Abstract
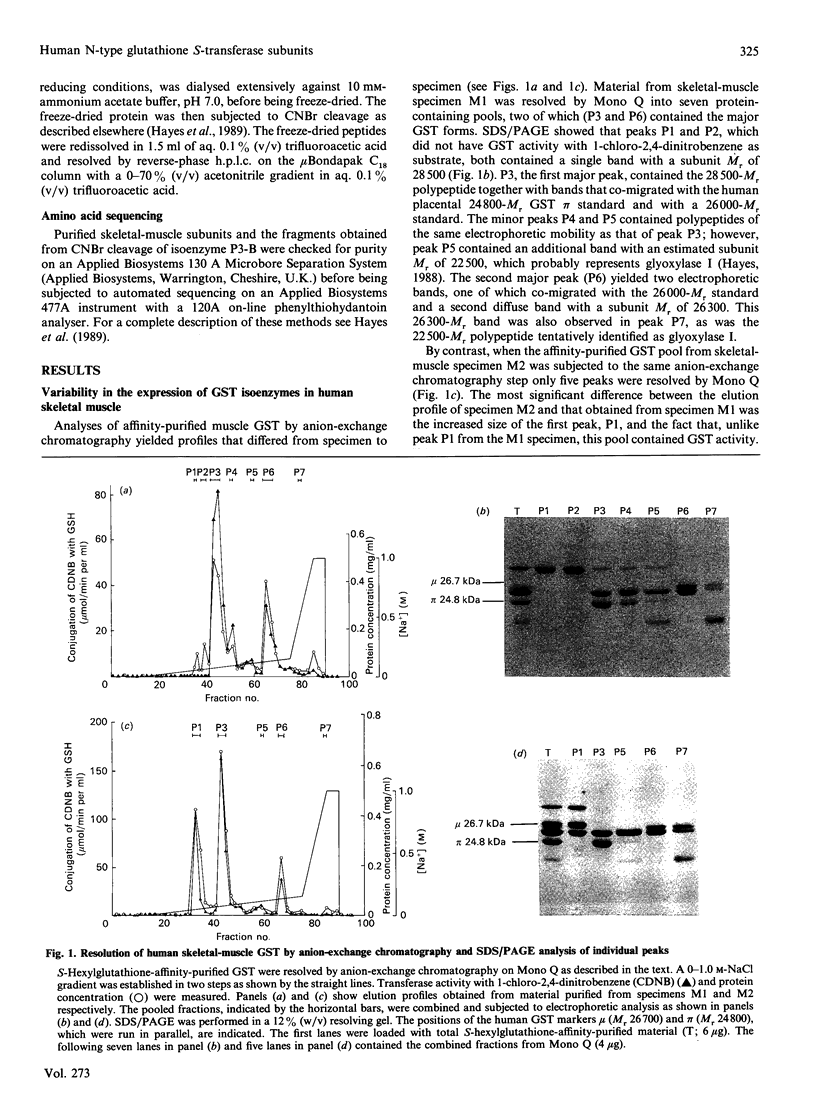
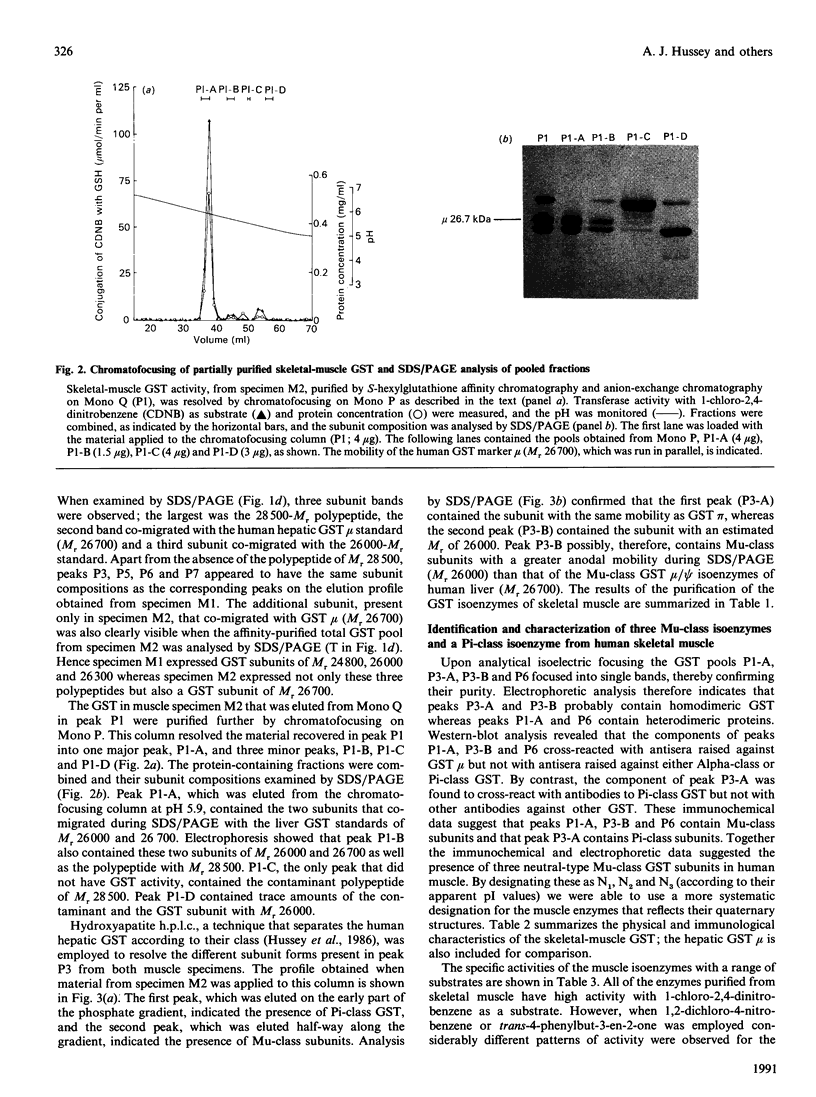
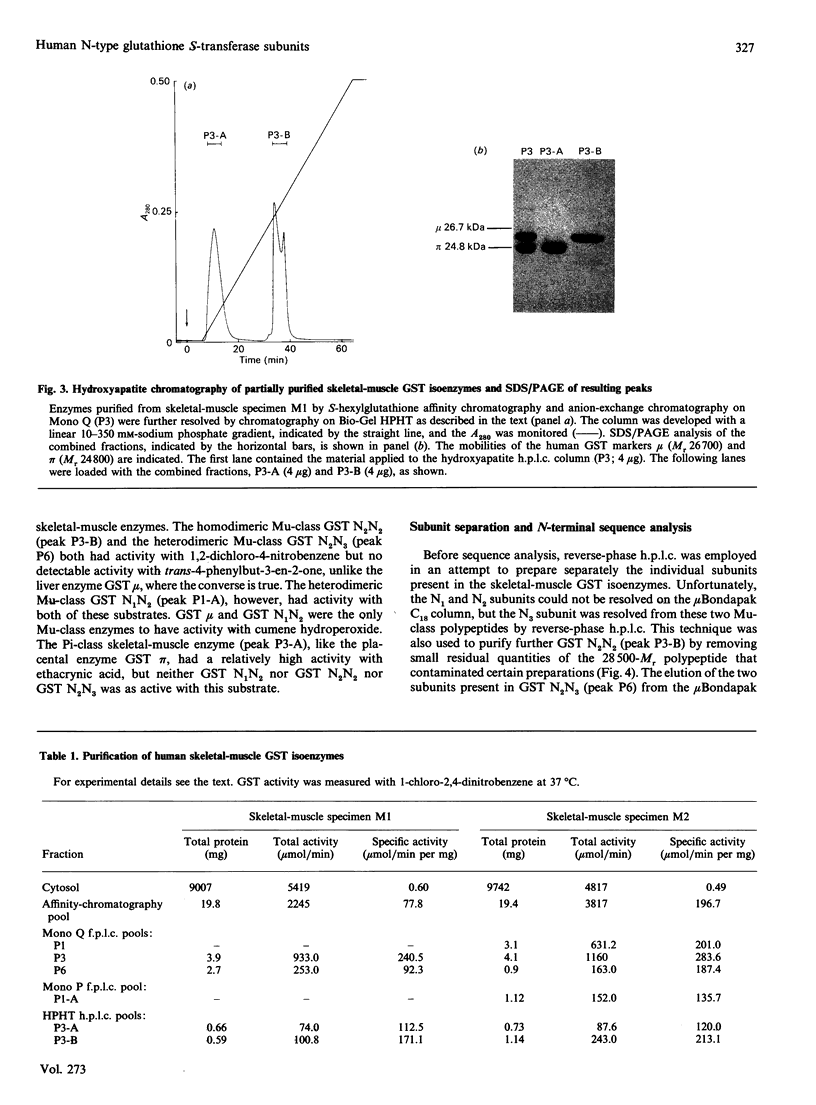
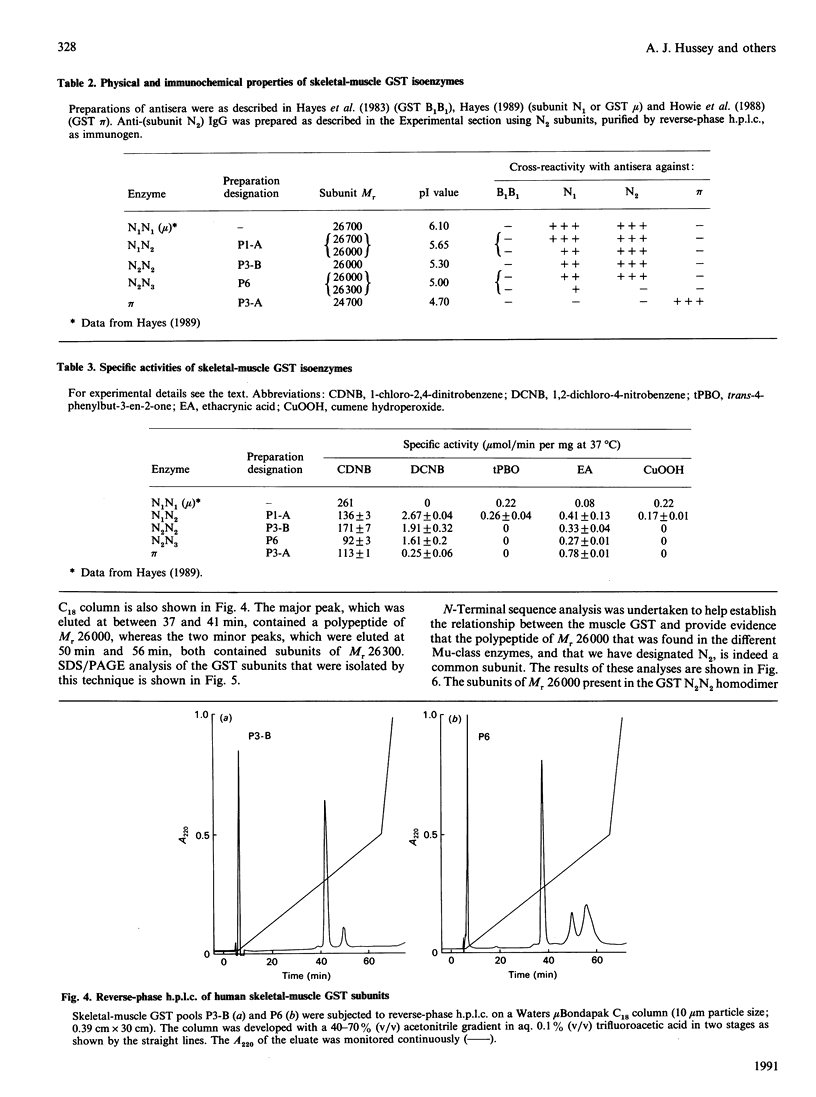
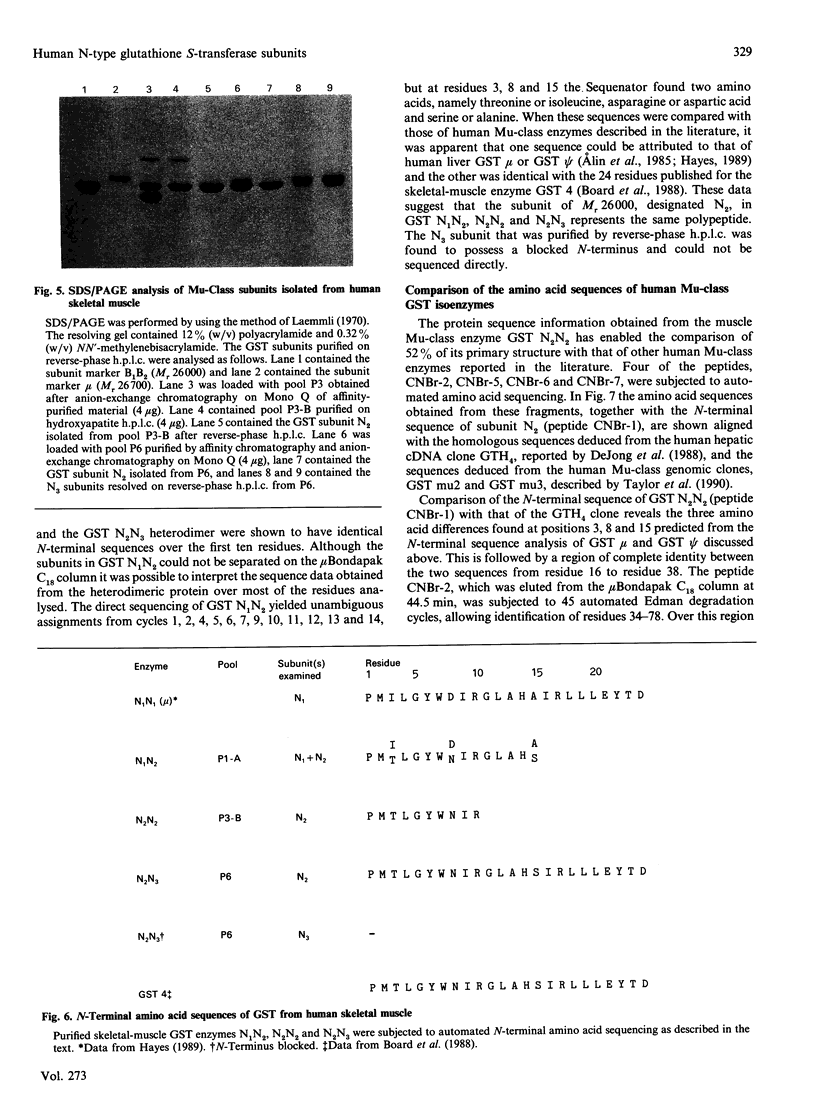
The cytosolic glutathione S-transferases (GST) from human skeletal muscle were purified by a combination of affinity chromatography and anion-exchange chromatography followed by either chromatofocusing or hydroxyapatite chromatography. Pi-class and Mu-class GST, but not Alpha-class GST, were isolated from muscle. In addition to a Pi-class GST subunit, which exists as a homodimer, this tissue also contains a total of three distinct neutral-type Mu-class GST subunits, which hybridize to form homodimers or heterodimers. The neutral-type subunits are referred to as N1-N3 and are defined by the decreasing isoelectric points of the homodimers; GST N1N1, N2N2 and N3N3 have estimated pI values of 6.1, 5.3 and less than 5.0 respectively. SDS/PAGE showed that N1, N2 and N3 have Mr values of 26,700, 26,000 and 26,300 respectively. The N1, N2 and N3 subunits are catalytically distinct, with N1 possessing a high activity for trans-4-phenylbut-3-en-2-one and N2 having high activity with 1,2-dichloro-4-nitrobenzene. In skeletal muscle the expression of the N1 subunit, but not of N2 and N3 subunits, was found to differ from specimen to specimen. The N1 subunit was absent from about 50% of samples examined, and the purification results from two different specimens are presented to illustrate this inter-individual variation. Skeletal muscle from one individual (M1), which did not express N1, contained only GST N2N2, N2N3 and pi, whereas the second sample examined (M2) contained GST N1N2, N2N2 and N2N3 as well as GST pi. N-Terminal amino acid sequence analysis supported the electrophoretic evidence that the N2 subunit in GST N1N2, N2N2 and N2N3 represents the same polypeptide. The peptides obtained from CNBr digests of N2 were subjected separately to automated amino acid sequencing, and the results indicate that N2 is distinct but closely related to the protein encoded by the human Mu-class cDNA clone GTH4 [DeJong, Chang, Whang-Peng, Knutsen & Tu (1988) Nucleic Acids Res. 16, 8541-8554]. GST N2N2 is probably identical with GST 4 [Board, Suzuki & Shaw (1988) Biochim. Biophys. Acta 953, 214-217], as over the 24 N-terminal residues of GST 4 there is complete identity between the two enzymes. Our data suggest that the GST 1 and GST 4 loci are part of the same multi-gene family.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alin P., Mannervik B., Jörnvall H. Structural evidence for three different types of glutathione transferase in human tissues. FEBS Lett. 1985 Mar 25;182(2):319–322. doi: 10.1016/0014-5793(85)80324-0. [DOI] [PubMed] [Google Scholar]
- Board P. G. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981 Jan;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Board P. G., Suzuki T., Shaw D. C. Human muscle glutathione S-transferase (GST-4) shows close homology to human liver GST-1. Biochim Biophys Acta. 1988 Apr 14;953(3):214–217. doi: 10.1016/0167-4838(88)90027-1. [DOI] [PubMed] [Google Scholar]
- Board P. G., Webb G. C., Coggan M. Isolation of a cDNA clone and localization of the human glutathione S-transferase 3 genes to chromosome bands 11q13 and 12q13-14. Ann Hum Genet. 1989 Jul;53(Pt 3):205–213. doi: 10.1111/j.1469-1809.1989.tb01786.x. [DOI] [PubMed] [Google Scholar]
- Bora P. S., Spilburg C. A., Lange L. G. Metabolism of ethanol and carcinogens by glutathione transferases. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4470–4473. doi: 10.1073/pnas.86.12.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Cowell I. G., Dixon K. H., Pemble S. E., Ketterer B., Taylor J. B. The structure of the human glutathione S-transferase pi gene. Biochem J. 1988 Oct 1;255(1):79–83. doi: 10.1042/bj2550079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong J. L., Chang C. M., Whang-Peng J., Knutsen T., Tu C. P. The human liver glutathione S-transferase gene superfamily: expression and chromosome mapping of an Hb subunit cDNA. Nucleic Acids Res. 1988 Sep 12;16(17):8541–8554. doi: 10.1093/nar/16.17.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulder C. G., Hirrell P. A., Hume R., Strange R. C. Studies of the development of basic, neutral and acidic isoenzymes of glutathione S-transferase in human liver, adrenal, kidney and spleen. Biochem J. 1987 Jan 1;241(1):221–228. doi: 10.1042/bj2410221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Gilligan D., Chapman B. J., Beckett G. J. Purification of human hepatic glutathione S-transferases and the development of a radioimmunoassay for their measurement in plasma. Clin Chim Acta. 1983 Oct 31;134(1-2):107–121. doi: 10.1016/0009-8981(83)90189-4. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Kerr L. A., Cronshaw A. D. Evidence that glutathione S-transferases B1B1 and B2B2 are the products of separate genes and that their expression in human liver is subject to inter-individual variation. Molecular relationships between the B1 and B2 subunits and other Alpha class glutathione S-transferases. Biochem J. 1989 Dec 1;264(2):437–445. doi: 10.1042/bj2640437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Use of immuno-blot techniques to discriminate between the glutathione S-transferase Yf, Yk, Ya, Yn/Yb and Yc subunits and to study their distribution in extrahepatic tissues. Evidence for three immunochemically distinct groups of transferase in the rat. Biochem J. 1986 Feb 1;233(3):779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Purification and physical characterization of glutathione S-transferase K. Differential use of S-hexylglutathione and glutathione affinity matrices to isolate a novel glutathione S-transferase from rat liver. Biochem J. 1986 Feb 1;233(3):789–798. doi: 10.1042/bj2330789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Selective elution of rodent glutathione S-transferases and glyoxalase I from the S-hexyglutathione-Sepharose affinity matrix. Biochem J. 1988 Nov 1;255(3):913–922. doi: 10.1042/bj2550913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie A. F., Hayes J. D., Beckett G. J. Purification of acidic glutathione S-transferases from human lung, placenta and erythrocyte and the development of a specific radioimmunoassay for their measurement. Clin Chim Acta. 1988 Sep 30;177(1):65–75. doi: 10.1016/0009-8981(88)90308-7. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Hayes J. D., Beckett G. J. The polymorphic expression of neutral glutathione S-transferase in human mononuclear leucocytes as measured by specific radioimmunoassay. Biochem Pharmacol. 1987 Nov 15;36(22):4013–4015. doi: 10.1016/0006-2952(87)90472-2. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Stockman P. K., Beckett G. J., Hayes J. D. Variations in the glutathione S-transferase subunits expressed in human livers. Biochim Biophys Acta. 1986 Nov 7;874(1):1–12. doi: 10.1016/0167-4838(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Islam M. Q., Platz A., Szpirer J., Szpirer C., Levan G., Mannervik B. Chromosomal localization of human glutathione transferase genes of classes alpha, mu and pi. Hum Genet. 1989 Jul;82(4):338–342. doi: 10.1007/BF00273994. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res. 1988 Dec;202(2):343–361. doi: 10.1016/0027-5107(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Kispert A., Meyer D. J., Lalor E., Coles B., Ketterer B. Purification and characterization of a labile rat glutathione transferase of the Mu class. Biochem J. 1989 Jun 15;260(3):789–793. doi: 10.1042/bj2600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laisney V., Nguyen Van Cong, Gross M. S., Frezal J. Human genes for glutathione S-transferases. Hum Genet. 1984;68(3):221–227. doi: 10.1007/BF00418392. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Morrow C. S., Cowan K. H., Goldsmith M. E. Structure of the human genomic glutathione S-transferase-pi gene. Gene. 1989 Jan 30;75(1):3–11. doi: 10.1016/0378-1119(89)90377-6. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Rhoads D. M., Zarlengo R. P., Tu C. P. The basic glutathione S-transferases from human livers are products of separate genes. Biochem Biophys Res Commun. 1987 May 29;145(1):474–481. doi: 10.1016/0006-291x(87)91345-3. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W., Markowitz M. M., Roush G., Miller D. G., Beattie E. J. Isoenzyme(s) of glutathione transferase (class Mu) as a marker for the susceptibility to lung cancer: a follow up study. Carcinogenesis. 1990 Jan;11(1):33–36. doi: 10.1093/carcin/11.1.33. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W., Miller D. G., Beattie E. J. A glutathione transferase in human leukocytes as a marker for the susceptibility to lung cancer. Carcinogenesis. 1986 May;7(5):751–753. doi: 10.1093/carcin/7.5.751. [DOI] [PubMed] [Google Scholar]
- Singh S. V., Ahmad H., Kurosky A., Awasthi Y. C. Purification and characterization of unique glutathione S-transferases from human muscle. Arch Biochem Biophys. 1988 Jul;264(1):13–22. doi: 10.1016/0003-9861(88)90564-4. [DOI] [PubMed] [Google Scholar]
- Singh S. V., Kurosky A., Awasthi Y. C. Human liver glutathione S-transferase psi. Chemical characterization and secondary-structure comparison with other mammalian glutathione S-transferases. Biochem J. 1987 Apr 1;243(1):61–67. doi: 10.1042/bj2430061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman P. K., Beckett G. J., Hayes J. D. Identification of a basic hybrid glutathione S-transferase from human liver. Glutathione S-transferase delta is composed of two distinct subunits (B1 and B2). Biochem J. 1985 Apr 15;227(2):457–465. doi: 10.1042/bj2270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman P. K., McLellan L. I., Hayes J. D. Characterization of the basic glutathione S-transferase B1 and B2 subunits from human liver. Biochem J. 1987 May 15;244(1):55–61. doi: 10.1042/bj2440055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R. C., Faulder C. G., Davis B. A., Hume R., Brown J. A., Cotton W., Hopkinson D. A. The human glutathione S-transferases: studies on the tissue distribution and genetic variation of the GST1, GST2 and GST3 isozymes. Ann Hum Genet. 1984 Jan;48(Pt 1):11–20. doi: 10.1111/j.1469-1809.1984.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Coggan M., Shaw D. C., Board P. G. Electrophoretic and immunological analysis of human glutathione S-transferase isozymes. Ann Hum Genet. 1987 May;51(Pt 2):95–106. doi: 10.1111/j.1469-1809.1987.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]
- Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–333. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]