Abstract
It is reported that adipose-derived stem cells (ADSCs) had multilineage differentiation potential, and could differentiate into neuron-like cells induced by special induction media, which may provide a new idea for restoration of erectile dysfunction (ED) after cavernous nerve injury. The aim of this research was to explore the neuronal differentiation potential of ADSCs in vitro. ADSCs isolated from inguinal adipose tissue of rat were characterized by flow cytometry, and results showed that ADSCs were positive for mesenchymal stem cell markers CD90 and CD44, but negative for hematopoietic stem cell markers. ADSCs maintained self-renewing capacity and could differentiate into adipocytes and neurocytes under special culture condition. In this research, two methods were used to induce ADSCs. In method 1, ADSCs were treated with the preinduction medium including epithelium growth factor, basic fibroblast growth factor, and brain derived neurotrophic factor (BDNF) for 3 days, then with the neurogenic induction medium containing isobutylmethylxanthine, indomethacin, and insulin. While in method 2, BDNF was not used to treat ADSCs. After induction, neuronal differentiation of ADSCs was evaluated. Neuronal markers, glial fibrillary acidic protein (GFAP), and β-tubulin III (Tuj-1) were detected by immunofluorescence and Western Blot analyses. The expressions of GFAP and Tuj-1 in method 1 were obviously higher then those in method 2. In addition, the positive rate of the neuron-like cells was higher in method 1. It suggested that ADSCs are able to differentiate into neural-like cells in vitro, and the administration of BDNF in the preinduction medium may provide a new way to modify the culture method for getting more neuron-like cells in vitro.
Keywords: Mesenchymal stem cells, Adipose-derived stem cells, Neuronal differentiation, Neuron-like cells
Introduction
More and more researches have focused on the human stem cells since it was first reported in 1998 (Thomson et al. 1998), and stem cell-based therapies for the repair and regeneration of various tissues and organs offer a paradigm shift that may lead to alternative therapeutic solutions for a number of diseases (Niapour et al. 2011; Corcos et al. 2011; Matthay et al. 2010). Erectile dysfunction (ED) is a pathological phenomenon which is difficult to achieve and maintain the penis to complete sexual intercourse. In western developed countries, prostate cancer is the second most malignant tumor in the male. For patients with localized prostate cancer, radical prostatectomy is probably the preferred selection. However, this surgical procedure decreases life quality of post-operation (Burnett et al. 2008). ED is the most common problem in prostate cancer patients after operation. An investigation indicated that nearly 71–89 % of patients undergoing radical prostatectomy suffered from ED at 2–5 years after operation, whose life quality decreased greatly (Penson et al. 2008). It is generally accepted that cavernous nerves (CNs) injury is the main cause of ED (Sezen et al. 2009). Because the neuronal cell has poor ability of self-renewal, it is difficult to return function to the damaged nerve. Recently regeneration therapy transplanting progenitor cells into necrotized tissues has been applied to various diseases, especially nervous system diseases. Various stem cells have been examined to obtain transplantable progenitors of neural cells for nerve regeneration therapy (Niapour et al. 2011; Maioli et al. 2011; Bae et al. 2011). However, many problems have emerged, for example: the insufficient cell population, problems of histocompatibility, inadequate tissue supply, and ethical concerns (Gottlieb 2002). Among various adult stem cells, adipose-derived stem cells (ADSCs) can differentiate into not only adipogenic, endotheliogenic, chondrogenic, cardiogenic, myogenic, and osteogenic cells, but also neuron-like cells in vitro under special culture conditions (Nagasaki et al. 2011; Lin et al. 2009; An et al. 2010; Madonna et al. 2009; WU et al. 2010; Huang et al. 2007). In addition, adipose tissues are plentiful and relatively easily obtained. Therefore, ADSCs as a donor show many advantages for they can be easily obtained in ubiquitously distributed adipose tissue with little harm to patient and are expandable in vitro (Kim et al. 2008). However, whether ADSCs can be induced to differentiate into neuron-like cells in vitro and have positive effects on the ED has not been clearly demonstrated. This study aimed to explore the potential of neural differentiation in ADSCs.
Materials and Methods
Animals
All animal procedures were approved by Animal Use and Care Committee of WHU (China). Sprague–Dawley rats (3–4 weeks old) of both sexes were used for these studies. Activities related to animal care were performed according to standard operating procedures accepted by Chinese National Animal Research Center.
Isolation and Culture of ADSCs
Adipose tissue was harvested according to the published methods (Safford et al. 2002). Briefly, adipose tissue was aseptically collected from the subcutaneous fat of a 1-month-old experimental rat under anesthesia. Tissues were washed extensively with phosphate-buffered saline (PBS), minced, and digested with collagenase type I (1 mg/mL; Invitrogen, UK) at 37 °C for 1 h. Enzyme activity was neutralized with α-modified eagle’s medium (DMEM) (Gibco, USA) containing 10 % FBS (Gibco, USA) and centrifuged at 1,200 rpm (300 g) for 10 min to obtain a high-density cell pellet. The pellet was resuspended in red blood cell (RBC) lysis buffer (Sigma, USA) and incubated at room temperature for 10 min to lyse contaminating RBCs. The stromal cell pellet was collected by centrifugation, as described above, and incubated overnight in DMEM with 10 % fetal bovine serum (FBS; Gibco, USA) at 37 °C with 5 % humidified CO2. Unattached cells and residual non-adherent red blood cells were removed after 24 h by washing with PBS. Medium was changed at 72 h intervals until the cells became confluent. After cells reached 90 % confluence, they were trypsinized and subcultured at a density of 10,000 cells/cm2 (passage 1). Cells were passaged repeatedly after achieving a density of 80–90 % until passage 3.
Flow Cytometry
ADSCs were examined for surface markers by Flow Cytometry. The third passage of ADSCs were trypsinized, centrifuged and resuspended to concentration of about 1 × 106 cells for each test. Thus, 10 μl each of a prediluted PE-conjugated mouse anti-rat CD34 (AbD Serotec), a FITC-conjugated mouse anti-rat CD45, CD90, and CD44 (all AbD Serotec) antibody was used in individual test. Negative control staining was performed using a FITC-conjugated mouse IgG1 isotype and a PE-conjugated mouse IgG1 isotype antibody respective the primary antibodies.
Differentiation Test of ADSCs
ADSCs were differentiated in culture under the conditions described below.
Adipogenic Differentiation
ADSCs were initially cultured and propagated up to 80–90 % confluence and then shifted to adipogenic medium (adipogenic medium is the elements which can induce adipose-derived stem cells differentiate into adipose cells) [DMEM low-glucose medium with 10 % FBS, 3 μg/ml insulin, 1 μM dexamethasone, 120 μM indomethacin, and 300 μM isobutylmethylxanthine (all from Sigma)], then adipogenic medium replaced every 3 days. This procedure repeated four times for 12 days.
Neurogenic Differentiation
We modified published protocols based on N2 medium supplemented with valproic acid or retinoic acid/forskolin, or Neural Induction Medium (Huang et al. 2007; Safford et al. 2004; Ning et al. 2006). We used two different methods to induce ADSCs. Method 1: Neurogenic differentiation was induced by culturing ADSCs in preinduction medium [DMEM low-glucose medium supplemented with 10 % FBS and 10 ng/ml epithelium growth factor (EGF, PeproTech, UK), 20 ng/ml basic fibroblast growth factor (bFGF, PeproTech) and 10 ng/ml brain derived neurotrophic factor (BDNF, PeproTech)] for 3 days. After preinduction, the cells were induced for up to 48 h in neurogenic medium [DMEM low-glucose medium with 10 % FBS, 120 μM indomethacin, 3 μg/ml insulin, 300 μM isobutyl-methylxanthine (all from Sigma)]; Method 2 was the same as Method 1, but BDNF was not used in preinduction medium. The cells were analyzed by immunofluorescence and Western Blot staining for the expression of Tuj-1 and GFAP.
Oil Red O Staining
The accumulation of neutral lipids was detected by staining ADSCs in a solution of 0.5 % Oil red O. Cells were fixed in 4 % paraformaldehyde for 1 h after cultured for 12 days in a six-well plate. The sample was then washed with 60 % isopropanol (in PBS). It was stained with a 0.5 % oil red-O solution (in PBS) for 3 h and washed with distilled water. The sample was tested under an optical microscope.
Immunofluorescence Staining
Cells were fixed in 4 % paraformaldehyde (PFA) for 20 min after exposure to NIM for 48 h, and then permeabilized with 0.05 % Triton X-100 for 10 min. Nonspecific binding sites were blocked using 5 % goat serum for 1 h. Slides were incubated overnight at 4 °C with mouse anti-rat Tuj-1 (SANTA CRUZ, sc-80005) (1:50) and mouse anti-rat GFAP (1:50) (SANTA CRUZ, sc-166481) antibodies diluted in PBS. Slides were washed three times in PBS and incubated in goat anti-mouse secondary antibody (from serotec) (1:100) for 1 h at 37 °C. Slides were washed three times in PBS, and then incubated in 4,6′-diamidino-2-phenylindole dihydrochloride (DAPI, 1:1000) for 3–5 min at room temperature. Finally, Slides were washed three times in PBS and mounted. For the negative control, primary antibodies were omitted. Labeled cells were examined by fluorescence microscopy (Nikon Eclipse Ti-U, Japan). Images were digitally recorded and processed using Image-Pro Plus (Media Cybernetics, USA).
Western Blot
Protein extracts were prepared from undifferentiated or differentiated ADSCs for Western blot analysis by lysing cells in 1 Laemmli (1–4 % SDS, 10 % glycerol, 100 mM dithiothreitol, 50 mM Tris, pH 6.8). All samples were sonicated and boiled for 5 min. Total protein (35–45 μg/ml) was resolved on 12.5 % acrylamide gel and electroblotted onto polyvinyldiethylfluoride (PVDF) membrane. Blots were stained with amido black (0.5 % w/v in 5 % acetic acid) and destained with water to confirm transfer and equal loading. We blocked blots in Tris-buffered saline (0.8 % NaCl, 2.7 mM KCl, 25 mM Tris, pH 7.4) with 0.05 % Tween 20 (TBST) and 1 % milk for 1 h at room temperature. The blot was probed overnight at 4 °C with mouse anti-rat Tuj-1 (1:500) and anti-GFAP (1:1,000) antibodies. The blots were washed 3 × 5 min in TBS-T and then incubated with a 1:3,000 dilution of peroxidase-conjugated secondary antibody (Chemicon) in blocking solution for 1 h at room temperature. We developed the blots by enhanced chemiluminescence (Pierce, Inc., Rockford, IL). β-Actin served as an internal control. Rat cavernous nerves served as a positive control.
Statistical Analyses
Observation five visions under low magnification at random after Immunofluorescence, counting each vision 50 cells within the positive cells rate of GFAP and Tuj-1. Results are expressed as the mean ± SD of two methods. Statistical analyses were performed by one way repeated measure ANOVA (using SigmaStat 3.1, SigmaPlot 9.0). Student’s t-test was used to compare the results between two methods. A value of p < 0.05 was considered significant.
Results
Characterization of ADSCs
Morphology of ADSCs
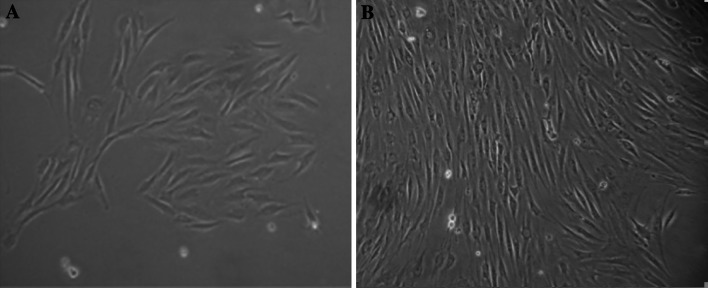
The primary ADSCs’ adherence after 24 h, exhibited a heterogeneous population of spindle-shaped cells morphologically (Fig. 1a). The third passage cells fusion to 80–90 % appeared as a monolayer of flat fibroblast-like cells under microscopy (Fig. 1b).
Fig. 1.
Morphology of ADSCs: a The primary ADSCs were cultured for 24 h, b The third passage ADSCs were 80–90 % confluent (×100)
Cell Surface Markers of ADSCs
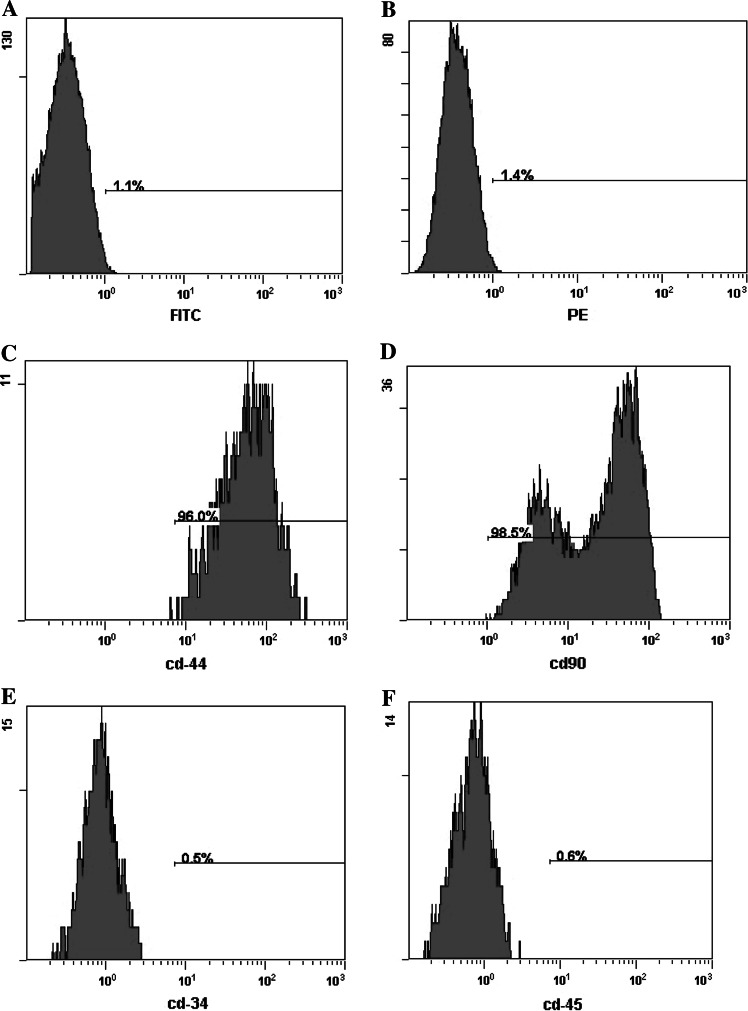
As shown in Fig. 2, the third passage of ADSCs expressed CD44 and CD90, and was negative for CD34 and CD45. The overwhelming majority (>95 %) of ADSCs expressed the mesenchymal cell surface markers CD90 and CD44.
Fig. 2.
The expression of mesenchymal stem cell surface-markers in ADSCs analyzed by flow cytometry a negative control for FITC-conjugated antibody, b negative control for PE-conjugated antibody, c CD44, d CD90, e CD34, f CD45
Multiplex Differentiation Capacity of ADSCs
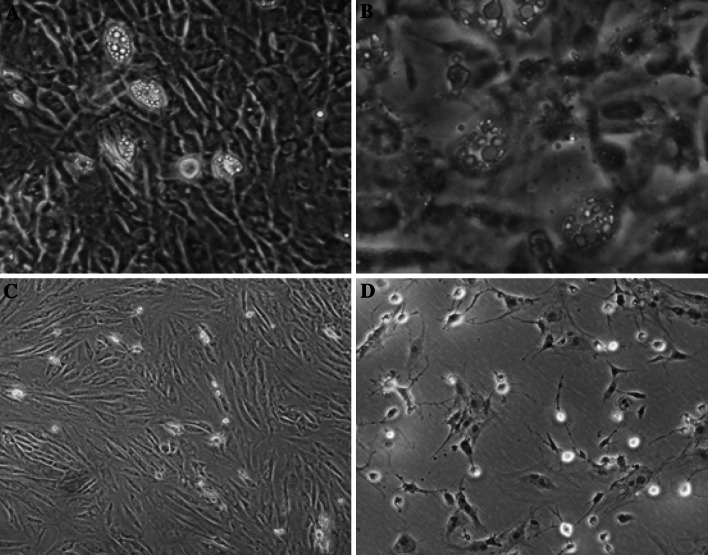
Adipogenic differentiation of ADSCs was apparent after 12 days of incubation with adipogenic medium. After 12 days, lipid droplets were seen in many cells under microscopy (Fig. 3a) and Oil red O-positive staining was observed (Fig. 3b). Before administration of neural differentiation medium, ADSCs displayed no changes in cellular morphology (Fig. 3c). After neurogenic differentiation induction, ADSCs displayed changes in cellular morphology including shrinkage of cytoplasm, formation of axons and dendrite-like cytoplasmic projections (Fig. 3d), and expressed the neuronal markers Tuj-1 (Fig. 4b, c) and GFAP (Fig. 4e, f) during neuronal differentiation in vitro.
Fig. 3.
Multiplex differentiation capacity of ADSCs: a Cells were cultured for 12 days in special adipogenic induction medium and lipid droplets could be observed (×100), b Lipid droplets in adipocytes were detected by oil-red O staining (× 200), c ADSCs were only treated with the preinduction medium (×100), d ADSCs were cultured in the neurogenic induction medium for 48 h after treated with the preinduction medium (×100)
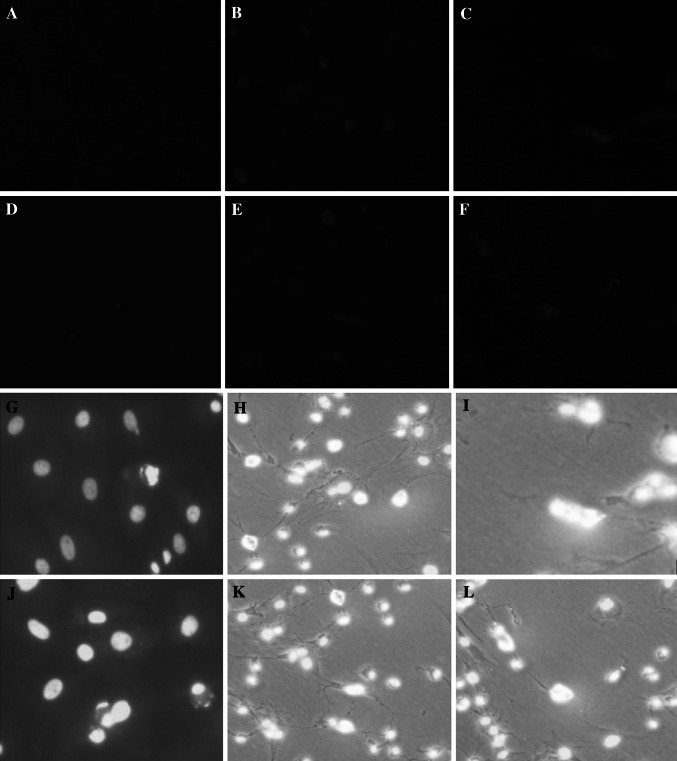
Fig. 4.
The expression of Tuj-1 and GFAP in ADSCs by two treatment methods detected by immunofluorescent staining (a–f) and nuclei were detected by DAPI (g–l). a, d ADSCs were treated only with the preinduction medium, b, e ADSCs were treated with method 1 for 48 h, then protein Tuj-1 and GFAP were detected, respectively, c, f ADSCs were treated with method 2 for 48 h, then protein Tuj-1 and GFAP were detected, respectively. In each group, the nuclei in ADSCs were stained at the same time (g–l)
Expression of Neural Markers in Differentiated ADSCs
Immunofluorescence Analysis
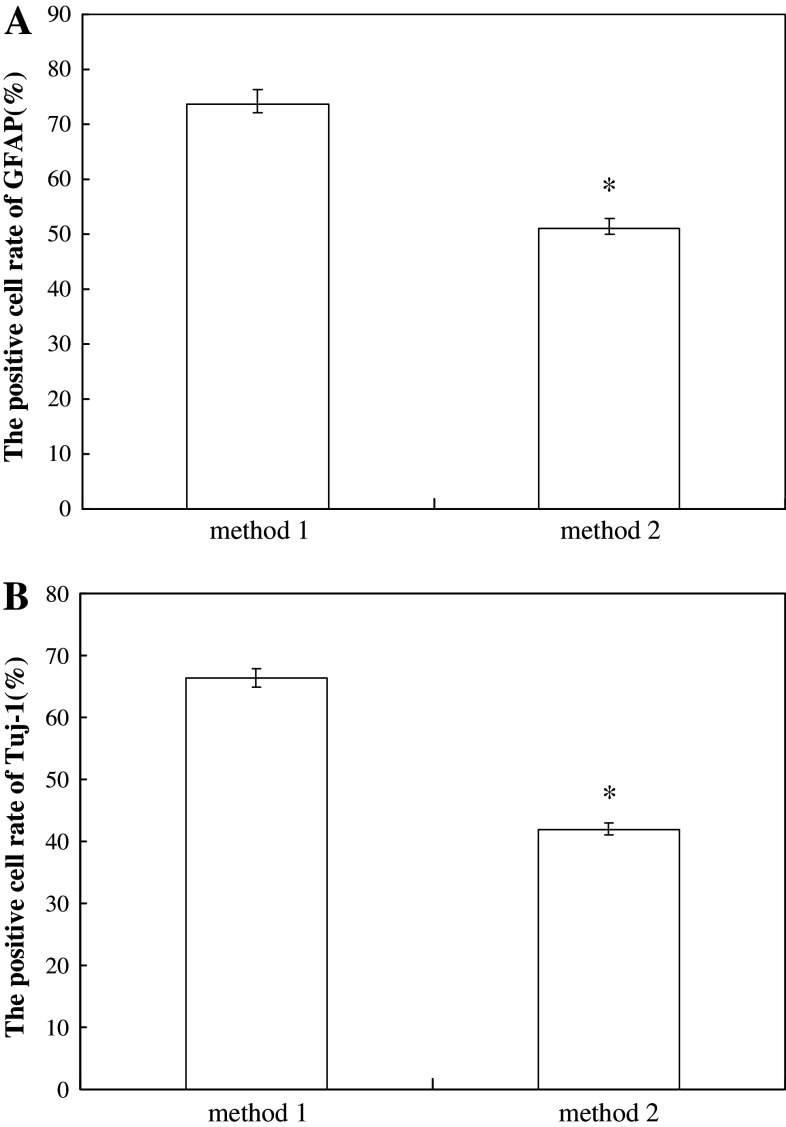
Expressions of neuron-like cell markers, GFAP and Tuj-1 were observed with the different induction time (Table 1). Immunofluorescence analyses indicated that expressions of GFAP and Tuj-1 of ADSCs in pre-induction medium were extremely low. After treatment of neuronal induction medium (NIM), expressions of GFAP and Tuj-1 were up-regulated with treatment time. GFAP emerged earlier than Tuj-1 in ADSCs with treatment of NIM for 6 h. The expression of GFAP showed robust expression in greater than 50 % of the cells after exposure to NIM for 24 and 48 h, while Tuj-1 showed low expression in more than 50 % of the cells after treatment for 24 h and moderate expression in greater than 50 % of the cells for 48 h. In method 1, the positive cell rates of GFAP and Tuj-1 with treatment as method 1 were much higher than those as method 2 (Fig. 5).
Table 1.
Expression of neural cell markers in rat ADSCs grown in preinduction medium and Exposed to Neural Induction Medium (NIM)
| Marker | Pre-induction | 6 h NIM | 24 h NIM | 48 h NIM |
|---|---|---|---|---|
| GFAP | ± | ++ | +++ | +++ |
| Tuj-1 | – | ± | + | ++ |
Changes of GFAP and Tuj-1 expression in ADSCs with treatment time increasing. Semi-quantitative assessment of marker expression was performed using the following criteria: +++, showed robust expression in more than 50 % cells; ++, showed moderate expression in more than 50 % cells; +, showed low expression in more than 50 % cells; ±, showed low expression in less than 50 % cells; −, showed no detectable expression
Fig. 5.
The positive rate of GFAP and Tuj-1 in ADSCs with two treatment methods. ADSCs were cultured for 48 h and the positive rates were shown as means ± SD. *P < 0.01, compared with the method 1 group
Western Blot Analysis
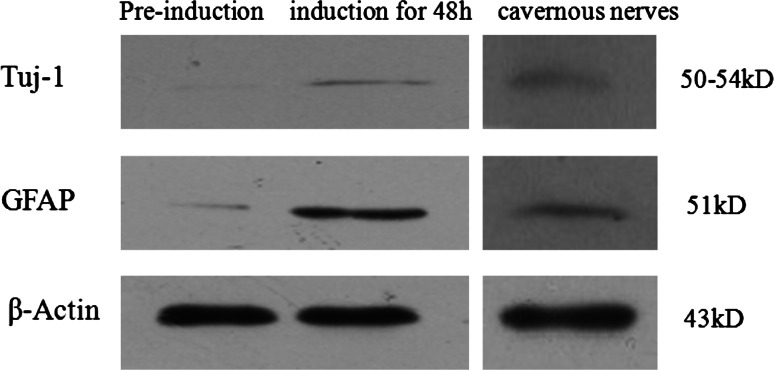
When ADSCs were treated with NIM for 48 h, expressions of GFAP and Tuj-1 increased obviously compared with those without treatment (Fig. 6). Rat cavernous nerves served as a positive control.
Fig. 6.
The expression of Tuj-1 and GFAP were detected by Western blots. Rat cavernous nerves served as a positive control and β-actin served as an internal control
Discussion
Our studies showed that rat ADSCs can be induced to differentiate into neuron-like cells. Using a series of immunofluorescence and Western blot experiments, we have found that rat ADSCs can be induced to undergo morphologic and phenotypic changes consistent with developing neuron-like cells. Isolation of ADSCs from adipose tissue was first reported by Zuk (Zuk et al. 2001). To identify the isolated ADSCs, Janq et al. (Janq et al. 2010) performed FACS analysis with various cell surface markers, including MSC-specific cell type markers and hematopoietic stem cell markers. The results showed that more than 95 % of the adipose tissue-derived stem cells expressed MSC-specific markers, including CD13, CD44, CD90, and CD166, but did not express markers for hematopoietic stem cells, such as CD14, CD34, and CD45. In our study, published methods (Lin et al. 2009) were used to isolate ADSCs and flow cytometry to identify ADSCs. Results showed that ADSCs could express not CD34 and CD45, but CD44 and CD90. It was suggested that ADSCs can be obtained by these methods. It is reported that ADSCs have the capacity to differentiate into multiple mesodermal lineages such as fat and nerve (Albersen et al. 2010; Yu et al. 2011). ADSCs were treated with adipogenic medium for 12 days, then Oil red O staining was used to detect lipid droplets. It was demonstrated that ADSCs could differentiate into adipocytes, which were consistent with the results of Nagasaki H et al. (Nagasaki et al. 2011).
It was reported that infant piglets and canine ADSCs were able to differentiate into neuron-like cells by neural induction medium (Huang et al. 2007; Sago et al. 2008). Janq et al. (Janq et al. 2010) and Yu et al. (Yu et al. 2007) also demonstrated that human ADSCs could differentiate into neuron-like cells, showing neuron-like cell morphology and expressing several proteins consistent with neuronal phenotype. In our study, with treatment of neural induction medium for 48 h, the shape of ADSCs changed from a fibroblast-like appearance to bipolar or multi-polar one; the expression of neural markers (GFAP and Tuj-1) were positive, which was similar to the changes detected in the developmental neurogenesis and gliogenesis.
Many different culture methods were used to induce ADSCs to differentiate into neuron-like cells. The induction media used by Kondo (Kondo et al. 2011) including bFGF, B27, Forskolin, isobutyl-methylxanthine, β-mercaptoethano, which could get higher induction rate, but Forskolin is too expensive to be widely used. Qian et al. (Qian et al. 2010) used β-mercaptoethano, BHA, DMSO for neurogenic differentiation of ADSCs, which got very low induction rate for DMSO caused cell toxicity. Yu et al. (Yu et al. 2007) treated ADSCs with BDNF and bFGF, which could not obtain enough neuronal-like cells. Other inducers such as indomethacin, isobutyl-methylxanthine, and insulin faced the same problem (Lin et al. 2009). In the present study, we used a two-step protocol to induce rat ADSCs to differentiate into neuron-like cells. The method 2 was the similar to what Wrage did (Wrage et al. 2008), and the method 1 was modification of the method 2. The only difference between two methods was that we added BDNF to the pre-induction medium in the method 1. Indomethacin and IBMX might work synergistically and upregulate the intracellular cAMP level, which can promote ADSCs to neural differentiation (Ning et al. 2006). EGF and bFGF were known as important factors for cell proliferation and differentiation. Therefore, in both methods ADSCs could be induced to differentiate into neuron-like cells. BDNF played major roles in the nerve development and adult neuroplasticity; it regulated neuronal survival, neurogenesis, neurite outgrowth, and synaptic plasticity. The mechanism may be BDNF binding with trkB-t activates a signaling pathway (involving a G-protein and protein kinase C) that induced neural stem cells to become glial progenitors and astrocytes (Cheng et al. 2007). In method 1, the positive cells rate of GFAP and Tuj-1 were (73.7 ± 2.5) % and (66.3 ± 1.5) %, respectively, while in method 2, they were (51 ± 2.0) % and (42 ± 1.0) %. The induction rate of method 1 was obviously higher than that in method 2. It was suggested that BDNF play the important role during the neural differentiation of ADSCs. High induction rate of ADSCs neural differentiation could guarantee sufficient neuron-like cells, which is foundation to restore erectile dysfunction following cavernous nerves (CNs) injury.
In conclusion, we have demonstrated that rat adipose tissue contains a population of stem cells, which could differentiate into neuron-like cells. We used BDNF as an inducer to get higher induction rate of ADSCs neural differentiation. We have established a rat model of ED following CN injury (Hu et al. 2010), our further research is to repair the injured CN with neuronal cells from ADSCs in a rat model.
Acknowledgments
This work was supported by the grants from the Natural Science Foundation of China (No. 81070483)
References
- Albersen M, Fandel TM, Lin G et al (2010) Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med 7:3331–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Cheng Y, Yuan Q et al (2010) IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng 38:1647–1654 [DOI] [PubMed] [Google Scholar]
- Bae KS, Park JB, Kim HS et al (2011) Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J 52:401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AL, Teloken PE, Briganti A et al (2008) Intraoperative assessment of an implantable electrode array for cavernous nerve stimulation. J Sex Med 5:1949–1954 [DOI] [PubMed] [Google Scholar]
- Cheng A, Coksayqan T, Tanq H et al (2007) Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J Neurochem 100:1515–1530 [DOI] [PubMed] [Google Scholar]
- Corcos J, Loutochin O, Campeau L et al (2011) Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn 30:447–455 [DOI] [PubMed] [Google Scholar]
- Gottlieb DI (2002) Large-scale sources of neural stem cells. Ann Rev Neurosci 25:381–407 [DOI] [PubMed] [Google Scholar]
- Hu W, Cheng B, Liu T et al (2010) Erectile function restoration after repair of excised cavernous nerves by autologous vein graft in rats. J Sex Med 7:3365–3372 [DOI] [PubMed] [Google Scholar]
- Huang T, He D, Kleiner G et al (2007) Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J Spinal Cord Med 30:S35–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janq S, Cho HH, Cho YB et al (2010) Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol 11:25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee MR, Kim JH et al (2008) IFATS collection: selenium induces improvement of stem cell behaviors in human adipose-tissue stromal cells via SAPK/JNK and stemness acting signals. Stem Cells 26:2724–2734 [DOI] [PubMed] [Google Scholar]
- Kondo T, Matsuoka AJ, Shimomura A et al (2011) Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem Cells 29:836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Banie L, Ning H et al (2009) Potential of adipose-derived stem cells for treatment of erectile dysfunction. J Sex Med 6:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna R, Geng YJ, De Caterina R et al (2009) Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol 29:1723–1729 [DOI] [PubMed] [Google Scholar]
- Maioli M, Rinaldi S, Santaniello S et al (2011) Radio frequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Tansplant. doi:10.3727/096368911X600966 [DOI] [PubMed]
- Matthay MA, Thompson BT, Read EJ et al (2010) Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 138:965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki H, Shang Q, Suzuki T et al (2011) Low-serum culture system improves the adipogenic ability of visceral adipose tissue-derived stromal cells. Cell Biol Int 35:559–568 [DOI] [PubMed] [Google Scholar]
- Niapour A, Karamali F, Nemati S et al (2011) Co-transplantation of human embryonic stem cell-derived neural progenitors and schwann cells in a rat spinal cord contusion injury model elicits a distinct neurogenesis and functional recovery. Cell Tansplant. doi:10.3727/096368911X593163 [DOI] [PubMed]
- Ning H, Lin G, Lue TF et al (2006) Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation 74:510–518 [DOI] [PubMed] [Google Scholar]
- Penson DF, McLerran D, Feng Z et al (2008) 5-Year urinary and sexual outcomes after radical prostatectomy: Results from the Prostate Cancer Outcomes Study. J Urol 179:S40–S44 [DOI] [PubMed] [Google Scholar]
- Qian DX, Zhang HT, Ma X et al (2010) Comparison of the efficiencies of three neural induction protocols in human adipose stromal cells. Neurochem Res 35:572–579 [DOI] [PubMed] [Google Scholar]
- Safford KS, Hicok KC, Safford SD et al (2002) Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 294:371–379 [DOI] [PubMed] [Google Scholar]
- Safford KM, Safford SD, Gimble JM et al (2004) Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol 187:319–328 [DOI] [PubMed] [Google Scholar]
- Sago K, Tamahara S, Tomihari M et al (2008) In vitro differentiation of canine celiac adipose tissue-derived stromal cells into neuronal cells. J Vet Med Sci 70:353–357 [DOI] [PubMed] [Google Scholar]
- Sezen SF, Lagoda G, Burnett AL (2009) Role of immunophilins in recovery of erectile function after cavernous nerve injury. J Sex Med 6:340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- Wrage PC, Tran T, To K et al (2008) The neuro-glial properties of adipose-derived adult stromal (ADAS) cells are not regulated by Notch 1 and are not derived from neural crest lineage. PLoS One 3:1453–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU G, Zheng X, Jiang Z et al (2010) Induced differentiation of adipose-derived stromal cells into myoblasts. J Huazhong Univ Sci Technolog Med Sci 30:285–290 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920 [DOI] [PubMed] [Google Scholar]
- Yu JM, Bunnell BA, Kang SK et al (2011) Neural differentiation of human adipose tissue-derived stem cells. Methods Mol Biol 702:219–231 [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–218 [DOI] [PubMed] [Google Scholar]