Abstract
Methyl mercury (MeHg) is a ubiquitous environmental pollutant leading to neurological and developmental deficits in animals and human beings. Bacopa monniera (BM) is a perennial herb and is used as a nerve tonic in Ayurveda, a traditional medicine system in India. The objective of the present study was to investigate whether Bacopa monniera extract (BME) could potentially inhibit MeHg-induced toxicity in the cerebellum of rat brain. Male Wistar rats were administered with MeHg orally at a dose of 5 mg/kg b.w. for 21 days. Experimental rats were given MeHg and also administered with BME (40 mg/kg, orally) for 21 days. After the treatment period, we observed that MeHg exposure significantly inhibited the activities of superoxide dismutase, catalase, glutathione peroxidase, and increased the glutathione reductase activity in cerebellum. It was also found that the level of thiobarbituric acid-reactive substances was increased with the concomitant decrease in the glutathione level in MeHg-induced rats. These alterations were prevented by the administration of BME. Behavioral interference in the MeHg-exposed animals was evident through a marked deficit in the motor performance in the rotarod task, which was completely recovered to control the levels by BME administration. The total mercury content in the cerebellum of MeHg-induced rats was also increased which was measured by atomic absorption spectrometry. The levels of NO2 − and NO3 − in the serum were found to be significantly increased in the MeHg-induced rats, whereas treatment with BME significantly decreased their levels in serum to near normal when compared to MeHg-induced rats. These findings strongly implicate that BM has potential to protect brain from oxidative damage resulting from MeHg-induced neurotoxicity in rat.
Keywords: Methyl mercury, Bacopamonniera, Cerebellum, Neuroprotection
Introduction
Methyl mercury (MeHg) is a highly neurotoxic compound leading to neurological and developmental deficits in animals and human beings (Clarkson et al. 2003). Even though MeHg-induced neurotoxicity is a widely reported phenomenon, the molecular mechanisms related to its toxicity are not completely understood. The most important mechanisms involved in MeHg neurotoxicity currently being explored are the impairment of intracellular calcium homeostasis (Sirois and Atchison 2000), oxidative stress, and the alteration of glutamate homeostasis (Ou et al. 1999; Aschner et al. 2000; Farina et al. 2003; Manfroi et al. 2004). Of particular importance, MeHg has been reported to increase the extracellular levels of glutamate (Juarez et al. 2002), and glutamate receptor antagonists have been reported to prevent MeHg-induced damage in the central nervous system of rodents (Juarez et al. 2005). It is noteworthy that MeHg-induced oxidative stress and MeHg-induced glutamate dyshomeostasis appear to be connected phenomena affecting each other (Aschner et al. 2007). Despite massive efforts in the search for new drugs that counteract mercurial toxicity, there are no effective treatments available that completely abolish its toxic effects. In MeHg poisoning, supportive care is given when necessary to maintain vital functions. In addition, the use of chelating agents assists the body’s ability to eliminate mercury from the tissues. However, these drugs are of limited use because of their adverse side effects (Tchounwou et al. 2003).
Methyl mercury (MeHg) intoxication is noted for Hunter–Russell syndrome (Minamata disease), the clinical manifestations of which are cerebellar ataxia, concentric constriction of visual fields, and sensory and auditory disturbances (Hunter et al. 1940; Hunter and Russell 1954). Among these disturbances, cerebellar ataxia is one of the most important clinical signs for diagnosis of this disease, and cerebellar degeneration is one of the most outstanding histopathological findings upon autopsy (Takeuchi 1968, 1982). The disease occurs worldwide because of industrial pollution, and many animal models of the disease have been developed (Nagashima 1997). However, the mechanism of cerebellar degeneration during MeHg intoxication is not well known, and an effective therapy for MeHg intoxication has not been established. Growing evidence suggests the involvement of oxidative stress in the brain and the cerebellum in neurodegenerative diseases such as Alzheimer disease and spinocerebellar degeneration (Rosen 1993; Yamashita et al. 2000). The cerebellum is a nitric oxide synthase-rich organ in which nitric oxide (NO) is produced. NO reacts with superoxide to form peroxynitrite and hydroxyl radicals, which are highly reactive free radical species inducing oxidative stress (Beckman et al. 1990). It has been suggested that NO production in the cerebellum may be strongly correlated with cerebellar degeneration during MeHg intoxication (Ikeda et al. 1999; Shinyashiki et al. 1998). Recently, a major target molecule of MeHg toxicity was reported in yeast cells (Miura et al. 1999). However, in mammalian cells, the target molecules causing MeHg toxicity have not yet been identified.
Recently, many studies have focused their effects on the protective effects of plants on diverse neuropathological conditions. In this regard, the plant Bacopa monniera (BM) has been shown to possess protective effects on several aspects of learning and mental function (Singh and Dhawan 1982, 1997; Vollala et al. 2010). Several clinical studies have confirmed the beneficial actions of BM (Russo and Borrelli 2005). Although studies have documented the various pharmacological activities of BM, very little is known about its interaction with MeHg-induced neurotoxicity. To gain more insight into the interaction of BM with MeHg toxicity, the oxidative stress, behavioral interference, and the level of NO2 − and NO3 − were studied. Taking into account of the absence of effective treatments for MeHg toxicity, the aim of this study was to determine the possible in vivo protective effects of BM against MeHg-induced neurotoxicity in rats.
Bacopa monnieri (Brahmi, Family: Scrophulariaceae), a traditional Ayurvedic medicinal plant, is extensively used for centuries for treatment of epilepsy, insomnia, and anxiety and also as a mild sedative and memory enhancer (Tripathi et al. 1996; Kishore and Singh 2005; Ernst 2006). Besides, BM displays antioxidant, antistress, and anxiolytic properties in experimental animals (Shanker and Singh 2000; Chowdhuri et al. 2002). Further, it improves the performance of rats in various learning situations such as shock-motivated brightness discrimination reaction, an active conditioned flight reaction, continuous avoidance response (Singh and Dhawan 1982), and attenuates experimentally induced amnesia in experimental animals (Kishore and Singh 2005; Saraf et al. 2008). Several clinical studies have confirmed the beneficial actions of BM (Russo and Borrelli 2005), and the pharmacological actions are mainly attributed to the saponin compounds present in the alcoholic extract of the plant. The major chemical constituents isolated and characterized from Bacopa are dammarane-type of tri terpenoid saponins. Several pharmacological (Singh et al. 1988; Singh and Dhawan 1997) and clinical studies (Nathan et al. 2001; Stough et al. 2001) on the extracts of BM standardized to the bacosides A and B have been reported. Bacoside A is shown to alleviate the amnesic effects of scopolamine (Russo and Borrelli 2005) and provide protection against phenytoin-induced deficit in cognitive function in mice (Vohora et al. 2000). Earlier studies have reported that BM revitalizes the intellectual functions among children (Sharma et al. 1987). Recently, preclinical studies have demonstrated cognitive enhancing effects with various BM extracts, although the precise mechanism(s) of its action is not clear (Stough et al. 2001; Roodenrys et al. 2002; Russo and Borrelli 2005). The neuroprotective and cognitive enhancing effects of BM extracts are explained to be due to several mechanisms such as chelation of metal ions (Tripathi et al. 1996), scavenging of free radicals (Russo et al. 2003), and enhanced antioxidative defense enzymes (Bhattacharya et al. 1999, 2000; Russo et al. 2003). Further, the antistress activity of BM in experimental animals is attributed to its propensity to modulate Hsp70 expression, cytochrome P450 levels, activity of SOD (Chowdhuri et al. 2002), enhanced kinase activity, neuronal synthesis coupled with restoration of synaptic activity, and nerve impulse transmission (Kishore and Singh 2005). Other biological effects of BM reported in animal model include hepatoprotection against morphine (Sumathy et al. 2001) and anti-ulcerogenic activity (Sairam et al. 2001).
Protective role of many compounds like melatonin (Kim et al. 2000), α-tocopherol (Yamashita et al. 2004), and docosahexaenoic acid (Parvinder et al. 2007) have been reported for MeHg-induced neurotoxicity. Plant extracts like Cipura paludosa (Greice et al. 2007) and Polygala paniculata (Farina et al. 2005) have also been reported for MeHg neurotoxicity. However, to the best of our knowledge, there are no data available in the scientific literature regarding the protective role of BM against MeHg-induced oxidative stress. The aim of this study was to determine for the first time the protective effects of BM against MeHg-induced oxidative stress in rats.
Materials and Methods
Chemicals
Methyl mercuric chloride was purchased from Sigma Chemicals. Glutathione reductase (GR), glutathione (GSH)-reduced form, glutathione oxidized form (GSSG), tert-butyl hydroperoxide, 5,5′-dithiobis-(2-nitrobenzoic acid (DTNB) were purchased from SRL. β-Nicotinamide adenine dinucleotide phosphate reduced (NADPH) was purchased from CDH. All the other chemicals used were of the analytical grade.
Preparation of BME
The plant material was collected at Chennai, Tamil Nadu, India, and was authenticated by Dr. A. Sasikala, Captain Srinivasa Murti Drug Research Institute for Ayurveda, Arumbakkam, Chennai, Tamil Nadu, India. The plant was shade dried, and coarsely powdered plant material (1 kg) was extracted with 90% ethanol in the cold (48 h). The extract was filtered and distilled on a cold water bath to obtain a dark green syrupy mass. It was finally dried in vacuo.
Dose Selection
A pilot study was conducted to establish the optimal dose of BME by evaluating behavioral and biochemical parameters. Rats were randomly divided into four groups of six animals each as follows: (1) control (double distilled water); (2) experimental group I (20 mg/kg BME); (3) experimental group II (40 mg/kg BME); and (4) experimental group III (80 mg/kg BME). The freshly prepared aqueous suspension was orally administered to the rats every day. After the treatment period, control and experimental rats were subjected to rotarod and other biochemical parameters. The optimal dosage for the present study was fixed as 40 mg/kg b.w.
Animals
Male Albino rats weighing 250–300 g were obtained from Central Animal House, Dr. ALMPGIBMS, University of Madras, Taramani campus, Chennai 113, Tamil Nadu, India. Rats were housed separately in polypropylene cages and fed standard pellet diet, kept under hygienic conditions. Rats were kept on a 12-h light and dark cycles with free access to water ad libitum. All experiments and protocols described in the present study were approved by the Institutional Animal Ethics Committee (IEAC) of Dr. ALMPGIBMS, University of Madras, Taramani campus, Chennai 113, Tamil Nadu, India. Rats were divided into four experimental groups of six animals each. Group I: control; group II: MeHg (5 mg/kg, b.w.) (Yamashita et al. 2004) orally for 21 days; group III: MeHg + BME (40 mg/kg, b.w.) orally 1 h prior to the administration of MeHg for 21 days; group IV: BME alone (40 mg/kg, b.w.) orally for 21 days.
Rotarod Task
The rats were subjected to rotarod task, which was based on the study of Duham and Miya (1957). Briefly, the apparatus consisted of a bar with a diameter of 2.5 cm, subdivided into four compartments by disks, 25 cm in diameter. After treatments, the rats were subjected to the rotarod task, and the time of permanence on the apparatus was recorded. The maximum time allowed on the rotarod apparatus was 60 s. Each rat was subjected to three different trials with 3 min of interval between each trial, and the mean of their falling latency values was used in the statistical analysis as actual value.
Tissue Preparation
After treatment period, experimental animals and control animals were killed by cervical dislocation. Brains were immediately taken out and washed with ice cold saline to remove blood and kept at −80°C. The cerebellum was rapidly dissected from the intact brain carefully on ice plate according to the stereotaxic atlas of Paxinos and Watson (1982). The cerebellum was homogenized individually in Tris buffer (pH 7.4). The tissue homogenate (10%) was made (w/v), which was centrifuged at 3,000×g for 10 min. The resulting pellet (P1) consisting of nuclear and cellular material was discarded. The supernatant (S1) containing mitochondria, synaptosomes, microsomes, and cytosol was further ultracentrifuged at 25,000×g for 1 h. Pellet had membrane fraction, while the supernatant had cytosol fraction. In this study, all biochemical estimations were performed in the cytosol fraction. Homogenates were kept at −80°C and thawed just before the start of biochemical estimation. All processes were carried out in cold conditions.
Total Mercury Determination
The tissues were weighed and wet-ashed in a Pyrex tube with a mixture of nitrate/sulfate/perchloric acids (1:4:1 v/v) at 160°C for 30 min. The concentrations of mercury in the cerebellum were determined by atomic absorption spectrometry (AAS), using stannous chloride as the reducing agent (Kim et al. 1995). The concentrations are expressed in μg/g tissue.
Biochemical Determinations
Thiobarbituric acid-reactive substances (TBARS), an index of lipid peroxidation, were estimated by the method of Okhawa et al. (1979). The amount of TBARS was determined spectrophotometrically at 532 nm and expressed as μmoles of TBARS/mg protein. Protein carbonyl levels were measured by the method of Levine et al. (1990) and expressed as nmoles/mg protein. The level of reduced glutathione (GSH) was measured by the method of Moron et al. (1979) on the basis of the reaction of 5,5′-dithiobis-2-nitrobenzoic acid which is readily reduced by sulfhydryls forming a yellow substance which was measured at 412 nm and expressed as μmoles/mg protein. The enzyme glutathione peroxidase (GPx) was assayed according to the method of Rotruck et al. (1973). The assay takes advantage of concomitant oxidation of NADPH by GR, which was measured at 340 nm. Enzyme activity was expressed as μg/min/mg protein. GR activity was assayed by the method of Carlberg and Mannervik (1985). The enzyme activity was quantitated at room temperature by measuring the disappearance of NADPH at 340 nm and was calculated as nmol NADPH oxidized/min/mg protein. Superoxide dismutase (SOD) activity, expressed as units/mg of protein, was based on the inhibition of superoxide radical reaction with pyrogallol (Marklund and Marklund 1974). Catalase (CAT) activity was determined by following the decrease in 240 nm absorption of hydrogen peroxide (H2O2). It was expressed as nanomoles of H2O2 reduced/min/mg of protein (Aebi 1984). The protein content was measured by Lowry et al. (1951).
Changes in NO2− and NO3− Levels in Serum
The NO2 − and NO3 − levels in the serum were measured by commercially available nitrite nitrate assay kit (Sigma Aldrich, USA) and were expressed as μM.
Statistical Analysis
Data represent mean ± SD. Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by Student’s t test using SPSS 10 version. If ANOVA analysis indicated significant differences, Tukey’s post hoc test was performed to compare mean values between treatment groups and control. A value of p < 0.05 was considered as statistically significant.
Results
Protective Effect of BME on Rotarod Task Describing Muscular Coordination
The performance of rats in the rotarod task before and after treatment was noted (Table 1). A marked deficit (p < 0.05) in the motor performance was observed for MeHg exposed animals when compared to control rats, whereas treatment with BME significantly (p < 0.01) abolished the deficit in the motor performance. The performance of BME alone treated resembled to that of control group.
Table 1.
Effects of BME on the rotarod performance of control and experimental rats
| Task | Time on the rotarod (s) | |||
|---|---|---|---|---|
| Control | MeHg | MeHg + BME | BME | |
| Phase 1 | 54.3 ± 0.27 | 53.6 ± 0.35 | 54.6 ± 0.26 | 53.4 ± 0.20 |
| Phase 2 | 55.1 ± 0.26 | 34.3 ± 0.20* | 50.4 ± 0.17** | 52.4 ± 0.38 |
Phases 1 and 2 represent the animals’ time of permanence on the rotarod task obtained before the beginning of treatments and after the treatment period, respectively. Data are expressed as mean ± SD of six rats in each group. The time of permanence on the rotarod expressed as seconds. * p < 0.05 significantly different from control group; ** p < 0.01 significantly different from MeHg-induced group, using one-way ANOVA with Tukey’s post hoc test
Determination of Total Mercury Content in Cerebellum of Control and MeHg-Treated Rats
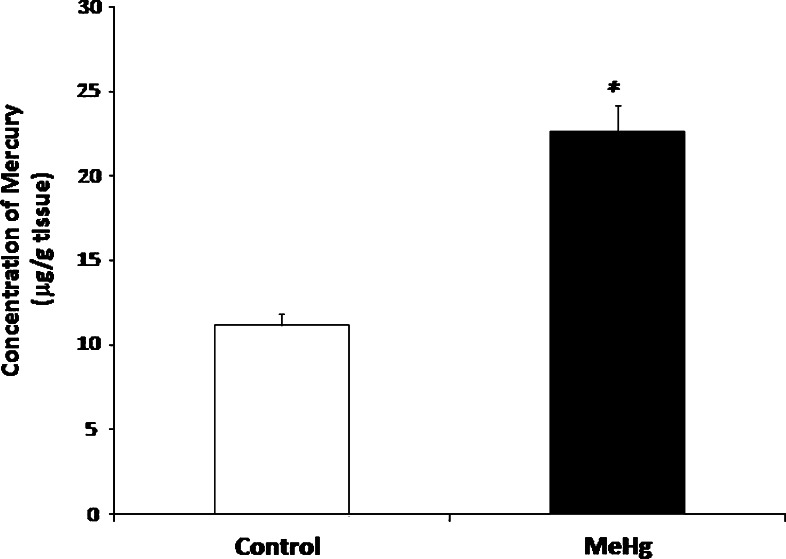
Experimental rats were given MeHg orally, and the content of mercury in the cerebellum was assayed and expressed in μg/g tissue (Fig. 1). The mercury concentration in the cerebellum of MeHg-treated group was found to be significantly higher (p < 0.05) than that of the control group
Fig. 1.
Concentration of mercury in the cerebellum of control and experimental rats. Data represent mean ± SD of six rats in each group. *p < 0.05 significantly different from control group, using one-way ANOVA with Tukey’s post hoc test. Concentration of mercury expressed as μg/g tissue
Effect of BME on MeHg-Induced Oxidative Changes on the Level of TBARS, Protein Carbonyls, and GSH in Cerebellum
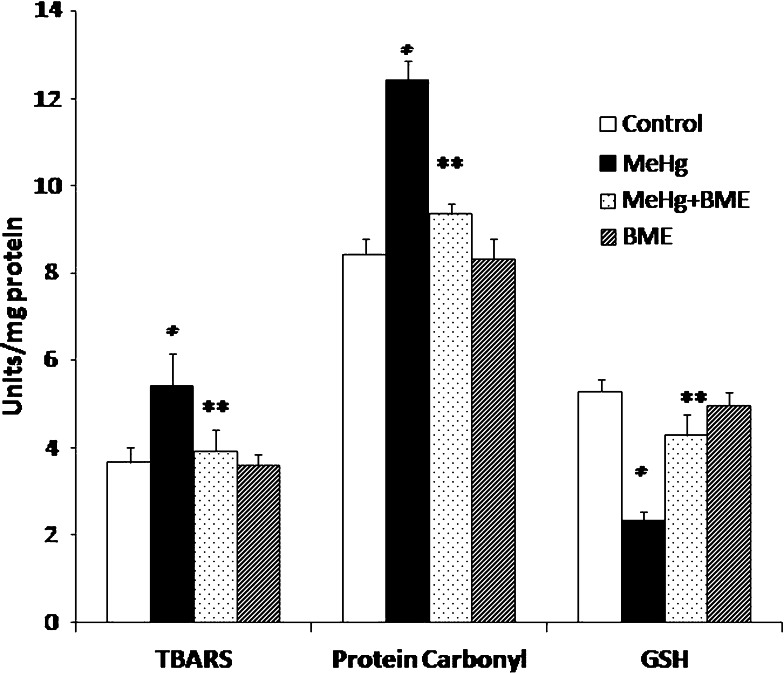
To assess neuroprotection, MeHg-treated rats were given BME orally 1 h prior to the administration of MeHg. The levels of TBARS and protein carbonyl content were found to be significantly increased (p < 0.05), whereas the level of GSH was significantly decreased in MeHg-induced rats when compared to control rats. Administration of BME significantly decreased the levels of the TBARS and protein carbonyl content, and the reduced GSH was completely restored. BME alone treated did not show any marked changes and resembled similar to control group (Fig. 2).
Fig. 2.
Effect of BME on the levels of TBARS, protein carbonyls, and GSH in the cerebellum of control and experimental rats. Data represent mean ± SD of six rats in each group. TBARS units expressed as μmoles of TBARS/mg protein. Protein carbonyls units expressed as nmoles/mg protein. GSH units expressed as μM/mg protein. *p < 0.05 significantly different from control group; ** p < 0.01 significantly different from MeHg-treated group, using one-way ANOVA with Tukey’s post hoc test. TBARS thiobarbituric acid-reactive substances, GSH reduced glutathione
MeHg-Induced Oxidative Damage on the Activities of GPx and GR in Cerebellum—Protective Effect of BME
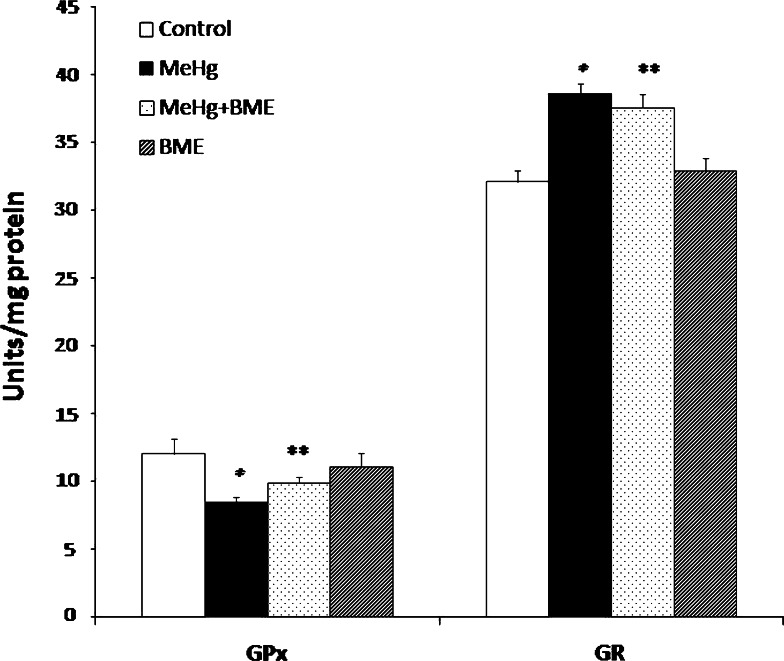
MeHg administration caused a significant decrease in GPx activity (p < 0.05), whereas the activity of GR was found to be significantly increased (p < 0.05) when compared to control rats. These alterations in the activity of GPx and GR activity were observed to be maintained at near normalcy when the rats were treated with BME (Fig. 3). Rats treated with BME alone did not show any alterations and was similar to that of control.
Fig. 3.
Effect of BME on the activities of GPx and GR in the cerebellum of control and experimental rats. Data represent mean ± SD of six rats in each group. GPx units expressed as μg/min/mg protein. GR units expressed as nmol NADPH oxidized/min/mg protein. *p < 0.05 significantly different from control group; ** p < 0.01 significantly different from MeHg-treated group, using one-way ANOVA with Tukey’s post hoc test. GPx glutathione peroxidase, GR glutathione reductase
Amelioration of MeHg-Induced Oxidative Impairments on the Activities of SOD and CAT by BME Treatment
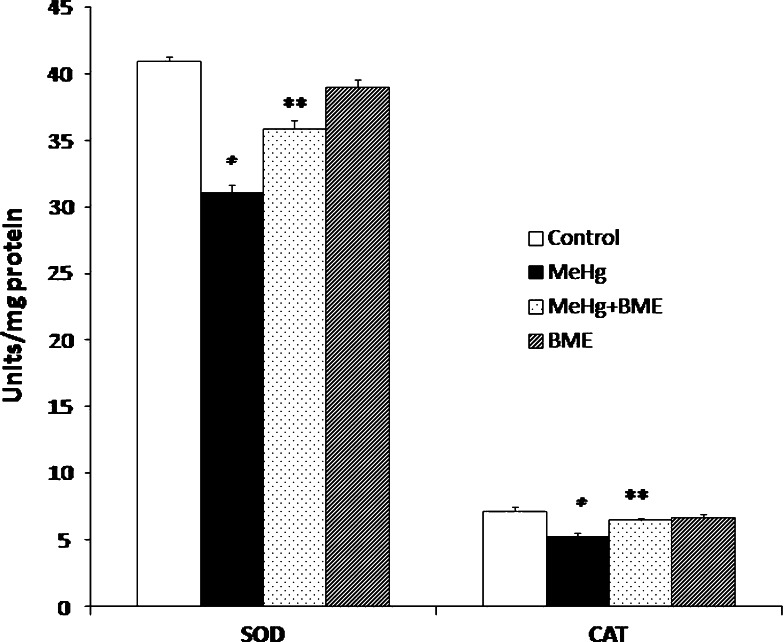
The activity of SOD and CAT was found to be significantly reduced (p < 0.05) in MeHg-treated rats, whereas the activity of SOD and CAT was observed to be increased after treatment with BME (Fig. 4). There were no significant changes in the activities of SOD and CAT in the BME alone treated group.
Fig. 4.
Effect of BME on the activities of SOD and CAT in the cerebellum of control and experimental rats. Data represent mean ± SD of six rats in each group. SOD units expressed as enzyme activity to inhibit 50% of pyrogallol auto-oxidation. CAT units expressed as nmol of H2O2 reduced/min/mg protein. *p < 0.05 significantly different from control group; **p < 0.01 significantly different from MeHg-treated group, using one-way ANOVA with Tukey’s post hoc test. SOD superoxide dismutase, CAT catalase
Protection of BME against MeHg-Induced Alterations in NO2− and NO3− Level in Serum
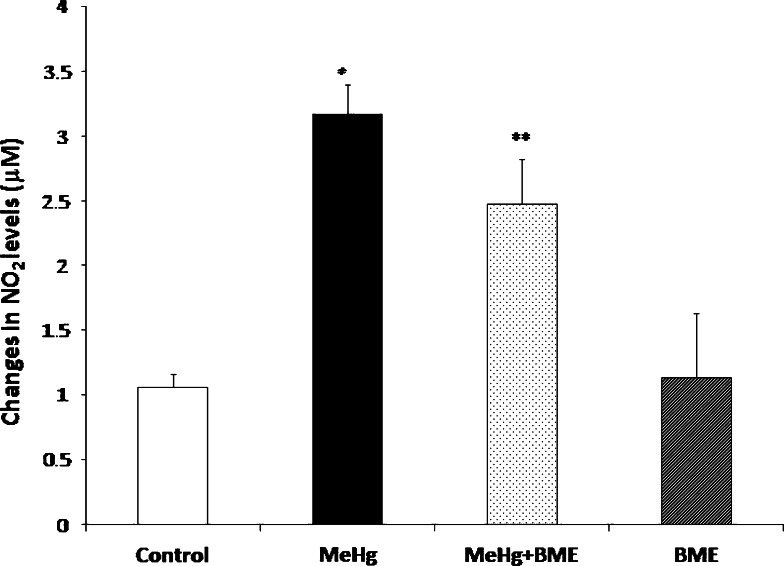
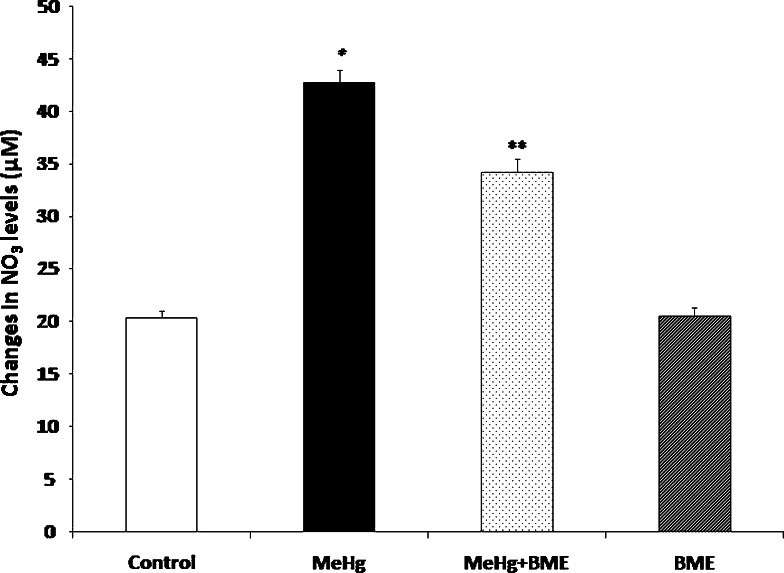
To investigate the changes in the free radical formation after MeHg administration, the levels of NO2 − (Fig. 5) and NO3 − (Fig. 6) in the serum of control and experimental rats were measured. The levels of NO2 − and NO3 − in serum were found to be significantly increased (p < 0.05) in MeHg-treated rats when compared to control rats. These levels were observed to be significantly reduced in the rats treated with BME. There were no significant changes noticed in the levels of serum NO2 − and NO3 − in BME alone administered rats.
Fig. 5.
Effect of BME in the NO2 − level in serum of control and experimental rats. Data represent mean ± SD of six rats in each group. *p < 0.05 significantly different from control group; **p < 0.01 significantly different from MeHg-treated group, using one-way ANOVA with Tukey’s post hoc test. NO2 − level expressed in μM
Fig. 6.
Effect of BME in the NO3 − level in serum of control and experimental rats. Data represent mean ± SD of six rats in each group. *p < 0.05 significantly different from control group; **p < 0.01 significantly different from MeHg-treated group, using one-way ANOVA with Tukey’s post hoc test. NO3 − level expressed in μM
Discussion
Although MeHg-induced neurotoxicity is a well-described phenomenon, there are still no effective treatments available for MeHg poisoning. In fact, treatments with chelating agents in order to eliminate mercury from the tissues are of limited use because of their adverse side effects (Tchounwou et al. 2003). We have shown for the first time that BME possesses protective effects against MeHg-induced neurotoxicity in rats. From a toxicological point of view, it is important to state that no visible signs of toxicity in the BME-treated rats were detected.
From our study, we found that the mercury concentration in the cerebellum of the MeHg-treated group was greater than that of the control group. Mercury levels in the cerebellums of MeHg-exposed dams were about ninefold higher than those found in the cerebellums of control dams (Franco et al. 2006). This type of exposure is much more common in humans. Recently, Passos et al. (2008) described the typical daily intake of MeHg in traditional riparian populations of the Tapajos River in Brazil as being about 70 μg/day. This level is much lower than that usually evaluated adverse effects of MeHg in animal models (Stringari et al. 2006).
Elevated levels of TBARS and protein oxidation as evaluated by protein carbonyl formation in the cerebellum after MeHg administration also indicate that lipid peroxidation and protein degeneration by oxidative stress are produced in the cerebellum during MeHg intoxication. It has been previously reported by Yamashita et al. (2004) that the elevation of TBARS and protein carbonyls were due to oxidative injury by free radicals. It is important to state that MeHg is able to react with and deplete thiol compounds such as GSH (Farina et al. 2005). In accordance with the Farina et al. results, our results also suggested that the level of GSH in rat cerebellum was decreased after MeHg exposure. This may lead to elevated levels of lipid and protein oxidative products. From our study, we found that the MeHg exposure also decreased the activity of cerebellar GPx. Because GPx is crucial for the detoxification of endogenous peroxides, one might suggest the occurrence of oxidative stress in this encephalic structure (Greice et al. 2007). Furthermore, oxidative and xenobiotic insults can lead to an increase in GSH synthesis, an effect that can mask thiol consumption by MeHg (Moskaug et al. 2005). It is interesting to note that BME abolished these changes in the antioxidant status treated by MeHg. Bacopa monniera extract (BME) significantly prevented the decrease in the activity of SOD, GPx, and CAT. These antioxidants play a pivotal role in preventing both free radical damage and generating oxidative stress-like conditions. Hence, we could presume that the elevation of these enzymes could be one mechanism by which BME counters MeHg neurotoxicity. Under the oxidative stress conditions, SOD has a crucial role in detoxifying superoxide radical to hydrogen peroxide (H2O2), which is then converted to H2O by GPx at the expense of GSH. GR is an important enzyme involved in the reduction of glutathione disulfide (GSSG) to glutathione (GSH), using NADPH as a reducing cofactor (Gul et al. 2000). Here, we showed that MeHg exposure increased GR activity in the cerebellum. Although studies on the effects of MeHg on GR activity are lacking in the literature, evidence shows that mercury is able to increase GR activity under in vivo conditions (Lash and Zarups 1996). This increment could be related, at least in part, to the direct oxidative effects of mercury on endogenous GSH, which leads to the enhancement in GR activity. In fact, the increase in GR activity could be interpreted as a protective response to preserve the homeostasis of intracellular thiol status. It is suggested that oxidative injury, especially lipid peroxidation, via a powerful oxidant (e.g., hydroxyl radicals), may play an important role in cerebellar degeneration during MeHg intoxication, and that BME may be one of the most useful nervine tonic and a protective antioxidant against the neurotoxicity of MeHg in the cerebellum.
The presence of free radical species including NO has been implicated in various forms of neurotoxicity (Dawson et al. 1991). NO can also react with superoxide anions from the electron transfer system at the mitochondria to generate peroxynitrite and hydroxyl radical, which are extremely reactive and cytotoxic (Beckman et al. 1990). Abnormally highly produced NO can be toxic to neurons as a free radical (Yamashita et al. 2004). From our study, we found that the levels of NO2 − and NO3 − in serum were elevated after MeHg administration, and BME administration decreased these levels to the extent when compared to the MeHg-treated rats, suggesting that these metabolites may become a marker of free radical injury and that NO production may play an important role in MeHg intoxication in the cerebellum.
Collectively, our findings provide reasonable evidence on the neuroprotective effect of BME in cerebellum, which is suggestive of the broad neuro therapeutic potential of this Ayurvedic herb in mitigating oxidative stress-mediated neuronal dysfunctions.
Conclusion
Bacopa monniera extract might be a potential candidate for reducing MeHg-induced oxidative stress in rats. These results provide evidence for the first time that BME exerts significant protection against MeHg-induced neurotoxicity. Although to date its precise site of action remains unclear, it can be presumed that the neuromodulatory properties of BME are likely to be responsible for the greater part of its protection against MeHg-induced neurotoxicity. However, these issues need further investigations.
Acknowledgments
The work described in this article was supported by Department of Medical Biochemistry, DR. ALMPGIBMS, University of Madras, Taramani Campus, Chennai 113, Tamil Nadu, India.
Conflict of interest
The authors declare that there is no conflict of interests.
References
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126 [DOI] [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH (2000) Methylmercury alters glutamate transport in astrocytes. Neurochem Int 37:199–206 [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M (2007) Involvement of glutamate and reactive oxygen species in methyl mercury neurotoxicity. Braz J Med Biol Res 40:285–291 [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Kumar A, Ghosal S (1999) Effect of Bacopa monniera on animal models of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. Res Commun Pharmacol Toxicol 4:1–12 [Google Scholar]
- Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S (2000) Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res 14:174–179 [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490 [DOI] [PubMed] [Google Scholar]
- Chowdhuri DK, Parmar D, Kakkar P, Shukla R, Seth PK, Srimal RC (2002) Antistress effects of bacosides of Bacopa monnieri: modulation of Hsp70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother Res 16:639–664 [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury current exposures and clinical manifestations. N Engl J Med 349:1731–1737 [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 88:6368–6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duham NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc 46:208–209 [DOI] [PubMed] [Google Scholar]
- Ernst E (2006) Herbal remedies for anxiety—a systematic review of controlled clinical trials. Phytomedicine 13:205–208 [DOI] [PubMed] [Google Scholar]
- Farina M, Dahm KC, Schwalm FD et al (2003) Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups:modulatory effect of ebselen. Toxicol Sci 73:135–140 [DOI] [PubMed] [Google Scholar]
- Farina M, Franco JL, Ribas CM, Meotti FC, Missau FC, Pizzolatti MG, Dafre AL, Antos ARS (2005) Protective effects of Polygala paniculata extract against methyl mercury induced neurotoxicity in mice. J Pharm Pharmacol 57:1503–1508 [DOI] [PubMed] [Google Scholar]
- Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Garcia Pomblum SC, Moro AM, Bohrer D, Bairros AV, Dafre AL, Santos ARS, Farina M (2006) Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ Res 102:22–28 [DOI] [PubMed] [Google Scholar]
- Greice MR, de Lucena S, Franco JL, Ribas CM, Azevedo MS, Meotti FC, Gadotti VM, Dafre AL, Santos AR, Farina M (2007) Cipura paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic Clin Pharmacol Toxicol 101:127–131 [DOI] [PubMed] [Google Scholar]
- Gul M, Kutay FZ, Temocin S, Hanninen O (2000) Cellular and clinical implications of glutathione. Indian J Exp Biol 38:625–634 [PubMed] [Google Scholar]
- Hunter D, Russell DS (1954) Focal cerebral and cerebellar atrophy in a human subject due to organic mercury compounds. J Neurol Neurosurg Psychiat 17:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D, Bomford RR, Russell DS (1940) Poisoning by methylmercury compounds. Quart J Med 9:193–213 [Google Scholar]
- Ikeda M, Komachi H, Sato I, Himi T, Yuasa T, Murota S (1999) Induction of neuronal nitric oxide synthase by methylmercury in the cerebellum. J Neurosci Res 55:352–356 [DOI] [PubMed] [Google Scholar]
- Juarez BI, Martinez ML, Montante M, Dufour L, Garcia E, Jimenez-Capdeville ME (2002) Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicol Teratol 24:767–771 [DOI] [PubMed] [Google Scholar]
- Juarez BI, Portillo-Salazar H, Gonzalez-Amaro R, Mandeville P, Aguirre JR, Jimenez ME (2005) Participation of N-methyl d-aspartate receptors on methylmercury-induced DNA damage in rat frontal cortex. Toxicology 207:223–229 [DOI] [PubMed] [Google Scholar]
- Kim CY, Watanabe C, Kasanuma Y, Satoh H (1995) Inhibition of glutamyl transpeptidase decreases renal deposition of mercury after mercury vapour exposure. Arch Toxicol 69:722–724 [DOI] [PubMed] [Google Scholar]
- Kim CY, Nakai K, Kameo S, Kurokawa N, Liu ZM, Satoh H (2000) Protective effect of melatonin on methyl mercury induced mortality in mice. Tohuko J Exp Med 191:241–246 [DOI] [PubMed] [Google Scholar]
- Kishore K, Singh M (2005) Effect of bacosides, alcoholic extract of Bacopa monniera Linn. (Brahmi), on experimental amnesia in mice. Indian J Exp Biol 43:640–645 [PubMed] [Google Scholar]
- Lash LH, Zarups RK (1996) Alterations in renal cellular glutathione metabolism after in vivo administration of a sub toxic dose of mercuric chloride. J Biochem Toxicol 11:1–9 [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the follin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JB, Frizzo ME, Souza DO, Farina M (2004) Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci 81:172–178 [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474 [DOI] [PubMed] [Google Scholar]
- Miura N, Kaneko S, Hosoya S, Furuchi T, Miura K, Kuge S, Naganuma A (1999) Overexpression of l-glutamine: d-fructose-6-phosphate amidotransferase provides resistance to methylmercury in Saccharomyces cerevisiae. FEBS Lett 458:215–218 [DOI] [PubMed] [Google Scholar]
- Moron M, Depierre JW, Mannervik BT (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78 [DOI] [PubMed] [Google Scholar]
- Moskaug JO, Carlson H, Myhrstad MC, Blomhoff R (2005) Polyphenols and glutathione synthesis regulation. Am J Clin Nutr 81:277S–283S [DOI] [PubMed] [Google Scholar]
- Nagashima KA (1997) Review of experimental methylmercury toxicity in rats: neuropathology and evidence for apoptosis. Toxicol Pathol 25:624–631 [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Clarke J, Lloyd J, Huchison CW, Downey L, Stough C (2001) The acute effects of an extract of Bacopa monnieri on cognitive function in healthy normal subjects. Hum Psychopharmacol 16:345–351 [DOI] [PubMed] [Google Scholar]
- Okhawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358 [DOI] [PubMed] [Google Scholar]
- Ou YC, White CC, Krejsa CM, Ponce RA, Kavanagh TJ, Faustman EM (1999) The role of intracellular glutathione in methylmercuryinduced toxicity in embryonic neuronal cells. Neurotoxicology 20:793–804 [PubMed] [Google Scholar]
- Parvinder K, Schulz K, Aschner M, Syversen T (2007) Role of docosahexaenoic acid in modulating methylmercury-induced neurotoxicity. Toxicol Sci 100(2):423–432 [DOI] [PubMed] [Google Scholar]
- Passos CJS, Sampaio DS, Lemire M, Fillion M, Guimaraes JRD, Lucotte M, Mergler D (2008) Daily mercury intake in fish eating populations in the Brazilian Amazon. Exp Sci Environ Epidemiol 18:76–87 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1982) The rat brain in sterotaxic coordinates. Academic Press, New York [Google Scholar]
- Roodenrys S, Booth D, Bulzomi S, Phipps A, Micallef C, Smoker J (2002) Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology 27:279–281 [DOI] [PubMed] [Google Scholar]
- Rosen DR (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364:362 [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Popa AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstar WG (1973) Selenium: biochemical role as a component of GPx. Science 179:588–590 [DOI] [PubMed] [Google Scholar]
- Russo A, Borrelli F (2005) Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine 12:305–317 [DOI] [PubMed] [Google Scholar]
- Russo A, Izzo AA, Borrelli F, Renis M, Vanella A (2003) Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res 17:870–875 [DOI] [PubMed] [Google Scholar]
- Sairam K, Rao CV, Goel RK (2001) Prophylactic and curative effects of Bacopamonniera in gastric ulcer models. Phytomedicine 8:423–430 [DOI] [PubMed] [Google Scholar]
- Saraf MK, Prabhakar S, Pandhi P, Anand A (2008) Bacopa monnieri ameliorates amnesic effects of diazepam qualifying behavioral–molecular partitioning. Neuroscience 155:476–484 [DOI] [PubMed] [Google Scholar]
- Shanker G, Singh HK (2000) Anxiolytic profile of standardized Brahmi extract. Indian J Pharmacol 32:152 [Google Scholar]
- Sharma R, Chaturvedi C, Tewari PV (1987) Efficacy of Bacopa monniera in revitalizing intellectual functions in children. J Res Edu Ind Med 1:12 [Google Scholar]
- Shinyashiki M, Kumagai Y, Nakajima H, Nagafune J, Homma-Takeda S, Sagai M, Shimojo N (1998) Differential changes in rat brain nitric oxide synthase in vivo and in vitro by methylmercury. Brain Res 798:147–155 [DOI] [PubMed] [Google Scholar]
- Singh HK, Dhawan BN (1982) Effect of Bacopamonniera Linn. (Brahmi) extract on avoidance responses in rat. J Ethnopharmacol 5:205–214 [DOI] [PubMed] [Google Scholar]
- Singh HK, Dhawan BN (1997) Neuropsychopharmacological effects of the ayurvedic nootropic Bacopa monniera Linn (Brahmi). Indian J Pharmacol 29:359–365 [Google Scholar]
- Singh HK, Rastogi RP, Srimal RC, Dhawan BN (1988) Effects of bacosides A and B on avoidance response in rats. Phytother Res 2:70–75 [Google Scholar]
- Sirois JE, Atchison WD (2000) Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol Appl Pharmacol 167:1–11 [DOI] [PubMed] [Google Scholar]
- Stough C, Lloyd J, Clarke J, Downey LA, Hutchison CW, Rodgers T, Nathan PJ (2001) The chronic effects of an extract of Bacopamonniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berlin) 156:481–484 [DOI] [PubMed] [Google Scholar]
- Stringari J, Meotti FC, Souza DO, Santos ARS, Farina M (2006) Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem Res 31:563–569 [DOI] [PubMed] [Google Scholar]
- Sumathy T, Subramanian S, Govindaswamy S, Balakrihna K, Veluchany G (2001) Protective role of Bacopa monnieri on morphine induced hepatotoxicity in rats. Phytother Res 15:643–645 [DOI] [PubMed] [Google Scholar]
- Takeuchi T (1968) Pathology of minamata disease. In: Kutsuna M (ed) Study group of minamata disease. Kumamoto University, Shuhan Publisher, Tokyo, pp 141–228 [Google Scholar]
- Takeuchi T (1982) Pathology of minamata disease. With special reference to its pathogenesis. Acta Pathol Jpn 32(1):73–99 [PubMed] [Google Scholar]
- Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175 [DOI] [PubMed] [Google Scholar]
- Tripathi YB, Chaurasia S, Tripathi E, Upadhyay A, Dubey GP (1996) Bacopa monniera Linn. as an antioxidant: mechanism of action. Indian J Exp Biol 34:523–526 [PubMed] [Google Scholar]
- Vohora D, Pal SN, Pillai KK (2000) Protection from phenytoin induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J Ethnopharmacol 71:383–390 [DOI] [PubMed] [Google Scholar]
- Vollala VR, Upadhya S, Nayak S (2010) Effect of Bacopamonniera Linn. (Brahmi) extract on learning and memory in rats: a behavioural study. J Vet Behav 5:69–74 [Google Scholar]
- Yamashita T, Ando Y, Obayashi K, Terazaki H, Sakashita N, Uchida K, Ohama E, Ando M, Uchino M (2000) Oxidative injury is present in Purkinje cells in patients with olivopontocerebellar atrophy. J Neurol Sci 175:107–110 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ando Y, Nakamura M, Obayashi K, Terazaki H, Haraoka K, Guo SX, Ueda M, Uchino M (2004) Inhibitory effect of α-tocopherol on methylmercury-induced oxidative stress. Environ Health Prev Med 9:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]