Abstract
Multiple sclerosis (MS) was characterized with widespread demyelination and axonal loss of central nervous system (CNS). Fibrinogen (fibrin) deposition was considered as one of the pathogenesis of MS. Therefore, we explored the effects of fibrinogen depleting agent batroxobin in experimental autoimmune encephalomyelitis (EAE) mice model. Our study showed that prevention and suppression with batroxobin significantly ameliorated clinical severity of EAE, reduced inflammatory cells infiltration, and demyelination, and suppressed the activation of astrocytes and macrophages comprising the CD11b+ population. Batroxobin treatment leads to reduced expression of p-Akt and increased expression of MBP as compared to control. In addition, batroxobin treatment partly reversed the dendric-like formation of macrophages irritated by fibrinogen in vitro. The reduced severity of EAE mice treated with batroxobin suggests that strategy targeting fibrin as a potential therapy for EAE may be beneficial for the treatment of MS patients.
Keywords: Multiple sclerosis, Fibrinogen, Fibrin, Experimental autoimmune encephalomyelitis
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of central nervous system (CNS), characterized with pathologically a range of inflammatory process including destruction of myelin sheaths, axonal damage, glial cells proliferation as well as fibrin deposition, and so on. Fibrin, as the final product of the coagulation process, plays not only a major role in blood clotting, but also the key driver of MS inflammation injury, identified as a mediator in the whole course of disease, especially the onset of inflammatory demyelination (Adams et al. 2007a, b; Claudio et al. 1995; Sobel and Mitchell 1989). Therefore, the development of therapeutics directing toward the inhibition of fibrin may provide a beneficial strategy against MS.
Experimental autoimmune encephalomyelitis (EAE) is a widely used animal model used for understanding the pathogenesis of MS, usually induced by myelin oligodendrocyte glycoprotein (MOG) in C57BL/6 mice (Lebar et al. 1986; Storch et al. 1998). Batroxobin (DF-521-Tobishi) is a thrombin-like substance isolated from the venom of Bothrops atrox moojeni. It acts on the fibrinogen in blood, and releases fibrinopeptide A to produce fibrin monomer, which forms soluble complex of fibrin monomer decomposed by serum plasmin and removed from blood circulation, and thus the so-called defibrinogenating state. Prior studies have reported that batroxobin, administrated prophylactically in EAE model induced with adoptive cell-transfected form or in a separate virus-induced model of demyelinating disease, demonstrated significantly suppressed clinical manifestation, delayed onset of symptoms, and decreased clinical scores (Inoue et al. 1996, 1997). However, whether fibrinogen depletion treatment by batroxobin has the prophylactic and therapeutic potential on MOG-induced EAE mice is unknown, and the application is debatable due to the chronic course of this disease and the potential of hemorrhagic side-effects. In order to determine the effects of batroxobin on clinical course and pathology of MOG-induced EAE mice, and investigate the mechanism of fibrin depleting on EAE, extended experiments were performed in EAE to find a way to a novel and potentially effective therapeutic strategy for inflammatory demyelinating disease including MS. Our results showed that batroxobin had a beneficial effect on EAE mice model.
Materials and Methods
Animals and Induction of EAE
EAE was induced in 12-week-old female C57BL/6 mice by subcutaneous immunization with 150 μg myelin oligodendrocyte glycoprotein (MOG)35–55 (MEVGWYRSPFSRVVHLYRNGK; Saibaisheng, Beijing, China) in complete Freund’s adjuvant (Sigma, USA) supplemented with 10 mg/ml mycobacterium tuberculosis (Tiantan Biological Institute, Beijing, China). In addition, the animal received 300 ng pertussis toxin (Sigma, USA) intraperitoneally (i.p.) at the time of immunization and 48 h later. Clinical signs of EAE were assessed every other day according to the following criteria: 0, no symptoms; 1, loss of tail tone or slightly ataxia; 2, hindlimb paresis; 3, hindlimb paralysis; 4, hindlimb and forelimb paralysis; and 5, moribund (Cross et al. 1994). Animals were kept in a specific pathogen-free facility at animal center, and all the experiments were carried out in accordance with guidelines of the standing committee of animals.
Administration of Batroxobin
Mice were depleted of fibrinogen by injection of batroxobin i.p. (30 BU/kg/day, TOBISHI Company, Japan) every other day at different stages: (i) starting immediately after disease induction (prevention/prophylaxis) (n = 8), (ii) starting soon after the appearance of clinical manifestations (suppression/therapy) (n = 8). While, in control animals, phosphate-buffer saline (PBS) were administrated at the same time (n = 12). Non-immunized mice were not induced to EAE, and not implanted batroxobin (n = 8).
Histopathologic Analysis
Spinal cord and cerebellum tissue samples from EAE mice at days 30, 40, and 60 post-immunization (p.i.) were separately collected after animals were anesthetized and perfused with saline and buffered 4% paraformaldehyde (PFA), one part of the samples were fixed in 4% PFA—cryoprotected in 30% sucrose—and another part without perfusion were stored in −70°C freezer until used. Sections were cut at 5–10 μm and performed for histological analysis. Inflammation, demyelination, and axonal loss were, respectively, assessed using hematoxylin/eosin (HE), Luxol Fast Blue (LFB), and Bielshowsky silver staining according to standard procedures. Histopathologic analysis and quantification of inflammation and demyelination were performed on frozen or paraffin sections as described previously (Akassoglou et al. 1998). Scoring for inflammation and demyelination was done in a blinded manner by a single observer on a scale of 0–5. For inflammation, the scoring system was 0 = no inflammation; 1 = a few inflammatory cells in the leptomeninges; 2 = organization of inflammatory cells around blood vessels; 3 = extensive perivascular cuffing with extension into the underlying parenchyma; 4 = large regions of white matter inflammation extending into the parenchyma covering between one-fourth and one-half cord white matter; and 5 = extensive inflammation covering more than one-half cord white matter. For demyelination, the following scale was used: 0 = none; 1 = a few subpial fibers affected; 2 = partial rim of subpial involvement, not into the parenchyma; 3 = extension beyond the subpial region into the parenchyma; 4 = large regions of white matter involvement, less than one-half cord cross section; and 5 = one-half or more than one-half cord white matter cross section involved.
In adjacent serial sections, immunohistochemistry was performed for myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) as a marker for myelin and astrocyte utilizing mouse anti-MBP monoclonal antibody (1:200, ab66188; abcam, USA), rabbit anti-GFAP polyclonal antibody (1:160, G9269; sigma, USA), followed by biotinylated goat anti-mouse or goat anti-rabbit IgG separately and standard avidin–biotin immuno-peroxidase method as previously documented (Gveric et al. 1999). Immunofluorescence was employed to identify fibrinogen (sheep anti-fibrinogen polyclonal antibody, 1:200, GenWay, USA). For quantitative analysis, sections stained with MBP, GFAP, and fibrinogen were all examined using immunohistochemical score (IHS) (Soslow et al. 2000). For image analysis, 2 or 3 lumbar spinal cord sections were selected according to regional landmarks from two different animals per group post-immunization days 20, 40, and 60 separately. Double-immunofluorescence was performed with a mixture of antibodies against MBP and GFAP. Images were collected and analyzed using the Axioplan Zeiss microscope (Hertifordshire, UK).
Western Blots
Tissue proteins were extracted by sonication in Radio Immunoprecipitation Assay (RIPA) buffer-contained protease inhibitors (Roche) and were assayed for concentration using the BCA protein assay kit (Thermo Scientific, USA). Protein extracts were subjected to a western blot analysis on SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore) following standard protocol as described previously (Akassoglou et al. 2002). Primary antibodies used include mouse anti-MBP (1:1000), rabbit anti-MHC class-I (1:2000, ab52749, abcam, USA), mouse anti-p-Akt (1:1000, cell signaling), and β-actin (Sigma).
Cell Culture
Murine RAW264.7 macrophage cell line was cultured in DMEM supplemented with 10% fetal bovine serum, and for 48 h either with addition of 1 mg/ml lipopolysaccharide (LPS, a potent activator of macrophage) (Sigma, L2654, USA) or on plates coated with 50 μg/ml plasminogen-free fibrinogen (Sigma, USA), then the latter also supplemented with batroxobin (0.1 BU/ml). For immunofluorescence, cells were cultured on glass coverslips, fixed with 4% PFA, blocked with 3% Bovine serum albumin (BSA) (Sigma, USA), and incubated with FITC-conjugated rat anti-mouse CD11b monoclonal antibody (1:100, MCA711G, Serotec, USA). Results represent the means of four independent experiments performed in triplicate.
Plasma Fibrinogen Concentration
Plasma fibrinogen concentration was measured by a modified weight method immediately after plasma sampling from ophthalmic vein of mice.
Statistical Analysis
Clinical scores were represented as mean ± SD, and the statistical analysis was performed using Graphprism software 4.0 (GraphPad Software Inc.) and SPSS 11.0 software. Two-way ANOVA non-parametric analyses were employed to analyze the value of inflammation, demyelination, axonal damage and IHS of MBP, GFAP and Fibrin. P < 0.05 was considered significant.
Results
Treatment of Batroxobin Ameliorated the Clinical Course of EAE
The experiments were conducted in C57BL/6 12-week-old mice immunized with MOG35–55 to assess the effects of batroxobin on the course of EAE. Prevention and suppression treatment with batroxobin (30 BU/kg) were applied by injections i.p. every other day. We found that the clinical manifestation of both prevention and suppression mice were more ameliorated than that of EAE mice, and displayed a significant reduction of clinical score as compared with control EAE mice. And the day of peek in control, prevention, and suppression groups separately occurred at days 29, 29, and 40 post-immunization (p.i.). Two-way ANOVA analysis revealed that there was a delay in disease in suppression group (Fig. 1a). The first signs of disease appeared at days 7 (peaking at days 29–40), and there was no relapse-remitting course. Both batroxobin prevention and suppression EAE animals exhibited decreased average clinical scores related to EAE-untreated mice at day 27 p.i. [average clinical score, 1.0 ± 0.12 for EAE control (n = 12) vs. 0.20 ± 0.39 for prevention (n = 8), 0.05 ± 0.47 for suppression (n = 8)] and at day 35 p.i. [average clinical score, 1.40 ± 0.52 for EAE control (n = 9) vs. 0.12 ± 0.35 for prevention (n = 5), 0.68 ± 0.43 for suppression (n = 8)]. The average clinical scores of both the batroxobin prevention and suppression EAE animals were lower than those of EAE-untreated control mice at days 27 and 35 p.i. (Fig. 1b) (P < 0.05). Two-way ANOVA analysis revealed that there was a significantly beneficial effect of batroxobin treatment, no matter whether in prevention or suppression treatment group.
Fig. 1.
Batroxobin administration ameliorated clinical score and clinical symptoms. Prevention and suppression of batroxobin-treated EAE ameliorated the clinical course compared with EAE-untreated control mice. a EAE was induced in 12-week-old C57BL/6 mice by subcutaneous immunization with 150 μg of MOG35–55 peptide in 4 mg/ml CFA. Pertussis toxin was given i.v. (300 ng per mouse) at the time of immunization and 48 h later. Batroxobin (30 BU/kg, i.p.) was given every other day. n = 8 animals in prevention and suppression group. Mice in control group received buffer accordingly (n = 12 animals). Batroxobin treatment by prevention and suppression ameliorated the clinical manifestations, and displayed a significant reduction of clinical score compared with control group as well. b Both of batroxobin prevention and suppression EAE animals exhibited decreased average clinical scores related to EAE untreated mice at day 27 [average clinical score, 1.0 ± 0.12 for EAE control (n = 12) vs. 0.20 ± 0.39 for prevention (n = 80, 0.05 ± 0.47 for suppression (n = 8)] and 35 p.i. (average clinical score, 1.40 ± 0.52 for EAE control (n = 9) vs. 0.12 ± 0.35 for prevention (n = 5), 0.68 ± 0.43 for suppression (n = 8)] (P < 0.05). Disease score measured was as follows: 0, no signs of disease; 1, loss of tone in the tail; 2, hindlimb paresis; 3, hindlimb paralysis; 4, tetraplegia; and 5, moribund. Clinical scores were calculated as the average of all the animals in each group
Treatment of Batroxobin Diminished Inflammation and Demyelination in Eae
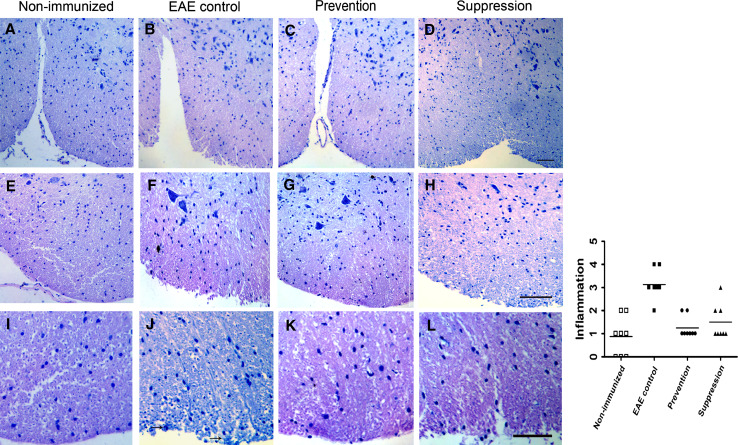
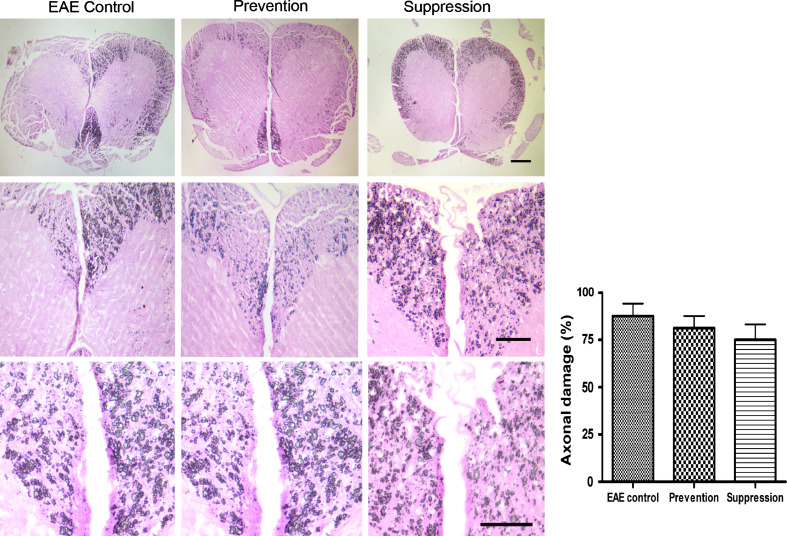
Spinal cords (SC) and cerebella from EAE-untreated (control) and EAE mice treated by batroxobin were subjected to both histology and immunohistochemistry analysis. We performed series of images from representative mice of non-immunized, control, prevention and suppression group, and each group HE staining images were demonstrated in SCs (Fig. 2). EAE-untreated control mice revealed slight or moderate inflammation, accompanied by parenchymal cells infiltration that indicated the inflammatory disease course (Fig. 2b, f). The degrees of CNS inflammation and demyelination were evaluated at Day 20, 40 and 60 p.i. in the SCs and cerebella in the prevention, suppression and the EAE-untreated control group. Inflammation was reduced significantly in prevention and suppression group mice compared with EAE control mice (Fig. 2) (P < 0.05). Infiltrated parenchymal cells were observed in the SCs of batroxobin-treated mice, but to a less extent than untreated mice. In addition, LFB staining demonstrated that demyelination reduced dramatically in mice of batroxobin prevention and suppression group compared with untreated controls (P < 0.05). Extensive areas of demyelination were detectable in EAE control mice (Fig. 3b, f), while slight demyelination was observed in prevention and suppression mice. However, Bielshowsky silver impregnation for axons showed indistinctive axonal density in the white matter of the spinal cord of EAE mice among control, prevention, and suppression mice (Fig. 4) (P > 0.05).
Fig. 2.
Spinal cords inflammation was significantly reduced in prevention and suppression group mice compared with control mice. Representative mice for non-immunized (a, e, i), control (b, f, j), prevention (c, g, k), and suppression (d, h, l) groups are shown. In the H&E-stained sections, inflammatory cells deeply infiltrated the CNS parenchyma and pia in the control group (j) (black arrows), while they were localized mainly to the meninges in the batroxobin-treated groups (c, g, d, h). Inflammation quantified in a blinded manner was reduced significantly in prevention and suppression group mice compared with control mice (P < 0.05). Horizontal lines indicate average values. Original scale bars, 200 μm (a–d) or 50 μm (e–h) or 30 μm (i–l)
Fig. 3.
Demyelination was significantly reduced in prevention and suppression group mice compared with control mice. Luxol fast blue (LFB) staining demonstrated myelin loss in the white matter of spinal cord of EAE control mice (b, f, j) (black arrows). Demyelination was reduced by prevention (c, g, k) or suppression (d, h, l) of using batroxobin in MOG-induced mouse EAE. Non-immunized (a, e, i) mice showed mainly intact myelin. Demyelination quantified in a blinded manner were reduced significantly in prevention and suppression group compared with control mice (P < 0.05)
Fig. 4.
There was no remarkable reduction of axonal density in prevention and suppression group mice compared with control mice. Bielschowsky’s silver impregnation for axons showed no remarkable reduction of axonal density in the white matter of the spinal cord of EAE mice compared with control mice. Quantification shows no distinctive significance in prevention and suppression group mice as compared with control mice (P > 0.05)
Immunohistochemical Characterization from EAE Mice and the Effects of Batroxobin Treatment
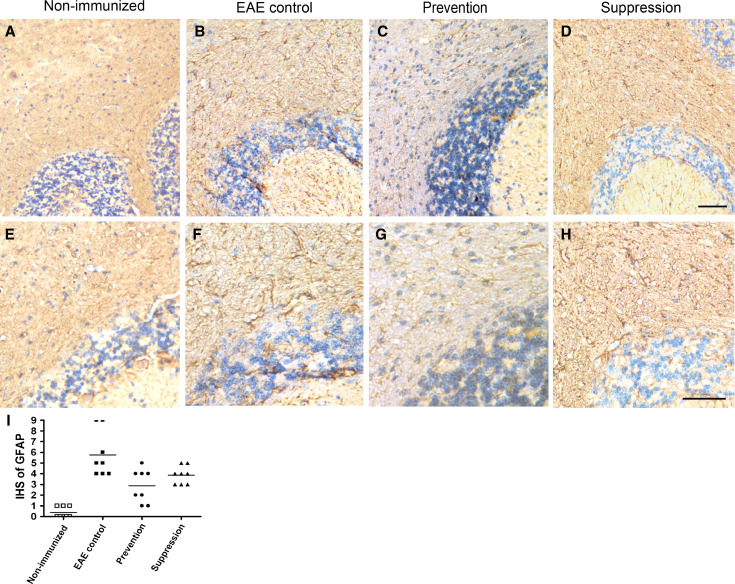
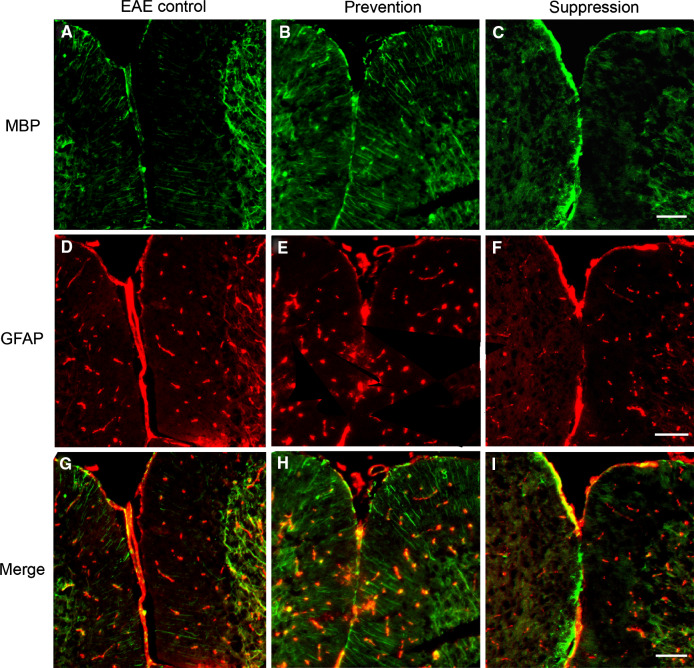
To confirm findings described above, myelin presence was visualized by using anti-myelin basic protein (MBP) antibody at days 20, 40, and 60 p.i. in the white matter of cerebella. As shown in Fig. 5, multifocal widespread areas of myelin damage were observed in the white matter in EAE mice, whereas a still larger number of MBP-positive myelinated areas were detected in prevention and suppression group mice (P < 0.05). In regions of myelin damage, astrocytes were activated in white matter of cerebella. As demonstrated in Fig. 6, the activation of astrocytes in prevention and suppression group had decreased as compared with untreated control mice (P < 0.05). Meanwhile, MBP and GFAP double immunofluorescences were used to detect myelin and astrocytes. MBP-positive myelin increased in the white matter of batroxobin-treated prevention and suppression group as compared with that of control mice. However, prevention and suppression group exhibited decreased GFAP-positive astrocytes (Fig. 7).
Fig. 5.
Increased MBP expression in the batroxobin-treated EAE mice compared with controls. Multifocal widespread areas of myelin damage were observed in white matter of EAE mice (a, d) by immunochemistry analysis. Higher number of MBP-positive myelin were detected in the white matter of prevention (b, e) and suppression (c, f) group mice. Quantitation (g) shows a remarkable increase in prevention and suppression group as compared with control mice (P < 0.05)
Fig. 6.
Reduced GFAP expression in the batroxobin-treated EAE mice compared with controls. Decreased activation of astrocytes in prevention (c, g) and suppression (d, h) group compared with control mice (b, f). Non-immunized (a, e) mice showed mainly normal astrocytes (detected by GFAP). Quantitation (i) shows a dramatic decrease in prevention and suppression group as compared with control mice (P < 0.05)
Fig. 7.
MBP and GFAP assay using double-labeling immunofluorescence. High expression of MBP (line-like/green) in the white matter of batroxibin-treated prevention and suppression mice compared with control. Decreased expression of GFAP (spot-like/red) in the white matter of prevention and suppression group mice related to the control mice (Color figure online)
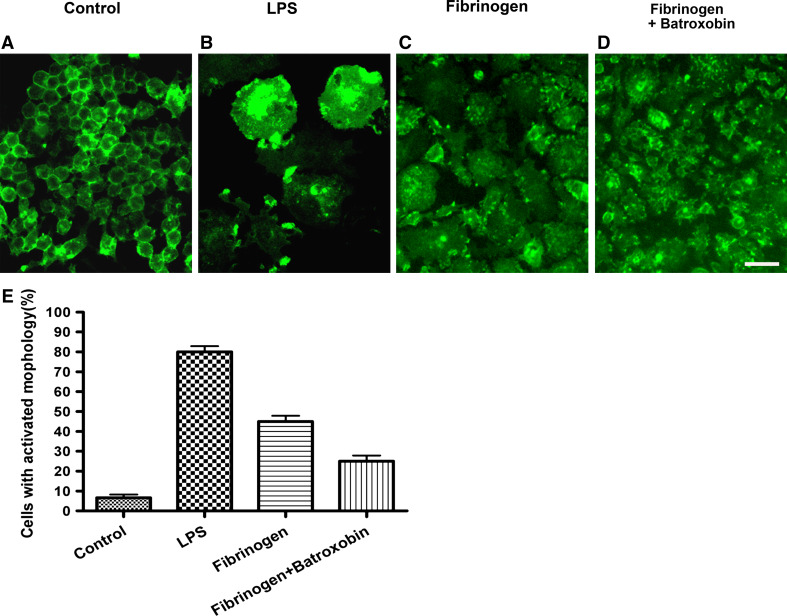
Batroxobin Treatment Partly Rescued the Activated Morphology of Macrophage Stimulated with LPS or Fibrinogen
The effects of fibrin depletion on macrophages, the major cell type that contributes to autoimmune-induced inflammatory demyelination, were investigated in this study. The murine macrophage cell line RAW 264.7 was used to evaluate the effects of fibrin depletion agent, because it acquired an activated dendritic morphology upon LPS induction or fibrinogen (Saxena et al. 2003). Immunofluorescence using an antibody against CD11b, a macrophage cell marker, showed that RAW264.7 cells acquire distinct dendritic-like morphology after 24 h in culture in the presence of LPS (Fig. 8b), compared with unstimulated RAW264.7 cells cultured on plastic (Fig. 8a). RAW264.7 cells cultured on fibrinogen-coated surface exhibit morphologic changes similar to those of the LPS-treated cells (Fig. 8c). While, the dendritic-like morphologic formation was reversed partly by batroxobin on RAW264.7 cells irritated by fibrinogen (Fig. 8d). Microscopy showed that the fibrinogen-induced activated morphologic alterations of macrophages were similar to that alterations induced by LPS, characterized by a dramatic increase in size and larger nuclei, compared with untreated control RAW264.7 cells. Qualification analysis revealed that 80.5 ± 3.5% of cells with activated morphology upon LPS induction, 46.2 ± 4.6% upon fibrinogen stimulation, 25.5 ± 6.1% upon fibrinogen-induced batroxobin supplement, and 5.6 ± 3.1% upon untreated control cells. Taken together, there was a remarkable decrease in activated morphological cells in fibrinogen supplemented with batroxobin (P < 0.05).
Fig. 8.
Batroxobin inhibited the activation of macrophages stimulated by fibrinogen. Untreated CD11b-immunostained RAW 264.7 macrophage cells showed undifferentiated, macrophage-like morphology (a). LPS-stimulated (1 mg/ml) RAW264.7 cells showed a morphological transformation to dendritic-like cells, cell size enlargement as well (b). Fibrinogen-stimulated cells showed morphologic alterations similar to those of LPS-stimulated cells (c). Batroxobin rescued the changes partly, the cells irritated by fibrinogen were reversed as round and small when treated with batroxobin (d). Qualification analysis reveals that 80.5 ± 3.5% of cells with activated morphology upon LPS induction, 46.2 ± 4.6% upon fibrinogen stimulation, 25.5 ± 6.1% upon fibrinogen-induced batroxobin supplement, and 5.6 ± 3.1% upon untreated control cells. Quantitation shows a remarkable decrease in activated morphological cells in fibrinogen supplemented with batroxobin (P < 0.05)
Batroxobin treatment decreased the deposition of fibrin in lesions, but the concentration of plasma fibrinogen had no significant differences.
In the cerebella of EAE-induced mice treated with batroxobin, either by the prevention or suppression, considerably less fibrin deposition was detected compared with the EAE-untreated control mice. Quantitative analysis showed a remarkable decrease in prevention and suppression as compared with control mice (Fig. 9a–d) (P < 0.05). However, the decreased fibrin deposition was not parallel with plasma fibrinogen concentration, which had no significant differences among the prevention (2.98 ± 0.82 g/l), suppression (2.78 ± 0.13 g/l) and control mice (3.15 ± 0.33 g/l) (Fig. 9e) (P > 0.05). Meantime, we observed that mice administered batroxobin do not show any hemorrhagic symptoms.
Fig. 9.
Decreased fibrin deposition in the white matter of prevention and suppression group mice. Decreased fibrin deposition of in lesions of prevention (b) and suppression (c) group after being treated with batroxobin as compared with control mice (a). Quantitation shows a remarkable decrease in prevention and suppression group as compared with control mice (d) (P < 0.05). The plasma fibrinogen concentrations of prevention (2.98 ± 0.82 g/l) and suppression (2.78 ± 0.13 g/l) group were lower than that of control mice (3.15 ± 0.33 g/l). However, there was no significant difference (P > 0.05) (e)
The Effect of Batroxobin on Expression of MHC-I, p-Akt, and MBP in the Spinal Cords
To investigate whether the fibrin depleting agent batroxobin induced effects by inhibition of fibrin deposition, together with decreased inflammatory responses or remyelination, major histocompatibility complex class I (MHC-I) molecular, p-Akt, and MBP expressions were specifically assessed by western blot (Fig. 10). MHC-I molecules belong to a large and extremely polymorphic family of cell-surface receptors in immune responses. In our experiment, there was no significant difference in MHC-I molecules expression among control, prevention, and suppression group. The p-Akt expression level was downregulated in prevention and suppression group compared with control group,; in contrast, MBP expression was upregulated in prevention and suppression mice as compared with control mice.
Fig. 10.
The effect of Batroxobin on expression of MHC-I, p-Akt, and MBP. The expression of MHC-I was not significantly different in the spinal cord among the control, prevention, and suppression mice. While the level of p-Akt expression was downregulated, the one of MBP expression was upregulated in prevention and suppression group as compared with control mice
Discussion
The MS lesions are characterized with demyelination and axonal loss, which correlated with blood brain barrier (BBB) disruption and extravascular fibrin deposition. Fibrin (fibrinogen) deposition may contribute to the initiation, progression, and the whole inflammatory process (Flick et al. 2004; Herwald et al. 2004; Kermode et al. 1990; Languino et al. 1993). In addition, fibrin deposition colocalizes with infiltrating macrophages within demyelinated plaques, as well as with damaged axons (Gay et al., 1997; Gveric et al., 2001). The mice genetically or pharmacologically being depleted of fibrinogen showed that fibrin may be a contributing factor during MS pathogenesis. The Fibγ377–395 peptides were subjected to EAE, which showed significantly reduced clinical scores and inflammation. Previous studies showed that such cases where fibrinogen was pharmacologically depleted also revealed a direct decrease of microglia activation in EAE (Adams et al. 2007a, b). The tumor necrosis factor (TNF) transgenic mice spontaneously develop inflammation and demyelination and which have a delay in the clinical onset of paralysis after being treated with ancord (snake venom-derived protease). Moreover, histopathologic analysis showed that ancord reduced both inflammation and demyelination (Akassoglou et al. 2004). Batroxobin, another snake venom protease, could reduce clinical scores in EAE induced by adoptive cell-transfected form or a separate virus-induced model (Inoue et al. 1996). In our study, we showed for the first time that fibrinogen depletion by batroxobin has the prophylactic and therapeutic potential on MOG-induced EAE mice, and mice administered with batroxobin do not show any hemorrhagic symptoms.
Fibrinogen-mediated initiation of inflammatory was supported by in vivo experiments. Fibrinogen knock out mice or mice genetically modified to abolish the binding of fibrinogen to CD11b/CD18 integrin showed reduced brain inflammation and clinical symptoms in animal models of MS (Adams et al. 2007a, b; Akassoglou et al. 2004). In our study, a marked reduction of the inflammation and demyelination in the spinal cords and cerebella were observed in the batroxobin-treated prevention and suppression mice in contrast to the EAE control mice. However, the mechanism by which fibrin mediates its effects on inflammatory demyelination is not well known, but one indication as regards its potential target comes from the observation that fibrin depletion reduces inflammation in EAE. Fibrinogen is involved in inflammatory reactions through the regulation of macrophage and astrocytes. Our immunofluorescence results revealed that fibrinogen induced a dramatic change in macrophage morphology from a quiescent to an activated state associated with an increase in cell size. The fibrinogen-mediated macrophage RAW264.7 activation was rescued at least partly after treatment with batroxobin in vitro, supporting the issue that inhibitory effect of batroxobin on the fibrinogen-mediated activation of macrophages including a fibrin-dependent activation of Akt signaling described previously (Akassoglou et al. 2004; Kornek and Lassmann 2003). Our results also confirmed that fibrin depletion with batroxobin could directly inhibit activation of CD11b+ macrophage resulting in decreased downstream p-Akt signaling pathway, which may ultimately ameliorate inflammatory response in EAE.
In this study, the potential effects of treatment of MS with batroxobin in an MOG-induced EAE animal model were examined. Batroxobin, as a defibrinogen drug, could significantly ameliorate both clinical symptoms and the severity of the inflammatory demyelinating response in the mice model. Batroxobin treatment could improve the clinical manifestations, displaye a significant reduction of clinical score, and reduce inflammatory demyelination, activation of astrocytes, and macrophages compared with untreated control mice. These results implied that strategy directing toward the inhibition of fibrin may have a beneficial effect against MS.
Although the mechanisms of fibrin's action are not yet fully understood, significant reduction in inflammation and demyelination after prophylactic and therapeutic fibrin depletion in EAE model were demonstrated in this study. Targeting fibrin could be a potential therapeutic strategy in MS and might be a safer approach than plasma activator (Chen and Strickland 1997). Further research to identify the cellular and molecular mechanisms that fibrin utilizes to mediate pathology of MS will provide pharmacologic targets, which will be beneficial for MS patients.
Moreover, these anti-inflammatory evidences in this study were consistent with other previous studies (Inoue et al. 1996). Prior investigation reported that in vivo administration of ancrod effectively results in 90% depletion of fibrinogen and does not completely deplete fibrinogen, nor any hemorrhagic symptoms (Lowe and Rumley 2001; Suh et al. 1995). The results of this study showed that plasma level of fibrinogen was not significantly different in both EAE control and in prevention and suppression animals, which would avoid main side-effect of potential bleeding as it might restrain the level of fibrinogen in serum.
In conclusion, batroxobin, as a potential fibrinogen-depleting agent, has a beneficial prophylactic and therapeutic effect on EAE mice model. Therefore, it is believed that targeting against fibrin represents a viable proposition for the development of new treatments for MS.
Acknowledgment
The work was supported by the general hospital of Chinese PLA and associated by the Academy of military medical sciences.
Abbreviations
- MS
Multiple sclerosis
- CNS
Central nervous system
- EAE
Experimental autoimmune encephalomyelitis
- MOG
Myelin oligodendrocyte glycoprotein
- BBB
Blood brain barrier
- BU
Batroxobin unit
- p.i.
Post-immunization
- MBP
Myelin basic protein
- GFAP
Glial fibrillary acidic protein
- IHS
Immunohistochemical score
- LPS
Lipopolysaccharide
- MHC-I
Major histocompatibility complex class I
References
- Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K (2007a) The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med 204:571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K (2007b) Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem 14:2925–2936 [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L (1998) Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol 153:801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Yu WM, Akpinar P, Strickland S (2002) Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron 33:861–875 [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S (2004) Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci USA 101:6698–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Strickland S (1997) Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 91:917–925 [DOI] [PubMed] [Google Scholar]
- Claudio L, Raine CS, Brosnan CF (1995) Evidence of persistent blood-brain barrier abnormalities in chronic-progressive multiple sclerosis. Acta Neuropathol 90:228–238 [DOI] [PubMed] [Google Scholar]
- Cross AH, Misko TP, Lin RF, Hickey WF, Trotter JL, Tilton RG (1994) Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest 93:2684–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL (2004) Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest 113:1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay FW, Drye TJ, Dick GW, Esiri MM (1997) The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain 120(Pt 8):1461–1483 [DOI] [PubMed] [Google Scholar]
- Gveric D, Cuzner ML, Newcombe J (1999) Insulin-like growth factors and binding proteins in multiple sclerosis plaques. Neuropathol Appl Neurobiol 25:215–225 [DOI] [PubMed] [Google Scholar]
- Gveric D, Hanemaaijer R, Newcombe J, van Lent NA, Sier CF, Cuzner ML (2001) Plasminogen activators in multiple sclerosis lesions: implications for the inflammatory response and axonal damage. Brain 124:1978–1988 [DOI] [PubMed] [Google Scholar]
- Herwald H, Cramer H, Morgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Bjorck L (2004) M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell 116:367–379 [DOI] [PubMed] [Google Scholar]
- Inoue A, Koh CS, Shimada K, Yanagisawa N, Yoshimura K (1996) Suppression of cell-transferred experimental autoimmune encephalomyelitis in defibrinated Lewis rats. J Neuroimmunol 71:131–137 [DOI] [PubMed] [Google Scholar]
- Inoue A, Koh CS, Yamazaki M, Yanagisawa N, Ishihara Y, Kim BS (1997) Fibrin deposition in the central nervous system correlates with the degree of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol 77:185–194 [DOI] [PubMed] [Google Scholar]
- Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P, McDonald WI (1990) Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain 113(Pt 5):1477–1489 [DOI] [PubMed] [Google Scholar]
- Kornek B, Lassmann H (2003) Neuropathology of multiple sclerosis-new concepts. Brain Res Bull 61:321–326 [DOI] [PubMed] [Google Scholar]
- Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC (1993) Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell 73:1423–1434 [DOI] [PubMed] [Google Scholar]
- Lebar R, Lubetzki C, Vincent C, Lombrail P, Boutry JM (1986) The M2 autoantigen of central nervous system myelin, a glycoprotein present in oligodendrocyte membrane. Clin Exp Immunol 66:423–434 [PMC free article] [PubMed] [Google Scholar]
- Lowe GD, Rumley A (2001) Fibrinogen and its degradation products as thrombotic risk factors. Ann N Y Acad Sci 936:560–565 [DOI] [PubMed] [Google Scholar]
- Saxena RK, Vallyathan V, Lewis DM (2003) Evidence for lipopolysaccharide-induced differentiation of RAW264.7 murine macrophage cell line into dendritic like cells. J Biosci 28:129–134 [DOI] [PubMed] [Google Scholar]
- Sobel RA, Mitchell ME (1989) Fibronectin in multiple sclerosis lesions. Am J Pathol 135:161–168 [PMC free article] [PubMed] [Google Scholar]
- Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT (2000) COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89:2637–2645 [DOI] [PubMed] [Google Scholar]
- Storch MK, Stefferl A, Brehm U, Weissert R, Wallstrom E, Kerschensteiner M, Olsson T, Linington C, Lassmann H (1998) Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol 8:681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh TT, Holmback K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL (1995) Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev 9:2020–2033 [DOI] [PubMed] [Google Scholar]