Abstract
N-methyl-d-aspartate receptor (NR) is involved in activity-dependent synaptic plasticity, such as associative long-term potentiation, and in related central functions, such as learning and memory. In this study, we observed effects of treadmill exercise on NR1 and doublecortin (DCX, a marker for neuroblast differentiation) in the subgranular zone of the dentate gyrus (DG). At 6 weeks of age, rats were put on a treadmill with or without running for 1 h/day for 5 consecutive days at 22 m/min for 5 weeks. Exercise increased NR1 immunoreactivity and protein level in the hippocampus. To identify the correlations between NR and neuroblasts, we intraperitoneally administered a NR antagonist, MK-801, to the exercised rats. MK-801 treatment reduced NR1 protein level in the hippocampus of the exercised rats. In addition, in the MK-801-treated group, the number of DCX cells was significantly decreased in the subgranular zone of the DG. These results suggest that NR may be one of the important factors that modulate neuroblast differentiation during exercise in rats.
Keywords: Doublecortin, N-methyl-d-aspartate receptor, Hippocampus, Neurogenesis, Subgranular zone
Introduction
There are distinct regions of active proliferation even in the adult mammalian brain, which are known to generate neurons continuously throughout life (Picard-Riera et al. 2004; Paizanis et al. 2007). The dentate gyrus (DG) of the hippocampus is one of neurogenic sites in the adult brain (Gould et al. 1997; Kempermann et al. 1997). Newly generated cells in the subgranular zone of the DG can proliferate, migrate, and finally differentiate into neurons, which extend axonal and dendritic projections and establish new synaptic connections to the existing hippocampal circuitry (Ramirez-Amaya et al. 2006; Kee et al. 2007; Laplagne et al. 2007). Neurogenesis is a dynamic process that is regulated both positively and negatively by various factors such as environmental, endocrine, and pharmacological stimuli (Jaako-Movits and Zharkovsky 2005; Kong et al. 2008; Garcez et al. 2009; Orendácová et al. 2009; Veena et al. 2009). It was reported that neurogenesis was enhanced by physical exercise such as wheel exercise and treadmill exercises (Kitamura et al. 2003; Lou et al. 2008; Yi et al. 2009).
Exercise is currently believed to support brain health, improve the status of patients with the risk of neurodegenerative diseases, increase neurogenesis, and enhance long-term potentiation, synaptic plasticity, and hippocampus-dependent learning and memory (van Praag et al. 1999; Lou et al. 2008; Deslandes et al. 2009; Devine and Zafonte 2009). It was reported that exercise stimulated neuronal activity, and upregulated expression of various genes such as N-methyl-d-aspartate receptor (NR), which may participate in neuronal plasticity or in cell signaling (Ghiani et al. 2007; Lou et al. 2008).
The subtype of NR is involved in activity-dependent synaptic plasticity, such as associative long-term potentiation (Bliss and Collingridge 1993), and in related central functions, such as learning and memory (Bliss and Collingridge 1993; Nakazawa et al. 2002; McHugh et al. 2007). In the rat hippocampus, NR1 subunit is mainly expressed in the pyramidal neurons, dentate granule cells, and some interneurons (Monyer et al. 1994; Gottlieb and Matute 1997). There are reports that activation of NR1 increases neurogenesis in the hippocampus (Kitamura et al. 2003; Joo et al. 2007; Maeda et al. 2007). However, there are some contradictory results about the effects of NR blockers on cell proliferation in the hippocampus of adult rodents. For example, blockade of NRs by antagonists increases cell proliferation in the hippocampus (Nacher and McEwen 2006; Nácher et al. 2007). In contrast, NR blockers inhibit seizure- and stroke-induced proliferation of neural progenitor cells in the hippocampus (Arvidsson et al. 2001).
In the present study, we investigated the effects of treadmill exercise on NR1 expression and neuroblast in the DG of rats. In addition, we also examined the effect of an NR blocker on neuroblast differentiation in treadmill-exercised rats.
Experimental Procedure
Experimental Animals
Male Wistar rats were purchased from Orient Bio Inc. (Seongnam, South Korea). They were housed in a conventional state under adequate temperature (23°C) and humidity (60%) control with a 12-h light/12-h dark cycle, and free access to food and water. The procedures for handling and caring for the animals adhered to the guidelines that are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85–23, 1985, revised 1996). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
Experimental Design for Treadmill Exercise
Male Wistar rats were randomly divided into 2 groups: sedentary (n = 12) and exercise (n = 12) groups. At 6 weeks of age, rats in both groups were familiarized with the treadmill running on a motorized treadmill (Model 1050 LS Exer3/6; Columbus Instruments, Columbus, OH) for 15 min/day at 15 m/min for 5 consecutive days. After the familiarization, electrical stimulation to encourage the rats to run was disconnected to avoid pain stress. The rats were run for 1 h/day/5 consecutive days at 22 m/min for 5 weeks and the speeds accelerated 2 m/min per 2 weeks. The sedentary rats were put on the treadmill without running for 1 h/day/5 consecutive days/5 weeks. Body weight over the course of the study was determined for each animal by summing the weekly averages.
Treatment with MK-801
To elucidate the effects of NR antagonist on the neuroblasts in exercised animals, the animals were divided into 2 groups (n = 7 in each group); vehicle (physiological saline) and 1 mg/kg MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate] (Sigma)-treated group received intraperitoneal injection once daily 30 min after exercise for last 1 week. The sedentary group was not included for experimental schedule to reduce the number of animals, and significant increase of NR1 expression and neuroblast numbers were identified.
Immunohistochemistry for NR1 and DCX
For immunohistological analysis, seven animals in each group were anesthetized with sodium pentobarbital and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were removed and postfixed in the same fixative for 6 h. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter, the frozen tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30-μm coronal sections, and they were then collected into six-well plates containing PBS.
For NR1 immunohistochemistry, sections were treated with pepsin (DAKO, Carpinteria, CA). Before pepsin pretreatment, 0.2 N HCl was warmed up to 37°C in a water bath. Frozen aliquots containing 100 mg/ml of pepsin solution were thawed just before use, and added to 0.2 N HCl. Then, the sections pre-warmed at 37°C in distilled water were transferred to the pepsin/HCl solution. After a given incubation time, the sections were briefly washed with PBS, and processed for immunohistochemistry (Watanabe et al. 1998). The sections were treated with 10% normal goat or rabbit serum in 0.05 M PBS for 30 min. They were then incubated with diluted rabbit anti-NR (1:1,000, Chemicon International, Temecula, CA) and goat anti-DCX (1:50, SantaCruz Biotechnology, Santa Cruz, CA) overnight at room temperature and subsequently exposed to biotinylated goat anti-rabbit or rabbit anti-goat IgG and streptavidin peroxidase complex (1:200, Vector, Burlingame, CA). They were then visualized with reaction to 3,3′-diaminobenzidine tetrachloride (Sigma, St. Louis, CA) in 0.1 M Tris–HCl buffer (pH 7.2) and mounted on gelatin-coated slides. The sections were mounted in Canada Balsam (Kanto, Tokyo, Japan) following dehydration.
For calculating numbers of DCX-immunoreactive cells in the DG, 15 sections were selected corresponding to Bregma −3.00 to −4.08 mm of rat brain atlas (Paxinos and Watson 2005). Images of all DCX-immunoreactive structures were taken from the DG through a BX51 light microscope (Olympus, Japan) equipped with a digital camera (DP71, Olympus, Japan) connected to a PC monitor. The number of DCX positive cells in the DG was counted by Optimas 6.5 software (CyberMetrics, Scottsdale, AZ). Data were represented by total DCX positive cells per animal.
Western Blot Analyses
To confirm changes in NR1 levels in the hippocampus, animals (n = 5 in each group) were killed and used for the western blot analysis. After killing them and removing the hippocampus, the tissues were homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol bis (2-aminoethyl ether)-N,N,N′,N′ tetraacetic acid (EGTA) (pH 8.0), 0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (EDTA) (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM dithiothreitol (DTT). After centrifugation, the protein level was determined in the supernatants using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, Rockford, IL). Aliquots containing 20 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. The aliquots were then loaded onto a 10% polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min, followed by incubation with rabbit anti-NR1 (1:50, Chemicon International), peroxidase-conjugated goat anti-rabbit IgG and ECL kit (Pierce Chemical, Rockford, IL). Loading controls were performed using antibodies against beta actin (Sigma). The results of the western blot analysis were scanned, and RODs were determined using Scion Image software (Scion Corp., Frederick, MD).
Statistical Analysis
The data shown here represent the means of experiments performed for each experimental area. Differences among the means were statistically analyzed by Student t-test in order to elucidate differences between each group.
Results
Effects of Treadmill Exercise on NR Immunoreactivity and Protein Level
In both the sedentary and exercise groups, NR1 immunoreaction was detected in the hippocampus (Fig. 1a, b). In the sedentary group, NR1 immunoreactivity was weak in the DG granule cell layer and in the pyramidal cell layer of the hippocampal CA3 region (CA3) (Fig. 1c, e); while, in the CA1, NR1 immunoreactivity was relatively moderate (Fig. 1g). In the exercised group, NR1 immunoreactivity was markedly increased in all subregions of the hippocampus (Fig. 1b). In the DG, strong NR1 immunoreactivity was observed in granule cells of the granule cell layer and in polymorphic cells of the polymorphic cell layer (Fig. 1c), and in the CA1-3, NR1 immunoreactivity was notably increased in pyramidal cells of the pyramidal cell layer (Fig. 1f, h). In addition, exercise markedly increased NR1 protein level in the hippocampus compared to that in the sedentary (Fig. 2).
Fig. 1.
Photomicrographs of NR1 immunoreactivity in the hippocampus in sedentary (a, c, e, and g) and exercised (b, d, f, and h) rats for 5 weeks. NR1 immunoreactivity (arrows) in the exercised rat is higher in all the subregions than that in the sedentary rat. CA cornus ammonis, DG dentate gyrus, GCL granule cell layer, ML molecular layer, PL polymorphic layer, SO stratum oriens, SP stratum pyramidale, SR stratum radiatum. Bar = 200 μm (a and b), 50 μm (c–h)
Fig. 2.
Western blot analysis of NR1 in the hippocampus of the sedentary exercise group. Relative optical density as percentage of immunoblot band is also represented (n = 5 per group; * P < 0.05, significantly different from the sedentary group). The bars indicate the means ± SE
Effect of Treadmill Exercise on DCX Immunoreactivity
In sedentary and exercised groups, DCX immunoreaction was detected in the subgranular zone of the DG (Fig. 3a–d); however, the number of DCX-immunoreactive cells dendrites differed significantly between groups (Fig. 4). In the exercised group, the absolute number of DCX-immunoreactive cells was significantly increased compared to that in the sedentary group (Figs. 3c, d, 4).
Fig. 3.
Photomicrographs of DCX immunoreactivity in the DG in sedentary (a and b) and exercised (c and d) rats for 5 weeks. DCX-immunoreactive cells are abundant in the exercised rat compared to that in the sedentary rat. GCL granule cell layer, PL polymorphic layer. Bar = 100 μm (a and c), 25 μm (b and d)
Fig. 4.
Analysis of DCX-immunoreactive cells in the DG of sedentary and exercised rats (n = 7 per group; * P < 0.05, significantly different from the sedentary group). The bars indicate the means ± SEM
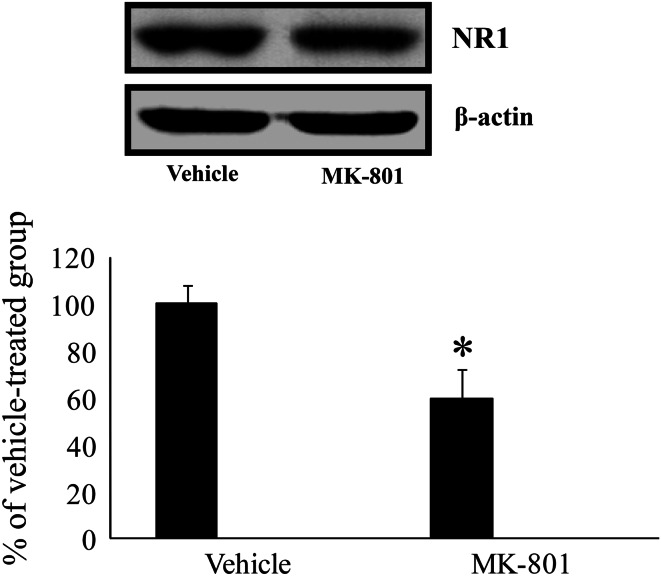
Effect of MK-801 on NR1 Protein Level
In the western blot analysis, NR1 protein level in the hippocampus of the treadmill-exercised group was significantly lower than that in the vehicle-treated group (Fig. 5).
Fig. 5.
Western blot analysis of NR1 in the hippocampus of the vehicle- and MK-801-treated group. Relative optical density as % of immunoblot band is also represented (n = 5 per group; * P < 0.05, significantly different from the vehicle-treated group). The bars indicate the means ± SEM
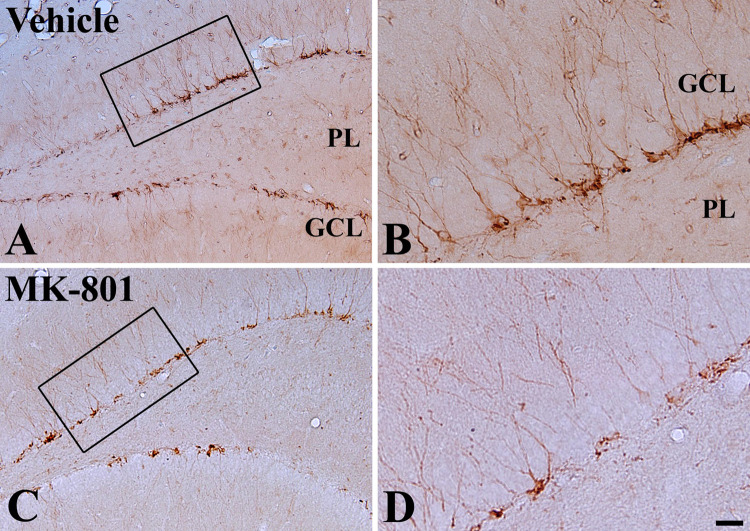
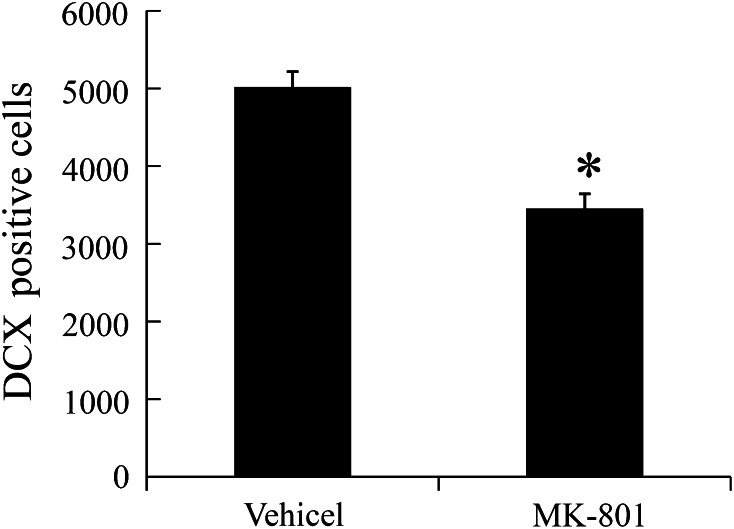
Effect of MK-801 on DCX Immunoreactivity
In both the vehicle- and MK-801-treated groups, DCX immunoreaction was detected in the subgranular zone of the DG (Fig. 6a–d). In the vehicle-treated group, DCX-immunoreactive cells were abundant (Fig. 6a, b). In the MK-801-treated group, the number of DCX-immunoreactive cells was significantly decreased compared to that in the vehicle-treated group (Figs. 6c, d, 7).
Fig. 6.
Photomicrographs of DCX immunoreactivity in the DG in vehicle (a and b) and MK-801 (c and d) treated exercised rats for 5 weeks. DCX-immunoreactive cells are less in the MK-801-treated rat compared to those in the vehicle-treated rat. GCL granule cell layer, PL polymorphic. Bar = 100 μm (a and c), 25 μm (b and d)
Fig. 7.
Analysis of DCX-immunoreactive cells in the DG of vehicle- and MK-801-treated exercised rats (n = 5 per group; * P < 0.05, significantly different from the vehicle-treated group). The bars indicate the means ± SEM
Discussion
There are several parameters that cause changes in neurogenesis in the adult hippocampus; for example, enriched environments or hippocampus-dependent learning increases neurogenesis (Gould et al. 1997; Kempermann et al. 1997), while aging or psychosocial stress decreases neurogenesis. In addition, growing evidence shows that the physiological activity of synaptic NRs promotes neuronal survival (Ciani et al. 1997; Ikonomidou et al. 1999, 2000) while loss of NR function in the hippocampus significantly impairs learning and memory (Huerta et al. 2000; Rampon et al. 2000; Nakazawa et al. 2002; McHugh et al. 2007).
Previous studies showed that low to moderate intensity treadmill running enhanced the expression of hippocampal NR1 mRNA (Lou et al. 2008) and NR2B mRNA in the DG (Farmer et al. 2004). In addition, exercise increased the capacity for the DG to express long-term potentiation in vitro and in vivo in adult animals (van Praag et al. 1999; Farmer et al. 2004; Christie et al. 2005). Similar to these studies, in the present study, we observed that NR1 expression significantly increased in the hippocampus after treadmill exercise.
In addition, we also observed the effects of exercise on neuroblast differentiation in the subgranular zone of the DG using DCX. DCX is frequently used as a marker for newly generated neurons because DCX expression is associated with the migration and differentiation of neuronal precursors (Francis et al. 1999; Gleeson et al. 1999). Treadmill exercise for 5 weeks significantly increased the number of DCX-immunoreactive cells in the DG. It was reported that treadmill exercise increased cell proliferation and differentiation in the DG and reduced blood glucose levels in a rat model of type II diabetes (Yi et al. 2009). These indicate that treadmill exercise could increase neuroblasts in the brain. On the other hand, treatment with celecoxib, a cyclooxygenase inhibitor which is a rate-limiting enzyme in synthesis of prostaglandins from arachidonic acid, into type 2 diabetic rats with treadmill exercise significantly decreases neuroblasts in the subgranular zone of the DG (Hwang et al. 2010).
In the present study, to investigate the relationship between NR and neuroblast differentiation in exercised rats, we treated MK-801 into the exercised rats. Treatment with MK-801 effectively reduced NR1 protein in the exercised rat DG. In a previous study, it was reported that subchronic MK 801 treatment (1 mg/kg) reduced NR1 mRNA level in the brain (Xi et al. 2009).
MK-801 treatment significantly reduced DCX-immunoreactive cells in the DG of the exercised rats. This result suggests that blockade of NR1 could reduce proliferation and differentiation of neuroblast in the hippocampus. Our result is supported by previous studies showing that neurogenesis was suppressed in the hippocampus of NR1 ε1 subunit knockout mice (Kitamura et al. 2003) and BrdU+ and S-phase cells were significantly decreased by MK-801 treatment (Joo et al. 2007; Maeda et al. 2007). MK-801 treatment reduced polysialylated neuronal cell adhesion molecule in the neuroblast of developing mouse striatum (Butler et al. 1998). Conversely, activation of NR on proliferating progenitors induced neuronal differentiation of adult-derived neural progenitor cells in vitro, and NR increased the fraction of microtubule associated protein-2-positive cells, suggesting that the maturation of post-mitotic neurons was altered by NR activation (Joo et al. 2007). In addition, MK-801 suppresses the increased neurogenesis following 2 h of focal ischemic insults by middle cerebral artery occlusion (Arvidsson et al. 2001).
However, some studies showed contradictory results in terms of the effects of MK-801 on neurogenesis, which may be due to differences in animal models used. In physiologically normal rodents, administration of MK-801 increased the density of [3H]thymidine-labeled cells in the DG of rats (Cameron et al. 1995) and the number of BrdU-labeled cells in tree shrews (Gould et al. 1997). In a photothrombosis model, MK-801 application during lesion induction significantly enhanced neurogenesis in the DG (Kluska et al. 2005).
In conclusion, exercise enhanced NR1 expression and neuroblast differentiation in the DG of the rat, and MK-801 treatment significantly reduced NR1 expression, neuroblast differentiation in exercised rats.
Acknowledgments
The authors would like to thank Mr. Seok Han, Mr. Seung Uk Lee, and Ms. Hyun Sook Kim for their technical help in this study. This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0010580), and by a grant (2010K000823) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, the Republic of Korea.
Footnotes
Jung Hoon Choi and Ki-Yeon Yoo contributed equally to this article.
Contributor Information
In Koo Hwang, Phone: +82-2-880-1271, FAX: +82-2-880-1213, Email: vetmed2@snu.ac.kr.
Moo-Ho Won, Phone: +82-33-250-8891, FAX: +82-33-256-1614, Email: mhwon@kangwon.ac.kr.
References
- Arvidsson A, Kokaia Z, Lindvall O (2001) N-methyl-d-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci 14(1):10–18 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361(6407):31–39 [DOI] [PubMed] [Google Scholar]
- Butler AK, Uryu K, Chesselet MF (1998) A role for N-methyl-d-aspartate receptors in the regulation of synaptogenesis and expression of the polysialylated form of the neural cell adhesion molecule in the developing striatum. Dev Neurosci 20(2–3):253–262 [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E (1995) Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci 15(6):4687–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A (2005) Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci 21(6):1719–1726 [DOI] [PubMed] [Google Scholar]
- Ciani E, Rizzi S, Paulsen RE, Contestabile A (1997) Chronic pre-explant blockade of the NMDA receptor affects survival of cerebellar granule cells explanted in vitro. Brain Res Dev Brain Res 99(1):112–117 [DOI] [PubMed] [Google Scholar]
- Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, Pompeu FA, Coutinho ES, Laks J (2009) Exercise and mental health: many reasons to move. Neuropsychobiology 59(4):191–198 [DOI] [PubMed] [Google Scholar]
- Devine JM, Zafonte RD (2009) Physical exercise and cognitive recovery in acquired brain injury: a review of the literature. PM R 1(6):560–575 [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR (2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124(1):71–79 [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J (1999) Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23(2):247–256 [DOI] [PubMed] [Google Scholar]
- Garcez RC, Teixeira BL, Schmitt Sdos S, Alvarez-Silva M, Trentin AG (2009) Epidermal growth factor (EGF) promotes the in vitro differentiation of neural crest cells to neurons and melanocytes. Cell Mol Neurobiol 29(8):1087–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Beltran-Parrazal L, Sforza DM, Malvar JS, Seksenyan A, Cole R, Smith DJ, Charles A, Ferchmin PA, de Vellis J (2007) Genetic program of neuronal differentiation and growth induced by specific activation of NMDA receptors. Neurochem Res 32(2):363–376 [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23(2):257–271 [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Matute C (1997) Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab 17(3):290–300 [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17(7):2492–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S (2000) Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 25(2):473–480 [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Kim IY, Kim YN, Song W, Moon SM, Won MH, Seong JK, Yoon YS (2010) Effects of treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2 diabetic rats: correlation with the neuroblasts. Brain Res 1341:84–92 [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283(5398):70–74 [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Stefovska V, Turski L (2000) Neuronal death enhanced by N-methyl-d-aspartate antagonists. Proc Natl Acad Sci USA 97(23):12885–12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaako-Movits K, Zharkovsky A (2005) Impaired fear memory and decreased hippocampal neurogenesis following olfactory bulbectomy in rats. Eur J Neurosci 22(11):2871–2878 [DOI] [PubMed] [Google Scholar]
- Joo JY, Kim BW, Lee JS, Park JY, Kim S, Yun YJ, Lee SH, Rhim H, Son H (2007) Activation of NMDA receptors increases proliferation and differentiation of hippocampal neural progenitor cells. J Cell Sci 120(Pt 8):1358–1370 [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10(3):355–362 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1997) Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA 94(19):10409–10414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H (2003) Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res 47(1):55–63 [DOI] [PubMed] [Google Scholar]
- Kluska MM, Witte OW, Bolz J, Redecker C (2005) Neurogenesis in the adult dentate gyrus after cortical infarcts: effects of infarct location, N-methyl-d-aspartate receptor blockade and anti-inflammatory treatment. Neuroscience 135(3):723–735 [DOI] [PubMed] [Google Scholar]
- Kong X, Li X, Cai Z, Yang N, Liu Y, Shu J, Pan L, Zuo P (2008) Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell Mol Neurobiol 28(4):569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Kamienkowski JE, Espósito MS, Piatti VC, Zhao C, Gage FH, Schinder AF (2007) Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci 25(10):2973–2981 [DOI] [PubMed] [Google Scholar]
- Lou SJ, Liu JY, Chang H, Chen PJ (2008) Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res 1210:48–55 [DOI] [PubMed] [Google Scholar]
- Maeda K, Sugino H, Hirose T, Kitagawa H, Nagai T, Mizoguchi H, Takuma K, Yamada K (2007) Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J Pharmacol Sci 103(3):299–308 [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S (2007) Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317(5834):94–99 [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12(3):529–540 [DOI] [PubMed] [Google Scholar]
- Nacher J, McEwen BS (2006) The role of N-methyl-d-aspartate receptors in neurogenesis. Hippocampus 16(3):267–270 [DOI] [PubMed] [Google Scholar]
- Nácher J, Varea E, Miguel Blasco-Ibáñez J, Gómez-Climent MA, Castillo-Gómez E, Crespo C, Martínez-Guijarro FJ, McEwen BS (2007) N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience 144(3):855–864 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S (2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297(5579):211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendácová J, Raceková E, Orendác M, Martoncíková M, Saganová K, Lievajová K, Abdiová H, Labun J, Gálik J (2009) Immunohistochemical study of postnatal neurogenesis after whole-body exposure to electromagnetic fields: evaluation of age- and dose-related changes in rats. Cell Mol Neurobiol 29(6–7):981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paizanis E, Kelaï S, Renoir T, Hamon M, Lanfumey L (2007) Life-long hippocampal neurogenesis: environmental, pharmacological and neurochemical modulations. Neurochem Res 32(10):1762–1771 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, 5th edn. Elsevier Academic Press, Boston, Amsterdam [Google Scholar]
- Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A (2004) Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res 76(2):223–231 [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA (2006) Integration of new neurons into functional neural networks. J Neurosci 26(47):12237–12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ (2000) Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci 3(3):238–244 [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2(3):266–270 [DOI] [PubMed] [Google Scholar]
- Veena J, Srikumar BN, Raju TR, Shankaranarayana Rao BS (2009) Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett 455(3):178–182 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y (1998) Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci 10(2):478–487 [DOI] [PubMed] [Google Scholar]
- Xi D, Zhang W, Wang HX, Stradtman GG, Gao WJ (2009) Dizocilpine (MK-801) induces distinct changes of N-methyl-d-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. Int J Neuropsychopharmacol 12(10):1395–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W, Lee IS, Won MH, Seong JK, Yoon YS (2009) Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res 34(6):1039–1046 [DOI] [PubMed] [Google Scholar]