Abstract
Chronic psychosocial isolation (CPSI) is known to cause several maladaptive changes in the limbic brain structures, which regulate the hypothalamic–pituitary–adrenal (HPA) axis activity. In this study, we focused our investigation on CPSI effects in the hypothalamus (HT) since it is a major driver of HPA axis activity. We also investigated whether the exposure to CPSI could alter the response to subsequent acute stress (30-min immobilization). In the HT, we followed cytosolic and nuclear levels of the glucocorticoid receptor (GR), as a mediator of HPA axis feedback inhibition, and its chaperones, the heat shock proteins (HSPs), hsp70 and hsp90. The CPSI did not cause any changes in either GR or HSPs levels. However, we observed increase of the GR and hsp70 in both HT cellular compartments as a response of naïve rats to acute stress, whereas the response of CPSI rats to acute stress was associated with elevation of the GR in the cytosol and decrease of HSPs in the nucleus. Thus, our data indicated reduced availability of HSPs to GR in both cytosol and nucleus of the HT under acute stress of CPSI animals, and therefore, pointed out to potentially negative effects of CPSI on GR function in the HT.
Keywords: Chronic stress, Hypothalamus, Glucocorticoid receptor, Heat shock proteins
Introduction
Stress response involves activation of the hypothalamic–pituitary–adrenal (HPA) axis, and the key driver of its activity is the hypothalamus (HT) (de Kloet et al. 2005; Sapolsky et al. 2000). The final products of the HPA axis activity, glucocorticoids (GCs), enable adaptive stress response and mediate feedback inhibition of the HPA axis activity, particularly via HT (de Kloet et al. 2005; Sapolsky et al. 2000). The HT is not only involved in regulation of stress response, but also plays a fundamental role in body’s homeostatic controls, and is fundamental for the integration of the nervous and endocrine systems (Lam et al. 2009; Tasker 2006).
The GCs act through the glucocorticoid receptor (GR), a ligand-dependent transcription factor that in the absence of ligand resides in cytoplasm bound within a multiprotein heterocomplex that includes molecular chaperones, the heat shock proteins (HSPs) hsp70 and hsp90 (DeFranco 2000; Pratt et al. 2004). In general, HSPs play important physiological roles in situations involving both systemic and cellular stress (Vamvakopoulos et al. 1993; Kultz 2005; Kregel 2002). Regarding the specific association of HSPs with GR, Hsp70 is essential for the folding of nascent chains of GR (Chen and Smith 1998; Grad and Picard 2007; Smith and Toft 1993), while hsp90 helps GR to achieve hormone-binding conformation (Cadepond et al. 1991; Grad and Picard 2007). Upon binding of GCs, GR translocates to the nucleus where it regulates transcription of a wide variety of genes. The processes of GR nuclear translocation (Galigniana et al. 2002; Pratt et al. 2004), as well as GR transcriptional activity (Kang et al. 1999; Stavreva et al. 2004), GR nuclear recycling (Liu and DeFranco 1999), and GR intranuclear mobility (Elbi et al. 2004) are also dependent on hsp70 and hsp90 chaperone machineries. Owing to the essential importance of HSPs in facilitating GR function, GR and its chaperones were intensely studied by numerous laboratories (Furay et al. 2006; Murphy et al. 2002; Noguchi et al. 2010; Patchev et al. 1994).
The current study is an extension of our previous study in which we investigated the effects of chronic psychosocial isolation (CPSI) stress in adult male Wistar rats (e.g., Adzic et al. 2009; Djordjevic et al. 2009). Models of chronic social isolation, although not widely studied, are of particular importance, since they appear to be relevant to certain subtypes of human depression (Wallace et al. 2009; Heinrich and Gullone 2006; Grippo et al. 2007; Hall 1998). In our previous reports, we investigated the effects of CPSI on the various aspects of GR signaling in the limbic brain structures, the hippocampus (HIPPO), and the prefrontal cortex (PFC) (Adzic et al. 2009; Djordjevic et al. 2009). Our experimental design (applied in this study, as well as in our previous ones) also included investigation of the effects of acute stress such as 30-min immobilization, with specific interest on how CPSI modulates response to the subsequent acute stress.
We have already shown that CPSI disturbs HSPs/GR ratio in the HIPPO and the PFC in tissue-specific manner (Djordjevic et al. 2009). In this study, we specifically investigated the effects of CPSI on the HT since this brain structure, like HIPPO and PFC, is of particular importance for understanding the effects of psychogenic stress (de Kloet et al. 2005; Swaab et al. 2005). Accordingly, in the HT, we analyzed protein levels of GR and its chaperones, hsp70 and hsp90, and their compartmental distribution in our CPSI model system.
Materials and Methods
Animals and Treatment
The experiments were performed on adult (3-month old) Wistar male rats (body mass 300–330 g) housed in standard size cages with four rats per cage. Food (commercial rat pellets) and water were provided ad libitum. Light was kept on continuously, between 07:00 and 19:00, and room temperature (RT) was maintained at 20 ± 2°C. All the animal procedures were approved by the Ethical Committee for the Use of Laboratory Animals of the VINCA Institute of Nuclear Sciences, according to the guidelines of the EU registered Serbian Laboratory Animal Science Association (SLASA).
The animals, 40 in total, were divided into four experimental groups (10 animals per group). Group I consisted of unstressed animals (control group). In group II, animals were exposed to acute immobilization for 30 min (acute stress). Group III animals were subjected to CPSI stress. During CPSI, animals were housed one per cage for 21 days and deprived from any tactile and visual contact. In group IV also animals were subjected to CPSI, but followed by 30 min of immobilization at the 21st day of isolation (combined stress).
All animals were euthanized by rapid decapitation immediately after stress treatments, between 09:00 and 11:00 under no additional stress conditions (such as sounds from immobilized animals or any scent cues) unless otherwise indicated.
Corticosterone Assay
Blood from each animal was collected at the time of euthanasia. Serum was prepared by 15-min centrifugation at 3,000 rpm. The individual corticosterone (CORT) concentrations were determined using the OCTEIA Corticosterone EIA kit according to the manufacturer’s instructions (American Laboratory Products Co.), and CORT concentrations were determined using standard curve and presented as ng/ml.
Preparation of Cytosolic and Nuclear Extracts of HT
Right away after decapitation, the examined brain tissue, the HT, was removed from each animal and immediately frozen in liquid nitrogen until further preparation.
Frozen tissues were weighed and homogenized (1:2 w/v) in ice-cold 20 mM Tris–HCl (pH 7.2) buffer containing 10% glycerol, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2 mM DTT, and protease inhibitors (20 mM Na2MoO4, 0.15 mM spermin, 0.15 mM spermidin, 0.1 mM PMSF, 5 μg/ml antipain, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, and 3 mM benzamidine), and phosphatase inhibitors (20 mM β-glycerophosphate, 5 mM Na4P2O7·10H2O, 2 mM Na3VO4, 25 mM NaF) with 20 strokes of Potter–Elvehjem Teflon-glass homogenizer. Samples were centrifuged for 10 min at 2,000×g at 4°C. Afterward, supernatants were ultracentrifuged for 1 h at 105,000×g, and the final supernatants were used as cytosolic fraction. Pellets were washed (three times) with 0.5 ml of homogenization buffer, and centrifuged for 10 min at 2,000×g at 4°C. Final pellets were weighed, resuspended (1:1 w/v) in the same buffer supplied with 0.5 M KCl, incubated for 1 h in ice-bath (with frequent vortexing), and centrifuged for 10 min at 8,000×g at 4°C. The supernatants were used as nuclear extracts.
Western Blot Detection of GR and HSPs
Protein concentrations in cytosolic and nuclear extracts were determined by the method of Lowry et al. (1951). The samples were prepared with denaturing buffer according to Laemmli (1970), boiled for 5 min, and 60 μg of proteins were subjected to electrophoresis on 7.5% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). After electrophoresis, proteins were transferred onto PVDF membrane (Immobilon-P membrane, Millipore) using a blot system (Transblot, BioRad). The membranes were incubated in blocking buffer, phosphate-buffered saline (PBS) containing 5% milk for 1 h at RT, and thereafter probed overnight at 4°C with specific primary antibodies diluted in PBS with 2.5% milk and 0.1% Tween 20. After washing three times in PBST, membranes were incubated with the respective secondary antibody for 2 h at 4°C, washed three times, soaked in enhanced chemiluminescence reagent (ECL, Pierce), and exposed to X-ray film. GR M-20 antibody (Santa Cruz Biotechnology) was used to detect GR; hsp70 (N27F3-4) (Santa Cruz Biotechnology) and hsp90 (F-8) (Santa Cruz Biotechnology) antibodies were used for the detection of hsp70 and hsp90, respectively. Rabbit polyclonal anti-β-actin (ab8227, Abcam), antibody for β-actin, was used as a loading control for both cellular compartments. Blots were developed with ECL Rabbit IgG, HRP-linked whole antibody, or ECL Mouse IgG, HRP-linked whole antibody (Amersham), whereever it was appropriate. Densitometry of protein bands on X-ray film was performed using Image J analysis PC software.
Statistical Analysis
Individual data for CORT are presented as mean ± SD. Owing to the non-normality of distributions of CORT levels, these data were analyzed by k-independent Kruskal–Wallis non-parametric test and subsequently by two independent samples Mann–Whitney non-parametric test. Because of multiple comparisons, Bonferroni correction was used, and consequently, the significance cut-off was set at 0.01 (there were five comparisons: control vs. acute, control vs. CPSI, control vs. combine, CPSI vs. combine, and acute vs. combine).
Regarding the levels of examined proteins (GR, hsp70, and hsp90) and ratios of hsp70/GR and hsp90/GR, data are presented as mean ± SEM from three independent measurements of pooled samples obtained from 10 animals in each group. Two-way ANOVA was used to assess the influence of different types of stressors (factors were acute and CPSI) and their interactions (acute × CPSI) under combined stress on these parameters. The post hoc Tukey test was used to determine differences between the experimental groups. The statistical significance was accepted at p < 0.05.
Results
Serum CORT Levels
CORT levels of each group of animals are represented in Table 1. The effect of stress on serum CORT levels was significant (Kruskal–Wallis χ² = 30.379, p < 0.001). In other words, 30 min of immobilization led to about five times increase of CORT levels when applied to naïve animals (Z = −3.356, p < 0.001) and about ten times increase when applied to CPSI animals (control vs. combine: Z = −3.46, p < 0.001; CPSI vs. combine: Z = −3.84, p < 0.001). On the other hand, CPSI led to a significant decrease of CORT serum levels (Z = −2.55, p = 0.01).
Table 1.
Serum CORT concentrations in Wistar rats under different types of stress: acute (A), CPSI (C) and combined (CA)
| Type of stress | Ctrl | A | C | CA |
|---|---|---|---|---|
| CORT (ng/ml) | 127.08 ± 57.56 | 635.18 ± 100.20* | 51.11 ± 33.41* | 610.22 ± 115.32*# |
Data are presented as mean ± SD (*p < 0.05, control vs. stress; # p < 0.05, CPSI vs. combined stress—judged by Mann–Whitney non-parametric test)
GR Levels in Cytosol and Nucleus of the HT
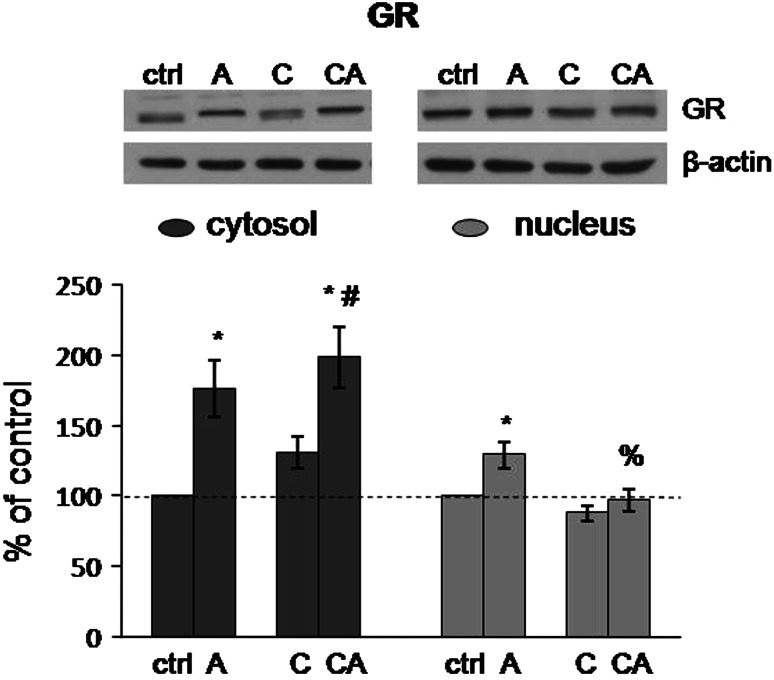
As it is seen from Fig. 1, only acute stress significantly influenced cytosolic GR levels in the HT (F = 20.36, p < 0.001). Precisely, 30 min of immobilization of both control and CPSI animals resulted in significant increase of cytosolic GR levels (control vs. acute: p < 0.01; control vs. combine: p < 0.001; CPSI vs. combine: p < 0.05). Considering nuclear GR levels (Fig. 1), they were affected by both acute and CPSI stress (acute stress: F = 9.63, p < 0.01; CPSI: F = 5.78, p < 0.05) and their interaction was very near to the level of significance (acute × CPSI: F = 3.69, p = 0.059). Nuclear GR levels in acutely stressed rats were significantly elevated compared to the control and combined stressed rats (control vs. acute: p < 0.05; acute vs. combine: p < 0.01).
Fig. 1.
Levels of GR in cytosol and nucleus of HT in Wistar rats under different types of stress. Acute (A), CPSI (C), and combined (CA) stress. Data are presented as mean ± SEM (*p < 0.05, control vs. stress; % p < 0.05, acute vs. combined stress; # p < 0.05, CPSI vs. combined stress—judged by post hoc Tukey test)
Hsp70 and Hsp90 Levels in Cytosol and Nucleus of the HT
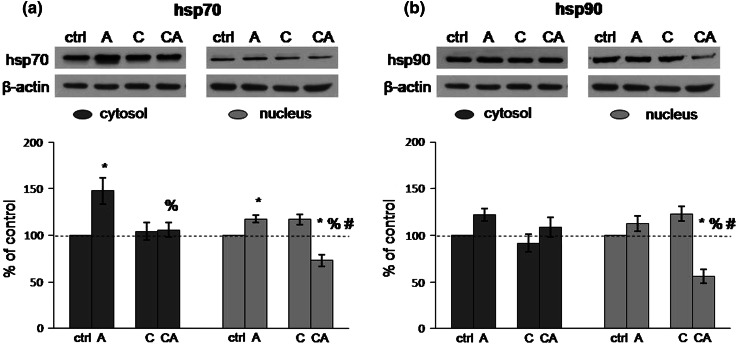
Quantification of hsp70 levels (Fig. 2a) showed significant elevation of this protein in both cytosol and nucleus of acutely stressed animals compared to the controls (cytosol: F = 7.17, p < 0.05; control vs. acute: p < 0.01; nucleus: F = 8.21, p < 0.01; control vs. acute: p < 0.05). On the other hand, CPSI did not exhibit any significant effect on the hsp70 levels in either cytosol or nucleus. However, under combined stress, the interaction between the two employed types of stress was revealed with respect to the hsp70 levels in both cytosol and nucleus (cytosol: acute × CPSI: F = 6.23, p < 0.05; nucleus: acute × CPSI: F = 44.62, p < 0.001). In particular, protein levels of hsp70 in the cytosol of CPSI animals subjected to subsequent acute immobilization were unchanged with regard to control and CPSI animals, but were lower than in acutely stressed animals (acute vs. combine: p < 0.05). The levels of nuclear hsp70 were significantly lower compared with all other animal groups (control vs. combine: p < 0.01; acute vs. combine: p < 0.001; CPSI vs. combine: p < 0.001).
Fig. 2.
Levels of hsp70 (a) and hsp90 (b) in cytosol and nucleus of HT in Wistar rats under different types of stress. Acute (A), CPSI (C), and combined (CA) stress. Data are presented as mean ± SEM (*p < 0.05, control vs. stress; % p < 0.05, acute vs. combined stress; # p < 0.05, CPSI vs. combined stress—judged by post hoc Tukey test)
There were no statistically significant differences in cytosolic hsp90 levels between analyzed groups (Fig. 2b). Considering nuclear hsp90 levels, the effect of each stress and their interaction under combined stress were revealed (acute stress: F = 15.71, p < 0.001; CPSI: F = 5.95, p < 0.05; acute × CPSI: F = 33.59, p < 0.001). Specifically, nuclear hsp90 levels were significantly decreased under combined stress compared to all other animal groups (control vs. combine: p < 0.001; acute vs. combine: p < 0.001; CPSI vs. combine: p < 0.001) (Fig. 2b).
Hsp70/GR and Hsp90/GR Ratios in Cytosol and Nucleus of the HT
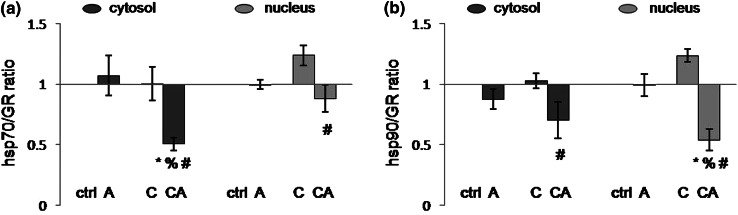
Analyzing hsp70/GR ratio in the cytosol (Fig. 3a), it was shown that effects of both types of stress were significant, as well as their interaction under combined stress (acute stress: F = 5.00, p < 0.05; CPSI: F = 7.07, p < 0.05; acute × CPSI: F = 9.00, p < 0.01). In other words, the hsp70/GR ratio was turned in favor of GR in the cytosol of HT under combined stress compared to all other animal groups (control vs. combine: p < 0.05; acute vs. combine: p < 0.01; CPSI vs. combine: p < 0.01). On the other hand, analyzing hsp70/GR ratio in the nucleus (Fig. 3a), the effect of only acute stress was statistically significant (F = 8.39, p < 0.01), as well as its interaction with CPSI under combined stress (acute × CPSI: F = 8.20, p < 0.01). Even though the effect of chronic stress was not detected in CPSI animals, a statistical trend of shifting hsp70/GR ratio in favor of hsp70 was revealed by post hoc test (control vs. CPSI: p = 0.06). However, after the subsequent acute immobilization, this ratio was turned in favor of GR (CPSI vs. combine: p < 0.001).
Fig. 3.
Ratios of hsp70/GR (a) and hsp90/GR (b) in cytosol and nucleus of HT in Wistar rats under under different types of stress. Acute (A), CPSI (C), and combined (CA) stress. Data are presented as mean ± SEM (*p < 0.05, control vs. stress; % p < 0.05, acute vs. combined stress; # p < 0.05, CPSI vs. combined stress—judged by post hoc Tukey test)
The cytosolic hsp90/GR ratio (Fig. 3b) was influenced solely by acute stress (F = 8.51, p < 0.01). In other words, the hsp90/GR ratio was altered in favor of GR in combined stress animal group compared to CPSI (p < 0.05). The nuclear hsp90/GR ratio (Fig. 3b) was also influenced by acute stress (F = 26.84, p < 0.001) and, under combined stress, the interaction between CPSI and acute stress was revealed (acute × CPSI: F = 15.79, p < 0.001). Although the effect of chronic stress on nuclear hsp90/GR ratio was not statically significant, post hoc test indicated that there was a statistical trend of turning hsp90/GR ratio in favor of hsp90 under CPSI (control vs. CPSI: p = 0.10). When CPSI animals were subjected to acute stress, nuclear hsp90/GR ratio was turned in favor of GR compared with all other groups (control vs. combine: p < 0.01; acute vs. combine: p < 0.05; CPSI vs. combine: p < 0.001).
Discussion
Our previous studies related to the investigation of CPSI effects in the HIPPO and the PFC of adult Wistar male rats suggested that CPSI have maladaptive effects (Adzic et al. 2009; Djordjevic et al. 2009). In the current study, we analyzed CPSI effects on the HT since this brain structure is the key regulator of HPA axis activity.
The changes in CORT serum levels under different stress conditions were in agreement with our previous data (Adzic et al. 2009; Djordjevic et al. 2009). The highly intensive stress of acute immobilization led to several fold increase of CORT levels with respect to control animals, which was also in agreement with other studies (Garcia et al. 2000; Noguchi et al. 2010). In CPSI rats, CORT levels were decreased, indicating HPA axis hypoactivity (discussed in other our articles, e.g., Adzic et al. 2009; Djordjevic et al. 2009; Radojcic et al. 2012). After subsequent acute immobilization, CPSI animals exhibited even higher HPA axis activation in comparison with naïve animals exposed to acute immobilization. This indicated HPA axis sensitization set by previous CPSI. Indeed, the increased response of HPA axis to a novel stress is often observed in chronically/repeatedly stressed animals (Bhatnagar and Dallman 1998; Bhatnagar and Vining 2003; Figueiredo et al. 2003; Gavrilovic unpublished data).
Similar to our previous results in the HIPPO and the PFC (Djordjevic et al. 2009), our current results in the HT also showed elevation of nuclear GR under acute immobilization, indicating that GR nuclear translocation in this brain structure was also governed by high CORT levels. However, contrary to the HIPPO and the PFC, under conditions of acute stress, cytosolic GR levels were elevated in the HT. Analyses of nuclear translocation in response to injections of CORT indicated that the decrease of hypothalamic cytosolic GR occurs at 2-h post-injection (Spencer et al. 2000). On the other hand, the study of Noguchi et al. (2010) suggested that GR nuclear translocation in medial basal HT could be observed at 30 min after the onset of immobilization, while changes in cytosolic GR levels were not observed even after 2 h of immobilization. It is important to note that our results, similar to Spencer’s et al. (2000), represent the effects on HT as a whole, and the failure to observe the changes, as was done by Noguchi et al. (2010), should be tempered by relative non-specificity of the whole HT dissection. Indeed, it is known that numerous HT nuclei express GR (Aronsson et al. 1988; Morimoto et al. 1996). Therefore, it might be important for some HT nuclei, at least other than those examined by Noguchi et al. (2010), and contrary to upper limbic structures, the HIPPO and the PFC (Adzic et al. 2009; Djordjevic et al. 2009), to possess enhanced sensitivity to GCs at the time point of 30 min of intense acute stress such as immobilization. In other words, this GR up-regulation in the HT under acute stress could indicate its potential role in other important processes regulated by HT, such as regulation of energy homeostasis and food intake (Ford et al. 2005; Stevens et al. 2010; Stricker-Krongrad and Beck 2002).
In any case, the increased protein levels of GR could be the result of its increased GR synthesis and/or stability in different subfields of HT. In favor of this assumption, are also our preliminary findings which demonstrated elevation of GR isoform phosphorylated at serine 246 (S246) in both cytosol and nucleus of HT after 30 min of immobilization (unpublished observations from our laboratory). In other words, in some pilot studies, it is shown that the c-Jun N-terminal kinases have a role in the stabilization of GR protein and the extension of its half-life, probably through GR phosphorylation at S246 (personal communication with Krstic-Demonacos).
Regarding the general roles of HSPs in cellular response to stress, our data indicated that acute immobilization of naïve rats led to significant increase of hsp70 in both cytosol and nucleus of the HT. The results revealed that this type of neuroendocrine stress induced, at the cellular level, proteotoxic stress and activation of cellular stress response (Kultz 2005; Nollen and Morimoto 2002). The levels of hsp90 in HT after 30-min immobilization were unchanged which was in accordance with a previous study (Noguchi et al. 2010).
Since the proper activity of the GR is dependent on its association with chaperones, we also analyzed the hsp70/GR and the hsp90/GR ratios which enabled us to estimate the availability of HSPs to GR. We observed that the acute stress did not change hsp70/GR and hsp90/GR ratios indicating that GR function was not compromised by acute immobilization at least according to the availability of HSPs to GR.
Under CPSI conditions, according to our results, the GR levels in both cellular compartments remained unchanged, similar to the results obtained in the HIPPO (Djordjevic et al. 2009). Also, CPSI did not alter the levels of hsp70 or hsp90 in either cellular compartment, as well. However, by calculating hsp70/GR and hsp90/GR ratios, the trends of turning both HSPs/GR ratios in favor of HSPs were detected in the nucleus of HT under CPSI. This could suggest that HSPs were more available to GR to participate in the stabilization of the liganded GR at DNA promoters (Stavreva et al. 2004), enhancing GR nuclear mobility (Elbi et al. 2004) and GR nuclear recycling (Liu and DeFranco 1999) and thereby, enhancing the overall GR transcriptional activity, in spite of unaltered nuclear GR levels under CPSI. Interestingly, such trend in the changes of nuclear HSPs/GR ratio, accompanied by unaltered nuclear GR levels in the HT under CPSI, closely resemble previously observed results in HIPPO (Djordjevic et al. 2009), which were furthermore related to the increased GR transcriptional activity (Adzic et al. 2009).
When CPSI animals were subjected to the subsequent acute 30-min immobilization, the changes of cytosolic GR levels were similar to those observed under acute immobilization of naïve rats. However, under combined stress, nuclear GR levels stayed unchanged and were lower than those observed in acutely stressed naïve rats. This could suggest that CPSI might have compromised nuclear translocation of GR, triggered by acute stress. Regarding the HSPs, their cytosolic levels remained unchanged, while their nuclear levels were significantly decreased compared to naïve animals subjected to acute stress. These data indicated that CPSI compromised activation of cellular stress response (Kregel 2002) and, again, pointed out the potentially maladaptive effect of CPSI on HT.
A marked result of this study was the change of HSPs/GR ratios when CPSI animals were subjected to novel acute stress. In other words, both HSPs/GR ratios were turned in favor of GR in the cytosol, as well as in the nucleus of HT, unlike in naïve animals subjected to acute stress. Reduced availability of HSPs to cytosolic GR, especially hsp70, could result in less properly folded GR (Smith and Toft 1993; Chen and Smith 1998) and therefore, in less GR hormone-binding capacity and its translocation to the nucleus (Pratt et al. 2004; Grad and Picard 2007). Indeed, we observed decreased nuclear GR levels under combined stress compared to acute stress. On the other hand, the diminished availability of HSPs to GR in nucleus, particularly the decreased availability of hsp90, could lead to a reduced overall GR transcriptional activity (Grad and Picard 2007; Stavreva et al. 2004). Since the GR in HT is a major mediator of feedback inhibition of the HPA axis activity, its decreased levels as well as decreased availability of its HSPs in the nucleus of HT could constitute at least one of the mechanisms of HPA axis hyperactivity in CPSI animals subjected to the subsequent acute stress. These alterations once more emphasized the potentially maladaptive effect of CPSI evaluated by response to later acute immobilization.
To conclude, CPSI reduced the availability of HSPs to the GR in response to the subsequent acute immobilization, in both cytosol and nucleus of the HT. The effect of CPSI on GR and HSPs in the HT confirmed the observations from our previous research carried out on HIPPO and PFC, that chronic stress such as CPSI may lead to maladaptive stress response and could impair reaction of an organism to a subsequent acute stress (Adzic et al. 2009; Djordjevic et al. 2009). Notwithstanding the fact that exact mechanisms of how chronic stress dysregulate HPA axis activity need further study, our data indicate that decreased availability of hsp70 and hsp90 to GR in the HT may play a role in altered response of chronically stressed organisms to a novel stress.
Acknowledgments
The authors are gratefully appreciative of the support provided by the Ministry of Education and Sciences of Serbia for this study (Grant III41029).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Adzic M, Djordjevic J, Djordjevic A, Niciforovic A, Demonacos C, Radojcic M, Krstic-Demonacos M (2009) Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J Endocrinol 202(1):87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsson M, Fuxe K, Dong Y, Agnati LF, Okret S, Gustafsson JA (1988) Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc Natl Acad Sci USA 85(23):9331–9335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M (1998) Neuroanatomical basis for facilitation of hypothalamic–pituitary–adrenal responses to a novel stressor after chronic stress. Neuroscience 84(4):1025–1039 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C (2003) Facilitation of hypothalamic–pituitary–adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav 43(1):158–165 [DOI] [PubMed] [Google Scholar]
- Cadepond F, Schweizer-Groyer G, Segard-Maurel I, Jibard N, Hollenberg SM, Giguere V, Evans RM, Baulieu EE (1991) Heat shock protein 90 as a critical factor in maintaining glucocorticosteroid receptor in a nonfunctional state. J Biol Chem 266(9):5834–5841 [PubMed] [Google Scholar]
- Chen S, Smith DF (1998) Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J Biol Chem 273(52):35194–35200 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–475 [DOI] [PubMed] [Google Scholar]
- DeFranco DB (2000) Role of molecular chaperones in subnuclear trafficking of glucocorticoid receptors. Kidney Int 57(4):1241–1249 [DOI] [PubMed] [Google Scholar]
- Djordjevic A, Adzic M, Djordjevic J, Radojcic MB (2009) Stress type dependence of expression and cytoplasmic-nuclear partitioning of glucocorticoid receptor, hsp90 and hsp70 in Wistar rat brain. Neuropsychobiology 59(4):213–221 [DOI] [PubMed] [Google Scholar]
- Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB (2004) Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci USA 101(9):2876–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP (2003) Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology 144(12):5249–5258 [DOI] [PubMed] [Google Scholar]
- Ford GK, Al-Barazanji KA, Wilson S, Jones DN, Harbuz MS, Jessop DS (2005) Orexin expression and function: glucocorticoid manipulation, stress, and feeding studies. Endocrinology 146(9):3724–3731 [DOI] [PubMed] [Google Scholar]
- Furay AR, Murphy EK, Mattson MP, Guo Z, Herman JP (2006) Region-specific regulation of glucocorticoid receptor/HSP90 expression and interaction in brain. J Neurochem 98(4):1176–1184 [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, Murphy PJ, Chinkers M, Radanyi C, Renoir JM, Zhang M, Pratt WB (2002) Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry 41(46):13602–13610 [DOI] [PubMed] [Google Scholar]
- Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A (2000) Recovery of the hypothalamic–pituitary–adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology 72(2):114–125 [DOI] [PubMed] [Google Scholar]
- Grad I, Picard D (2007) The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol 275(1–2):2–12 [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS (2007) Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32(8–10):966–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS (1998) Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol 12(1–2):129–162 [DOI] [PubMed] [Google Scholar]
- Heinrich LM, Gullone E (2006) The clinical significance of loneliness: a literature review. Clin Psychol Rev 26(6):695–718 [DOI] [PubMed] [Google Scholar]
- Kang KI, Meng X, Devin-Leclerc J, Bouhouche I, Chadli A, Cadepond F, Baulieu EE, Catelli MG (1999) The molecular chaperone Hsp90 can negatively regulate the activity of a glucocorticosteroid-dependent promoter. Proc Natl Acad Sci USA 96(4):1439–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92(5):2177–2186 [DOI] [PubMed] [Google Scholar]
- Kultz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685 [DOI] [PubMed] [Google Scholar]
- Lam CK, Chari M, Lam TK (2009) CNS regulation of glucose homeostasis. Physiology (Bethesda) 24:159–170 [DOI] [PubMed] [Google Scholar]
- Liu J, DeFranco DB (1999) Chromatin recycling of glucocorticoid receptors: implications for multiple roles of heat shock protein 90. Mol Endocrinol 13(3):355–365 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275 [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M (1996) Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res 26(3):235–269 [DOI] [PubMed] [Google Scholar]
- Murphy EK, Spencer RL, Sipe KJ, Herman JP (2002) Decrements in nuclear glucocorticoid receptor (GR) protein levels and DNA binding in aged rat hippocampus. Endocrinology 143(4):1362–1370 [DOI] [PubMed] [Google Scholar]
- Noguchi T, Makino S, Matsumoto R, Nakayama S, Nishiyama M, Terada Y, Hashimoto K (2010) Regulation of glucocorticoid receptor transcription and nuclear translocation during single and repeated immobilization stress. Endocrinology 151(9):4344–4355 [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI (2002) Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci 115(Pt 14):2809–2816 [DOI] [PubMed] [Google Scholar]
- Patchev VK, Brady LS, Karl M, Chrousos GP (1994) Regulation of HSP90 and corticosteroid receptor mRNA by corticosterone levels in vivo. Mol Cell Endocrinol 103(1–2):57–64 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Harrell JM, DeFranco DB (2004) Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal 16(8):857–872 [DOI] [PubMed] [Google Scholar]
- Radojcic M, Adzic M, Niciforovic A, Djordjevic J, Djordjevic A, Demonacos C, Krstic-Demonacos M (2012) Effects of chronic psychosocial isolation on limbic brain structures of Wistar rats. In: Costa A, Villalba E (eds) Horizons in neuroscience research, vol 5. Nova Science Publishers, New York, pp 97–126 [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21(1):55–89 [DOI] [PubMed] [Google Scholar]
- Smith DF, Toft DO (1993) Steroid receptors and their associated proteins. Mol Endocrinol 7(1):4–11 [DOI] [PubMed] [Google Scholar]
- Spencer RL, Kalman BA, Cotter CS, Deak T (2000) Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using western blot analysis. Brain Res 868(2):275–286 [DOI] [PubMed] [Google Scholar]
- Stavreva DA, Muller WG, Hager GL, Smith CL, McNally JG (2004) Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol Cell Biol 24(7):2682–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A (2010) Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 151(8):3652–3664 [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Beck B (2002) Modulation of hypothalamic hypocretin/orexin mRNA expression by glucocorticoids. Biochem Biophys Res Commun 296(1):129–133 [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ (2005) The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4(2):141–194 [DOI] [PubMed] [Google Scholar]
- Tasker JG (2006) Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity (Silver Spring) 14(Suppl 5):259S–265S [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Fukuhara K, Patchev V, Chrousos GP (1993) Effect of single and repeated immobilization stress on the heat shock protein 70/90 system of the rat: glucocorticoid-independent, reversible reduction of Hsp90 in the liver and spleen. Neuroendocrinology 57(6):1057–1065 [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ (2009) CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci 12(2):200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]