [DOI] [PubMed] [Google Scholar]
- Schaller BJ (2007) Influence of age on stroke and preconditioning-induced ischemic tolerance in the brain. Exp Neurol 205(1):9–19 [DOI] [PubMed] [Google Scholar]
- Schwartz M, Butovsky O, Bruck W, Hanisch UK (2006) Microglial phenotype: is the commitment reversible? Trends Neurosci 29(2):68–74 [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Takeda Y, Masui K, Taninishi H, Sasaki T, Danura T, Morita K (2011) Effect of fentanyl on ischemic depolarization and ischemic neuronal damage of hippocampal CA1 in the gerbil. J Anesth 25(4):540–548 [DOI] [PubMed] [Google Scholar]
- Steward O, Torre ER, Tomasulo R, Lothman E (1992) Seizures and the regulation of astroglial gene expression. Epilepsy Res Suppl 7:197–209 [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M (1998) Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol 56(2):149–171 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH (2002) Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma 19(1):85–98 [DOI] [PubMed] [Google Scholar]
- Szilagyi G, Simon L, Wappler E, Magyar K, Nagy Z (2009) (-)Deprenyl-N-oxide, a (-)deprenyl metabolite, is cytoprotective after hypoxic injury in PC12 cells, or after transient brain ischemia in gerbils. J Neurol Sci 283(1–2):182–186 [DOI] [PubMed] [Google Scholar]
- Tamagaki C, Murata A, Asai S, Takase K, Gonno K, Sakata T, Kinoshita T (2000) Age-related changes of cornu ammonis 1 pyramidal neurons in gerbil transient ischemia. Neuropathology 20(3):221–227 [DOI] [PubMed] [Google Scholar]
- Tortosa A, Ferrer I (1994) Poor correlation between delayed neuronal death induced by transient forebrain ischemia, and immunoreactivity for parvalbumin and calbindin D-28k in developing gerbil hippocampus. Acta Neuropathol 88(1):67–74 [DOI] [PubMed] [Google Scholar]
- Tripathi M, Vibha D (2010) Stroke in young in India. Stroke Res Treat 2011:368629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, Banwait S, Jin K (2006) Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res 1123(1):237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Nonoguchi N, Ikemoto T, Miyatake S, Kuroiwa T (2010) Asialoerythropoietin attenuates neuronal cell death in the hippocampal CA1 region after transient forebrain ischemia in a gerbil model. Neurol Res 32(9):957–962 [DOI] [PubMed] [Google Scholar]
- Yan BC, Park JH, Lee CH, Yoo KY, Choi JH, Lee YJ, Cho JH, Baek YY, Kim YM, Won MH (2011) Increases of antioxidants are related to more delayed neuronal death in the hippocampal CA1 region of the young gerbil induced by transient cerebral ischemia. Brain Res 1425:142–154 [DOI] [PubMed] [Google Scholar]
- Yoo KY, Lee CH, Li H, Park JH, Choi JH, Hwang IK, Kang IJ, Won MH (2011) Ethyl acetate extracts of raw and steamed Codonopsis lanceolata protects against ischemic damage potentially by maintaining SOD1 and BDNF levels. Int J Neurosci 121(9):503–509 [DOI] [PubMed] [Google Scholar]
- Yu JT, Lee CH, Yoo KY, Choi JH, Li H, Park OK, Yan B, Hwang IK, Kwon YG, Kim YM, Won MH (2010) Maintenance of anti-inflammatory cytokines and reduction of glial activation in the ischemic hippocampal CA1 region preconditioned with lipopolysaccharide. J Neurol Sci 296(1–2):69–78 [DOI] [PubMed] [Google Scholar]
- Zemtsova I, Gorg B, Keitel V, Bidmon HJ, Schror K, Haussinger D (2011) Microglia activation in hepatic encephalopathy. Hepatology 54(1):204–215 [DOI] [PubMed] [Google Scholar]
- Zhang YB, Kan MY, Yang ZH, Ding WL, Yi J, Chen HZ, Lu Y (2009) Neuroprotective effects of N-stearoyltyrosine on transient global cerebral ischemia in gerbils. Brain Res 1287:146–156 [DOI] [PubMed] [Google Scholar]