Abstract
Consisting of a fragment of ACTH(4–7) and C-terminal PGP tripeptide, the polypeptide Semax is successfully used for acute stroke therapy. Previous experiments showed rapid induction of Bdnf, Ngf, and TrkB expression in intact rat hippocampus following Semax treatment. To investigate the mRNA expression of neurotrophins and their receptors after treatment with either Semax or PGP, the rat brains were analyzed at three time points following a permanent middle cerebral artery occlusion (pMCAO). We have shown for the first time that both Semax and PGP activate the transcription of neurotrophins and their receptors in the cortex of rats subjected to pMCAO. The profiles of transcription alteration under PGP and Semax treatment were partially overlapped. Semax enhanced the transcription of Bdnf, TrkC, and TrkA 3 h after occlusion, Nt-3 and Ngf 24 h after occlusion, and Ngf 72 h after occlusion. PGP enhanced the transcription of Bdnf and TrkC 3 h after pMCAO and Ngf, TrkB, TrkC, and TrkA 24 h after pMCAO. The analysis of the transcription alterations under PGP and Semax treatment in the cortex of rats without surgery, sham-operated rats and rats subjected to pMCAO revealed that Semax selectively affected the transcription of neurotrophins and their receptors in the ischemic rat cortex, whereas the influence of PGP was mainly unspecific.
Keywords: Neurotrophin, Trk receptor, Semax, PGP, Cerebral ischemia
Introduction
It is well known that melanocortins, a family of neuropeptides derived from pro-opio(melano)cortin, have an influence on the functioning of the central nervous system (De Wied 1999). Peptide ACTH(4–10), a member of this family, has neurotrophic, behavioral, and cognitive effects and shows none of the hormonal activity of ACTH (De Wied 1977). As an analog of ACTH(4–10), synthetic heptapeptide Semax (Met-Glu-His-Phe-Pro-Gly-Pro) consists of a fragment of ACTH(4–7) and a C-terminal PGP (Pro-Gly-Pro) tripeptide that augments the peptide’s stability (Ashmarin et al. 1995). The effects of Semax on the functioning of the central nervous system have been assessed in numerous studies. It was well demonstrated that Semax stimulates learning and memory formation in rodents and humans (Kaplan et al. 1996). Semax has a marked nootropic effect (Ashmarin et al. 1997). Under the conditions of global ischemia in rats, Semax reduced neurological deficiency at the first 6.5 h after surgery; the survival rate for the experimental rats was raised (Yakovleva et al. 1999). Recently, it has been shown in vitro that Semax increases the survival of cerebellar granule cells under the conditions of glutamate neurotoxicity (Storozhevykh et al. 2007). Intranasal administration of Semax decreased the volume of photoinduced cortical infarction in rats (Romanova et al. 2006). Although Semax has been successfully used for some years for the clinical treatment of stroke, brain hypoxia, and brain trauma (Ashmarin et al. 1997; Gusev and Skvortsova 2002), the molecular mechanisms of Semax’s action are still not completely understood. It has been suggested that the neuroprotective effects of Semax are related to neurotrophin gene expression: the rapid induction of Bdnf and Ngf mRNAs in rat glial cell cultures has been observed after Semax treatment (Shadrina et al. 2001). Subsequently, it was demonstrated in vivo that Semax application increases both protein and mRNA levels of Bdnf, and TrkB, and mRNA level of Ngf in rat hippocampus (Dolotov et al. 2006; Agapova et al. 2007). At the same time, the studies of Semax degradation in rat serum and its entry to the rat brain (Potaman et al. 1991, 1993; Dolotov et al. 2004) and the studies of self-maintained effects of PGP and other proline-containing peptides (Lyapina et al. 2000; Samonina et al. 2001; Zhuikova et al. 2003; Storozhevykh et al. 2007) raise the question of whether C-terminal PGP tripeptide contributes to the effects of Semax. Recently we reported that both Semax and PGP affected the mRNA expression of some growth factors and their receptor genes under conditions of an experimental focal cerebral ischemia in rats (Dmitrieva et al. 2008b). In this study, we investigated the effect of Semax and PGP on expression of mRNAs encoding neurotrophins in rats subjected to a permanent middle cerebral artery occlusion (pMCAO). The Bdnf, Nt-3, Ngf, TrkB, TrkC, and TrkA mRNA expression levels were analyzed in the frontoparietal cortex from the rats without occlusion, sham-operated rats and rats with pMCAO 3, 24, and 72 h after treatment with saline, Semax, or PGP.
Materials and Methods
Animals
The animal experiments were performed in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publ. no. 80-23, revised 1996). Adult male Wistar rats (270–320 g) were maintained under natural light with free access to food and water. Animals were randomly divided into groups: controls without surgery treated with saline (n = 15), controls without surgery treated with Semax (n = 15), controls without surgery treated with PGP (n = 15), sham-operated controls treated with saline (n = 15), sham-operated controls treated with Semax (n = 15), sham-operated controls treated with PGP (n = 15), rats subjected to focal cerebral ischemia and treated with saline (n = 17), rats subjected to focal cerebral ischemia and treated with Semax (n = 15), rats subjected to focal cerebral ischemia and treated with PGP (n = 15). These groups were subdivided into groups treated for 3, 24, and 72 h, each of which included at least five animals.
Focal Cerebral Ischemia
Focal cerebral ischemia was induced as previously described (Dmitrieva et al. 2008a). The irreversible electrical coagulation of the distal segment of the left middle cerebral artery (pMCAO) was performed under anesthesia with chloral hydrate (300 mg/kg). Sham-operated animals underwent the same surgical procedure, except for the arterial occlusion. All animals (controls without surgery, sham-operated controls, and rats subjected to focal cerebral ischemia) were administered intraperitoneal injections of either saline, Semax (100 μg/kg), or PGP (37.5 μg/kg) at 15 min and 1 h; the group of 24 h received additional injections at four and 8 h; the group of 72 h received additional injections at 4, 8, 24, 28, 32, 48, 52, and 56 h. The rats were decapitated under anesthesia with ethyl ether three, 24, and 72 h after the operation, and the cortex tissues of both hemispheres were removed and frozen in liquid nitrogen.
RNA Extraction and cDNA Synthesis
Total RNA was isolated from tissue samples using guanidine thiocyanate (Chomczynski and Sacchi 1987). RNA integrity was assessed by analyzing the ratio between rRNA bands after agarose gel electrophoresis under denaturing conditions. RNA samples were stored at −70°C under ethanol. Residual genomic DNA was removed from the total RNA samples by treating with RNase-free DNase I (MBI Fermentas, Vilnius, Lithuania) in accordance with the supplier’s recommendations. RNA was then extracted with a 1:1 phenol/chloroform mix and precipitated with sodium acetate (3.0 M, pH 5.2). DNase I-treated total RNA samples (5 μg) were taken for cDNA synthesis with an oligo(dT)18 primer using a RevertAid First Strand cDNA Synthesis Kit (MBI Fermentas) in accordance with the manufacturer’s instructions.
RT-PCR and Real-Time PCR Quantitation
First-strand cDNA was used as a template for the polymerase chain reaction (PCR) with gene-specific primers. Gene-specific primers were designed using OLIGO Primer Analysis software (Wojciech and Piotr Rychlik copyright, version 6.31) (Table 1). Aliquots of the PCR products were size-fractionated on silver-stained 6% PAAG as previously described (Vladychenskaya et al. 2004). For real-time PCR quantitation, equal amounts of cDNA were added to a set of PCR with mastermix Buffer B SYBR Green I (Syntol, Russia) and primers for glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as the internal control and genes examined. Reactions were carried out on Mx3000P (Stratagene, USA). The following real-time PCR protocol was used for all genes: denaturation: 95°C for 10 min; amplification and quantification repeated 40 cycles: of 95°C for 30 s, 64°C for 30 s, 72°C for 30 s with a single fluorescence measurement; melting curve: 95°C for 1 min, 64°C for 30 s, 95°C for 30 s; cooling: down to 25°C. Melting temperature analysis was used to verify the appropriate PCR products. To determine crossing points (CP) the default settings of Mx3000P software (Stratagene, USA) were used.
Table 1.
Nucleotide sequence and location of the primers corresponding to rat genes analyzed
| Primer | Sequence | GenBank accession no. | Location, nucleotides |
|---|---|---|---|
| Bdnf (F) | 5′-AGGCACTGGAACTCGCAATG-3′ | NM_012513 | 859–879 |
| Bdnf (R) | 5′-TGTCTATCCTTATGAACCGCC-3′ | 937–957 | |
| TrkB (F) | 5′-CACCAACCATCACATTTCTC-3′ | NM_012731 | 1520–1540 |
| TrkB (R) | 5′-ATCTGTCTCTCGTCCTTCCC-3′ | 1765–1785 | |
| Nt-3 (F) | 5′-GGAAGATTATGTGGGCAACC-3′ | NM_031073 | 681–701 |
| Nt-3 (R) | 5′-GTGTCCCCGAATGTCAATGG-3′ | 818–838 | |
| TrkC (F) | 5′-CATTGAGTTTGTGGTGCGTG-3′ | NM_019248 | 1011–1031 |
| TrkC (R) | 5′-TAGTGGGTGGGCTTGTTGAA-3′ | 1144–1164 | |
| Ngf (F) | 5′-AGATAGCAATGTCCCAGAGG-3′ | XM_001067130 | 1266–1276 |
| Ngf (R) | 5′-ATTGGTTCAGCAGGGGCACT-3′ | 1357–1377 | |
| TrkA (F) | 5′-GTCTCCTTCCCAGCCAGTGT-3′ | NM_021589 | 911–932 |
| TrkA (R) | 5′-GGGTTGTCCATAAAGGCAGC-3′ | 1175–1196 | |
| Gapdh (F) | 5′-TGCCATCAACGACCCCTTCA-3′ | NM_017008 | 936–955 |
| Gapdh (R) | 5′-ACTCAGCACCAGCATCACCC-3′ | 1104–1123 |
The statistical significance of the differences in mRNA expression of the examined genes between groups was analyzed by a randomization test using the Relative Expression Software Tool (REST©) (Pfaffl et al. 2002). This software performs group-wise comparison and statistical analysis of gene expression normalized by a reference gene. The expression ratio is 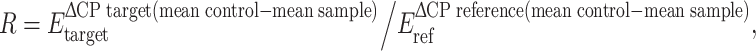 where E is the efficiency. Real-time PCR efficiencies were estimated by the amplification of a standardized dilution series of cDNA (three replicates) and calculated by REST©.
where E is the efficiency. Real-time PCR efficiencies were estimated by the amplification of a standardized dilution series of cDNA (three replicates) and calculated by REST©.
The results are expressed as a percentage of the relative expression level of control animals without surgery treated with saline (100%) and presented as mean ± SE. The statistically significant difference was accepted at P < 0.05.
Results
The transcription changes of neurotrophins and their receptors genes expression pattern were analyzed in the cortex of rats with occlusion where the damaged area was localized and in the same cortex areas of sham-operated rats and rats without surgery. A histological analysis showed the damaged area was localized in the ipsilateral frontoparietal cortex of the ischemic animals (Fig. 1; Dmitrieva et al. 2008a).
Fig. 1.
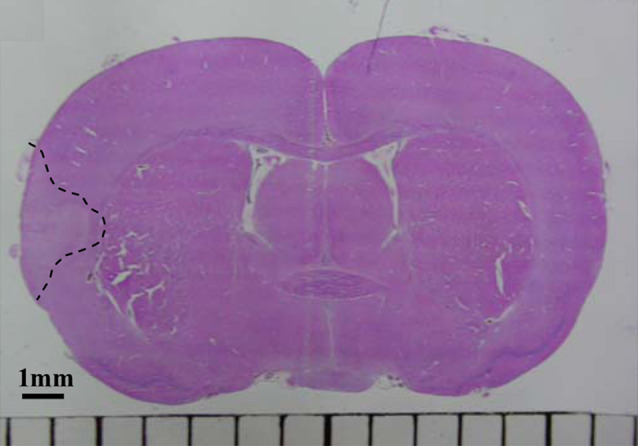
Photomicrograph of an H&E-stained section of a rat brain after 3 h pMCAO. A coronal rat brain section on the level −0.5 mm from bregma. The dashed line indicates the damaged area involving the entire cortical layer
Neurotrophin mRNA Expression in the Rat Brain Following pMCAO
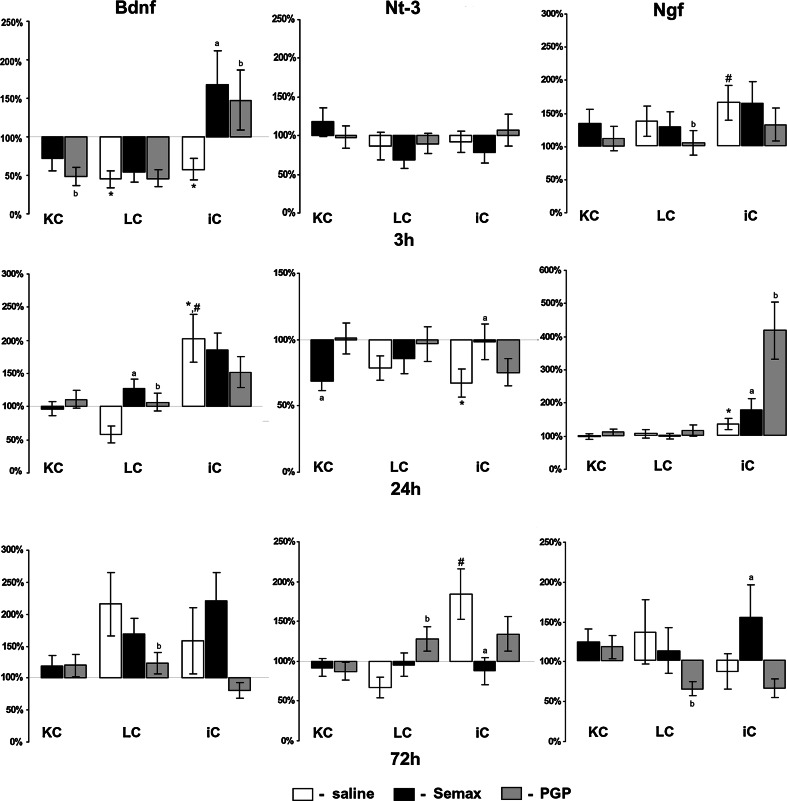
The expression of Bdnf mRNA in the cortex of ischemic rats and in the cortex of sham-operated controls was decreased 3 h after the operation compared with the controls without surgery (Fig. 2). At the same time, no changes in Nt-3 mRNA expression in the cortex of sham-operated controls and rats with occlusion were observed. The expression of Ngf mRNA in the cortex of ischemic rat brains was increased 3 h after occlusion compared with the sham-operated controls (Fig. 2).
Fig. 2.
Semax and PGP effects on expression of Bdnf, Ngf, and Nt-3 transcripts in rat brain. Analysis of neurotrophin mRNA expression in the cortex of rat brains 3, 24, and 72 h after pMCAO. KC—cortex of control without surgery, LC—cortex of sham-operated rats, iC—cortex of rats with occlusion. The expression was normalized by the reference gene Gapdh. The data are expressed as percentage values relative to the expression level in control animals without surgery treated with saline (100%) and presented as mean ± SE. The significant differences (P < 0.05) are marked with a comparison: * time-matched control rats without surgery treated with saline, # time-matched sham-operated control treated with saline, a proper group (control without surgery, sham, iC) after treatment with Semax, b proper group after treatment with PGP
Twenty-four hours after surgery, Bdnf mRNA expression in the cortex of sham-operated controls did not significantly differ from the level of the controls without surgery (Fig. 2). In ischemic rats, the expression of Bdnf mRNA was more than double that of the sham-operated controls and the controls without surgery. Twenty-four hours after occlusion, mRNA expression in the cortex of ischemic rats was decreased for Nt-3, but was increased for Ngf compared with the controls without surgery.
Seventy-two hours after occlusion, the changes of neurotrophin mRNA expression in ischemic rat brain were observed only for the Nt-3 gene: the Nt-3 transcription level was increased in iC compared with the sham-operated controls (Fig. 2). It should be noted that, in the sham-operated controls 72 h after surgery, the expression of Bdnf mRNA was increased compared with the controls without surgery, but the increase was not statistically significant.
Neurotrophin mRNA Expression in the Cortex of Rats Treated with Semax
Under Semax treatment, the changes in Bdnf and Ngf mRNA expression in the cortex of rats without surgery were not observed at the time points used (Fig. 2). A reduced level of Nt-3 mRNA expression in the cortex of rats treated with Semax without surgery was observed at 24 h. In the cortex of sham-operated controls, Semax treatment enhanced the expression of Bdnf mRNA 24 h after surgery.
Semax treatment enhanced the expression of Bdnf mRNA in the cortex of ischemic rats 3 h after occlusion (Fig. 2). The changes of Nt-3 mRNA expression were followed in an ischemic rat brain under Semax treatment and an increase 24 h after occlusion and a decrease 72 h after occlusion were observed. Semax treatment enhanced the expression of Ngf mRNA in the cortex of ischemic rats 24 and 72 h after occlusion.
Neurotrophin mRNA Expression in the Cortex of Rats Treated with PGP
A decreased level of Bdnf mRNA expression was observed in the cortex of rats without surgery after 3 h of PGP treatment compared with rats treated with saline (Fig. 2). Changes in Nt-3 and Ngf mRNA expression in the cortex of rats under PGP treatment without surgery were not observed at the chosen time points. In the cortex of sham-operated controls, PGP treatment enhanced the expression of Bdnf mRNA 24 h after surgery and reduced it 72 h after surgery. PGP treatment enhanced the expression of Nt-3 mRNA in the cortex of the sham-operated controls at 72 h. Under PGP treatment, the expression of Ngf mRNA in the cortex of sham-operated rats was reduced 3 and 72 h after surgery.
Three hours after occlusion, PGP treatment enhanced the expression of Bdnf mRNA in the cortex of ischemic rats (Fig. 2). PGP did not influence the Nt-3 mRNA expression in ischemic rat brains at the chosen time points. Twenty-four hours after occlusion, PGP treatment enhanced the expression of Ngf mRNA in the cortex of ischemic rats by more than fourfold.
Neurotrophin Receptor mRNA Expression in the Rat Brain Following pMCAO
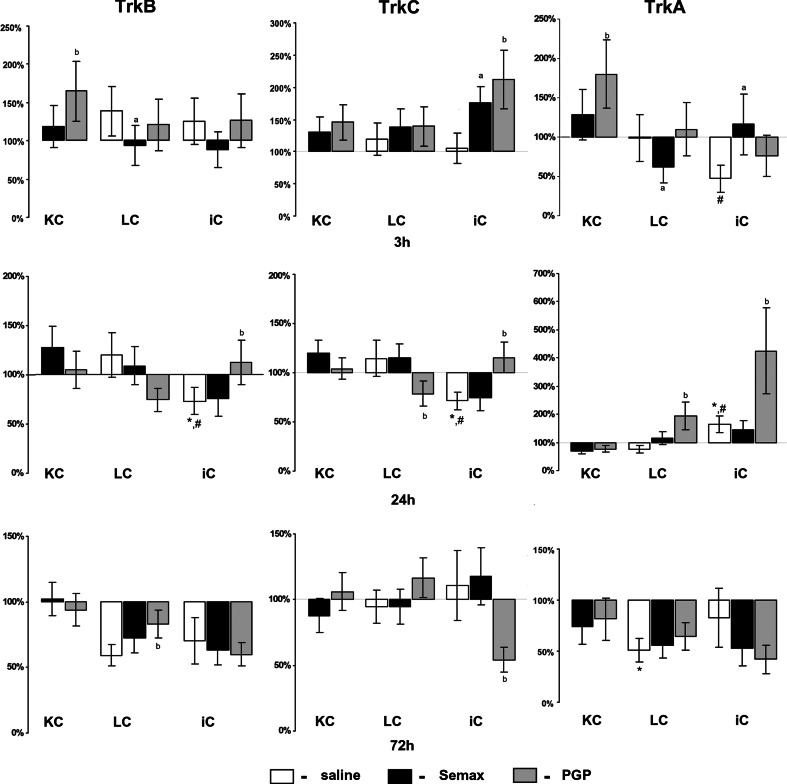
The expression of TrkA mRNA in the cortex of ischemic rats was decreased 3 h after occlusion compared with the sham-operated controls (Fig. 3). Twenty-four hours after occlusion, TrkA mRNA expression in the cortex of ischemic rat brains was increased compared with the sham-operated controls and controls without surgery. It should be noted that, in the sham-operated control rats 72 h after the operation, the expression of TrkA mRNA was significantly decreased compared with the controls without surgery. The expression of TrkB and TrkC mRNA in the cortex of ischemic rat brains was decreased 24 h after occlusion compared with the sham-operated controls and controls without surgery (Fig. 3).
Fig. 3.
Semax and PGP effects on the expression of TrkB, TrkC, and TrkA transcripts in a rat brain. An analysis of neurotrophin receptors mRNA expression in the cortex of rat brains 3, 24, and 72 h after pMCAO. KC—cortex of control without surgery, LC—cortex of sham-operated rats, iC—cortex of rats with occlusion. The expression was normalized by the reference gene Gapdh. The data are expressed as percentage values relative to the expression level in control animals without surgery treated with saline (100%) and presented as mean ± SE. The significant differences (P < 0.05) are marked with a comparison: * time-matched control rats without surgery treated with saline, # time-matched sham-operated control treated with saline, a proper group (control without surgery, sham, iC) after treatment with Semax, b proper group after treatment with PGP
Neurotrophin Receptor mRNA Expression in the Cortex of Rats Treated with Semax
An influence of Semax treatment on TrkB, TrkC, and TrkA gene expression in the cortex of rats without surgery was not observed (Fig. 3). Reduced expression of TrkB and TrkA mRNA in the cortex of sham-operated controls was observed under Semax treatment 3 h after surgery. Semax treatment increased the expression of TrkC and TrkA mRNA in iC 3 h after occlusion.
Neurotrophin Receptor mRNA Expression in the Cortex of Rats Treated with PGP
Under PGP treatment, an increased level of TrkB and TrkA gene expression in the cortex of rats without surgery was observed at 3 h (Fig. 3). Changes of TrkC mRNA expression in the cortex of rats without surgery under PGP treatment were not observed at the chosen time points. TrkB gene expression in the cortex of sham-operated rats was increased under PGP treatment 72 h after the operation. Twenty-four hours after surgery, PGP treatment reduced TrkC gene expression in the cortex of sham-operated rats but it enhanced the expression of the TrkA gene.
Under PGP treatment, an increase in TrkB mRNA expression in the cortex of ischemic rats was observed 24 h after occlusion (Fig. 3). PGP treatment enhanced the expression of TrkC mRNA in iC 3 h after occlusion. Twenty-four hours after occlusion, the level of TrkC mRNA in iC remained increased under PGP treatment, but 72 h after occlusion, it was decreased. The expression of TrkA mRNA in the cortex of ischemic rat brains was significantly increased under PGP treatment 24 h after occlusion. Here, in the iC of PGP-treated rats, the level of TrkA mRNA was more than fourfold greater than in rats treated with saline.
Discussion
Thus far, a growing amount of data supports the hypothesis that neurotrophins constitute a neuroprotective mechanism in brain ischemia (Kim et al. 2005; Wu 2005; Zhang et al. 2008). Neuroprotective functions of neurotrophins were confirmed in the models of focal cerebral ischemia in rats (Schabitz et al. 1997; Zhang and Pardridge 2001; Ferrer et al. 2001). Considering the influence of Semax on the expression of neurotrophins and their receptors revealed in cell cultures and in the hippocampi of intact rats, it has been assumed that the neuroprotective effect observed in Semax stroke therapy could also be related to the neurotrophic factors and their receptors.
It is well documented that the expression of neurotrophins and their receptors is affected by cerebral ischemia (Gusev and Skvortsova 2002). In our study, the transcription of neurotrophins was also altered in the ischemic cortex of rats subjected to pMCAO. With regard to the similar decrease in Bdnf transcription in the cortex of sham-operated rats registered 3 h after surgery, it could be supposed that this downregulation of Bdnf transcription in the cortical tissues is related to surgical stress, including narcosis. The upregulation of Bdnf transcription in the ischemic cortex, including the damaged area and peri-infarction zone, was shown in our study to correspond with other evidence (Kokaia et al. 1995; Ferrer et al. 2001; Pera et al. 2005; Kim et al. 2005). The transcription of Nt-3 in the ischemic cortex was altered in a manner similar to that observed for Bdnf but in a later period (decrease—at 24 h, increase—at 72 h). Unlike the decrease in Bdnf, the downregulation of Nt-3 transcription could not be related to surgical stress. This downregulation of Nt-3 transcription following cerebral ischemia was confirmed by other evidence (Lindvall et al. 1992). In our study, the transcription of Ngf was increased during the first day. Consistent with the data presented by Cui et al. (1999) and Lindvall et al. (1992), our results also show an early increase in Ngf transcription followed by its complete recovery 72 h after occlusion.
In our study, we did not observe any increase in TrkB transcription at the earliest time point (3 h) reported in the other studies (Majda et al. 2001; Kokaia et al. 1995). Moreover, the transcription of TrkB and TrkC was downregulated 24 h after surgery in the ischemic cortex of rats with occlusion. After transient focal cerebral ischemia in rats, Lee et al. (1998) observed the induction of TrkA expression in the ischemic cortex when Ngf expression was depressed. In our study, the Ngf and TrkA mRNA expression profiles had inverse features: the upregulation of Ngf transcription was accompanied by a decrease in TrkA transcription. Thus, we observed that under ischemic conditions the transcription upregulation of neurotrophins investigated was accompanied by the transcription downregulation of their high-affinity receptors registered at the same time point (Bdnf and TrkB, Ngf and TrkA) or earlier (Nt-3 and TrkC). Because neurotrophins regulate neuronal survival by interacting with their high-affinity receptors, the coexpression of neurotrophins and these Trk receptors is thought to be an underlying factor of neuroprotection. Indeed, the induction of neurotrophin receptors expression after an ischemic injury may play an important role in regulating the responsiveness of ischemic neurons to neurotrophins for survival. Thus, despite the neurotrophin transcription induction registered in the ischemic cortex, the cells localized in this area have a slim chance of survival because of the decreased neurotrophin receptor transcription.
Previously, Dolotov et al. (2006) did not reveal any influence of Semax on Bdnf transcription in the cortex of intact rats, and the decrease in Ngf transcription was registered by Agapova et al. (2007). Indeed, despite the different type of infusion and time points used in our study, similar features were observed as a result of the influence of Semax: in the cortex of rats without surgery Semax did not show any effect on the expression of Bdnf and Ngf and their receptors and inhibited the transcription of Nt-3. Similar to Semax, the PGP treatment of intact rats also caused the downregulation of neurotrophin transcription (Bdnf) in the cortex. At the same time, in contrast to Semax, PGP stimulated the transcription of neurotrophin receptors (TrkB, TrkA) in the cortex of the intact adult rat brain.
Taking into account that surgery, including narcosis, causes severe stress, we assessed the effects of Semax and PGP on mRNA expression of neurotrophins and their receptors in rat brain subjected to a sham operation. The comparison the effects of Semax and PGP in rats without surgery and in sham-operated rats led to the conclusion that, under the conditions of surgical stress, both peptides affected more genes and for a longer period. The only same effect of Semax and PGP was observed in the cortex of sham-operated rats: the upregulation of Bdnf mRNA expression at 24 h. Semax treatment in sham-operated rats inhibited the transcription of neurotrophin receptors (TrkB, TrkA) in the cortex after 3 h. The influence of PGP on the transcription of neurotrophins and their receptors in the cortex of rats subjected to surgical stress had modulating features and was mostly observed at 24 and 72 h after sham surgery.
Under Semax treatment the transcription of neurotrophins and their receptors was upregulated in the ischemic cortex of rats with occlusion: the levels of Bdnf, TrkA, and TrkC mRNA at 3 h, and the levels of Nt-3 and Ngf mRNA at 24 h. Later, at 72 h, Semax treatment promoted an increase in Ngf transcription in the ischemic cortex, but the level of Nt-3 was decreased. Considering the trends of gene transcription investigated in rats with occlusion treated with saline, it could be concluded that Semax treatment promoted the earlier upregulation of Bdnf, TrkC, and TrkA, and prevented the decrease in Nt-3 and Ngf transcription. Thus, Semax contributes to the survival of ischemic cells by enhancing the transcription not only of neurotrophins, but also of their high-affinity receptors. Here, it could be supposed that the success of urgent Semax therapy for stroke is mostly accounted for by the early upregulation of the transcription neurotrophins and their receptors.
Same as under the Semax treatment, the expression of Bdnf, TrkC and Ngf mRNAs was elevated in the ischemic cortex after 3 and 24 h of PGP treatment, respectively. However, PGP treatment for 24 h, in contrast to Semax, additionally enhanced the transcription of neurotrophin receptors genes (TrkB, TrkC, TrkA) in the ischemic cortex.
In conclusion, Semax and PGP showed an activating influence on the expression of the system of neurotrophins and their receptors that promotes cell neuroprotection and survival in neural tissue after cerebral ischemia. This activating influence was mostly observed 3 and 24 h after the ischemic attack when the cells in the penumbra still retained their functional activity and were able to survive (Lipton 1999; Lu et al. 2003). Semax and PGP effects on the expression of genes investigated only partly overlap and have special characteristics of selectivity and extensity. It seems that treatment with Semax leads to changes in the transcription of neurotrophins and their receptors specifically in the ischemic rat cortex, whereas the influence of PGP was mainly unspecific and more broadly affected gene activity in rats without surgery, sham-operated rats and rats subjected to pMCAO. It should be mentioned that Semax undergoes rapid enzymatic degradation resulting in a mixture of different derivative peptides (Shevchenko et al. 2006). PGP was predominant in the brain tissues 1 h after the Semax intranasal injection. From the similar effects of Semax and PGP observed in our study, it could be supposed that some Semax effects were mediated by the PGP component that appeared during Semax degradation. In addition, it has been previously reported that the Semax and PGP effects on the transcription of growth factors and their receptor genes also only partially overlapped (Dmitrieva et al. 2008b). Hence, Semax and PGP probably alter the transcription of neurotrophins and their receptors by their own mechanisms. The success of Semax stroke therapy observed in clinical reports could be related to the activating effects of Semax on the transcription of neurotrophins and their receptor genes as observed in the ischemic cortex of rats following middle cerebral artery occlusion. Our findings suggest that Semax probably has a specific target, which activates the mechanisms of neural maintenance and plasticity under ischemic conditions.
Acknowledgments
This study was supported by the Russian Foundation for Basic Research (project no. 08-04-01279), Molecular and Cell Biology program of the Russian Academy of Sciences, and the Federal Support of Leading Schools of the Russian Ministry of Science and Education.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- pMCAO
Permanent middle cerebral artery occlusion
- Bdnf
Brain-derived neurotropic factor
- Ngf
Nerve growth factor
- Gapdh
Glyceraldehyde-3-phosphate dehydrogenase
References
- Agapova TY, Agniullin YV, Shadrina MI, Shram SI, Slominsky PA, Lymborska SA, Myasoedov NF (2007) Neurotrophin gene expression in rat brain under the action of Semax, an analogue of ACTH 4–10. Neurosci Lett 417(2):201–205 [DOI] [PubMed] [Google Scholar]
- Ashmarin IP, Nezavibat’ko VN, Levitskaya NG, Koshelev VB, Kamensky AA (1995) Design and investigation of ACTH(4–10) analog deprived of D-aminoacids and hydrophobic radicals. Neurosci Res Commun 16:105–112 [Google Scholar]
- Ashmarin IP, Nezavibat’ko VN, Miasoedov NF, Kamenskii AA, Grivennikov IA, Ponomareva-Stepnaia MA, Andreeva LA, Kaplan AYa, Koshelev VB, Riasina TV (1997) A nootropic adrenocorticotropin analog 4–10-semax: 15 years experience in its design and study. Zh Vyssh Nerv Deiat Im I P Pavlova 47(2):420–430 [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- Cui JK, Hsu CY, Liu PK (1999) Suppression of postischemic hippocampal nerve growth factor expression by a c-fos antisense oligodeoxynucleotide. J Neurosci 19(4):1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wied D (1977) Behavioral effects of pituitary peptides. Acta Physiol Pol 28(15):77–91 [PubMed] [Google Scholar]
- De Wied D (1999) Behavioral pharmacology of neuropeptides related to melanocortins and the neurohypophyseal hormones. Eur J Pharmacol 375(1–3):1–11 [DOI] [PubMed] [Google Scholar]
- Dmitrieva VG, Torshina EV, Yuzhakov VV, Povarova OV, Skvortsova VI, Limborska SA, Dergunova LV (2008a) Expression of sphingomyelin synthase 1 gene in rat brain focal ischemia. Brain Res 1188:222–227 [DOI] [PubMed] [Google Scholar]
- Dmitrieva VG, Dergunova LV, Povarova OV, Skvortsova VI, Limborskaya SA, Myasoedov NF (2008b) The effect of Semax and the C-terminal peptide PGP on expression of growth factor genes and receptors in rats under conditions of experimental cerebral ischemia. Dokl Akad Nauk 422(2):402–405 [DOI] [PubMed] [Google Scholar]
- Dolotov OV, Zolotarev IuA, Dorokhova EM, Andreeva LA, Alfeeva LIu, Grivennikov IA, Miasoedov NF (2004) The binding of Semax, ACTH 4–10 heptapeptide, to plasma membranes of the rat for a brain basal nuclei and biodegradation. Biorg Khim 30(3):241–246 [DOI] [PubMed] [Google Scholar]
- Dolotov OV, Karpenko EA, Inozemtseva LS, Seredenina TS, Levitskaya NG, Rozyczka J, Dubynina EV, Novosadova EV, Andreeva LA, Alfeeva LY, Kamensky AA, Grivennikov IA, Myasoedov NF, Engele J (2006) Semax, an analog of ACTH(4–10) with cognitive effects, regulates BDNF and trkB expression in the rat hippocampus. Brain Res 1117(1):54–60 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Krupinski J, Goutan E, Marty E, Ambrosio S, Arenas E (2001) Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol 101:229–238 [DOI] [PubMed] [Google Scholar]
- Gusev EI, Skvortsova VI (2002). Neuroprotectors in complex therapy of ischemic insult. Ther Neurol Dis 3(8)
- Kaplan AYa, Kochetova AG, Nezavibat`ko VN, Rjasina TV, Ashmarin IP (1996) Synthetic ACTH analogue semax displays nootropic-like activity in humans. Neurosci Res Commun 19:115–123 [Google Scholar]
- Kim M-W, Bang M-S, Han T-R, Ko Y-J, Yoon B-W (2005) Exercise increased bdnf and trkB in contralateral hemisphere of the ischemic rat brain. Brain Res 1052:16–21 [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Zhao Q, Kokaia M, Elmer E, Metsis M, Smith ML, Siesjo BK, Lindvall O (1995) Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol 136:73–88 [DOI] [PubMed] [Google Scholar]
- Lee TH, Kato H, Chen ST, Kogure K, Itoyama Y (1998) Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke 29(8):1687–1696 [DOI] [PubMed] [Google Scholar]
- Lindvall O, Ernfors P, Bengzon J, Kokaia Z, Smith ML, Siesjo BK, Persson H (1992) Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc Natl Acad Sci USA 89(2):648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79(4):1431–1568 [DOI] [PubMed] [Google Scholar]
- Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR (2003) Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab 23(7):786–810 [DOI] [PubMed] [Google Scholar]
- Lyapina LA, Pastorova VE, Samonina GE, Ashmarin IP (2000) The effect of prolyl-glycyl-proline (PGP) peptide and PGP-rich substances on haemostatic parameters of rat blood. Blood Coagul Fibrinolysis 11(5):409–414 [DOI] [PubMed] [Google Scholar]
- Majda BT, Meloni BP, Rixon N, Knuckey NW (2001) Suppression subtraction hybridization and northern analysis reveal upregulation of heat shock, trkB, and sodium calcium exchanger genes following global cerebral ischemia in the rat. Brain Res Mol Brain Res 93(2):173–179 [DOI] [PubMed] [Google Scholar]
- Pera J, Malgorzata Z, Kaminska B, Szczudlik A (2005) Neurotrophic factor expression after focal brain ischemia preceded by different preconditioning strategies. Cerebrovasc Dis 19:247–252 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potaman VN, Antonova LV, Dubynin VA, Zaitsev DA, Kamensky AA, Myasoedov NF, Nezavibatko VN (1991) Entry of the synthetic ACTH(4–10) analogue into the rat brain following intravenous injection. Neurosci Lett 127(1):133–136 [DOI] [PubMed] [Google Scholar]
- Potaman VN, Alfeeva LY, Kamensky AA, Nezavibatko VN (1993) Degradation of ACTH/MSH(4–10) and its synthetic analog semax by rat serum enzymes: an inhibitor study. Peptides 14(3):491–495 [DOI] [PubMed] [Google Scholar]
- Romanova GA, Silachev DN, Shakova FM, Kvashennikova YN, Viktorov IV, Shram SI, Myasoedov NF (2006) Neuroprotective and antiamnesic effects of Semax during experimental ischemic infarction of the cerebral cortex. Bull Exp Biol Med 142(6):663–666 [DOI] [PubMed] [Google Scholar]
- Samonina GE, Kopylova GN, Sergeev VI, Zhuikova SE, Bakaeva ZV (2001) Correction of the stomach blood flow as a mechanism of anti-ulcer effects of short proline-containing peptides. Ross Fiziol Zh Im I M Sechenova 87(11):1488–1492 [PubMed] [Google Scholar]
- Schabitz WR, Schwab S, Spranger M, Hacke W (1997) Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 17(5):500–506 [DOI] [PubMed] [Google Scholar]
- Shadrina MI, Dolotov OV, Grivennikov IA, Slominsky PA, Andreeva LA, Inozemtseva LS, Limborska SA, Myasoedov NF (2001) Rapid induction of neurotrophin mRNAs in rat glial cell cultures by Semax, an adrenocorticotropic hormone analog. Neurosci Lett 308(2):115–118 [DOI] [PubMed] [Google Scholar]
- Shevchenko KV, Nagaev IIu, Alfeeva LIu, Andreeva LA, Kamenskiĭ AA, Levitskaia NG, Shevchenko VP, Grivennikov IA, Miasoedov NF (2006) Kinetics of Semax penetration into the brain and blood of rats after its intranasal administration. Bioorg Khim 32(1):64–70 [DOI] [PubMed] [Google Scholar]
- Storozhevykh TP, Tukhbatova GR, Senilova YE, Pinelis VG, Andreeva LA, Myasoyedov NF (2007) Effects of semax and its Pro-Gly-Pro fragment on calcium homeostasis of neurons and their survival under conditions of glutamate toxicity. Bull Exp Biol Med 143(5):601–604 [DOI] [PubMed] [Google Scholar]
- Vladychenskaya IP, Dergunova LV, Dmitrieva VG, Limborska SA (2004) Human gene MOB: structure specification and aspects of transcriptional activity. Gene 338(2):257–265 [DOI] [PubMed] [Google Scholar]
- Wu D (2005) Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx 2(1):120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva EV, Kuzenkov VS, Fedorov VN, Skvortsova VI, Koshelev VB, Gusev EI, Ashmarin IP (1999) Study of the efficacy of semax in global cerebral ischemia in vivo. Biull Eksp Biol Med 127(8):172–174 [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM (2001) Neuroprotection in transient focal brain ischemia after delayed intravenous administration of brain-derived neurotrophic factor conjugated to a blood-brain barrier drug targeting system. Stroke 32(6):1378–1384 [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Wang RZ, Wang RZ, Li GL, Wei JJ, Li ZJ, Feng M, Kang J, Du WC, Ma WB, Li YN, Yang Y, Kong YG (2008) Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci Lett 444(3):227–230 [DOI] [PubMed] [Google Scholar]
- Zhuikova SE, Badmaeva KE, Samonina GE, Plesskaia LG (2003) Semax and some glyproline peptides accelerate the healing of acetic ulcers in rats. Eksp Klin Gastroenterol 4:88–92 [PubMed] [Google Scholar]