Abstract
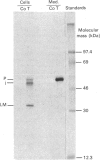
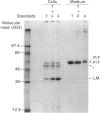
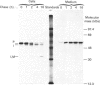
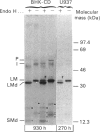
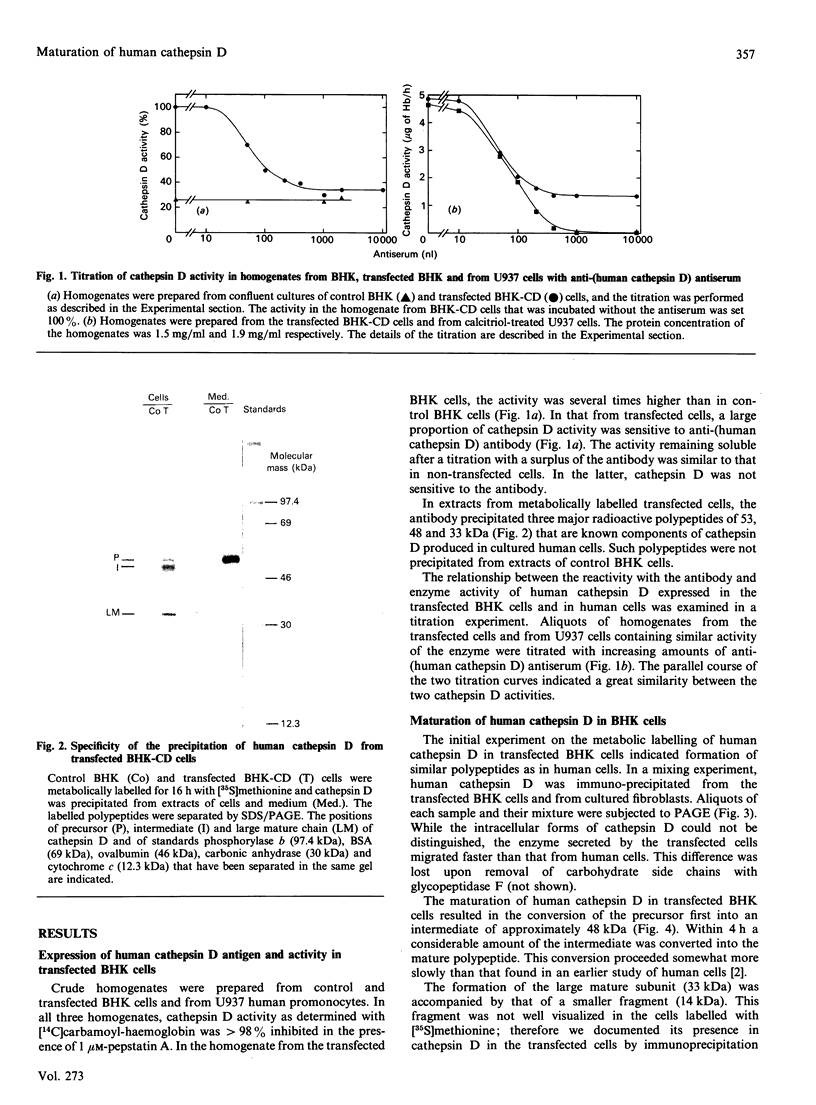
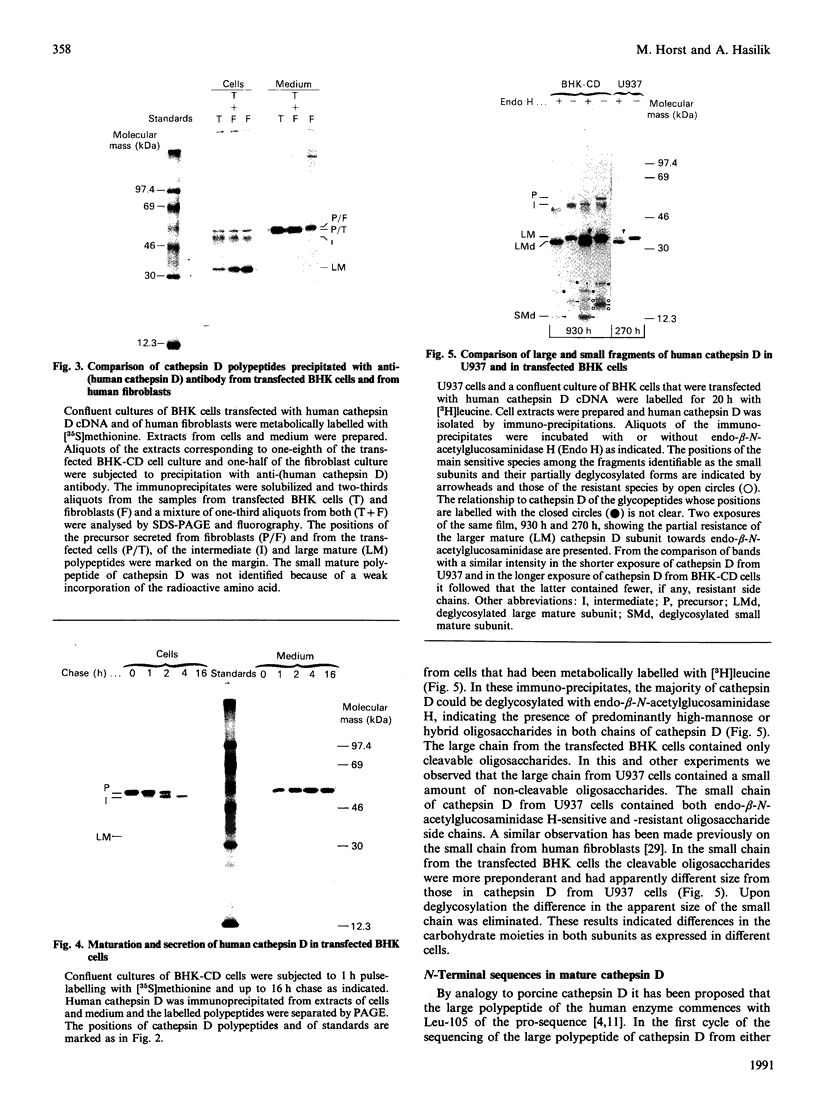
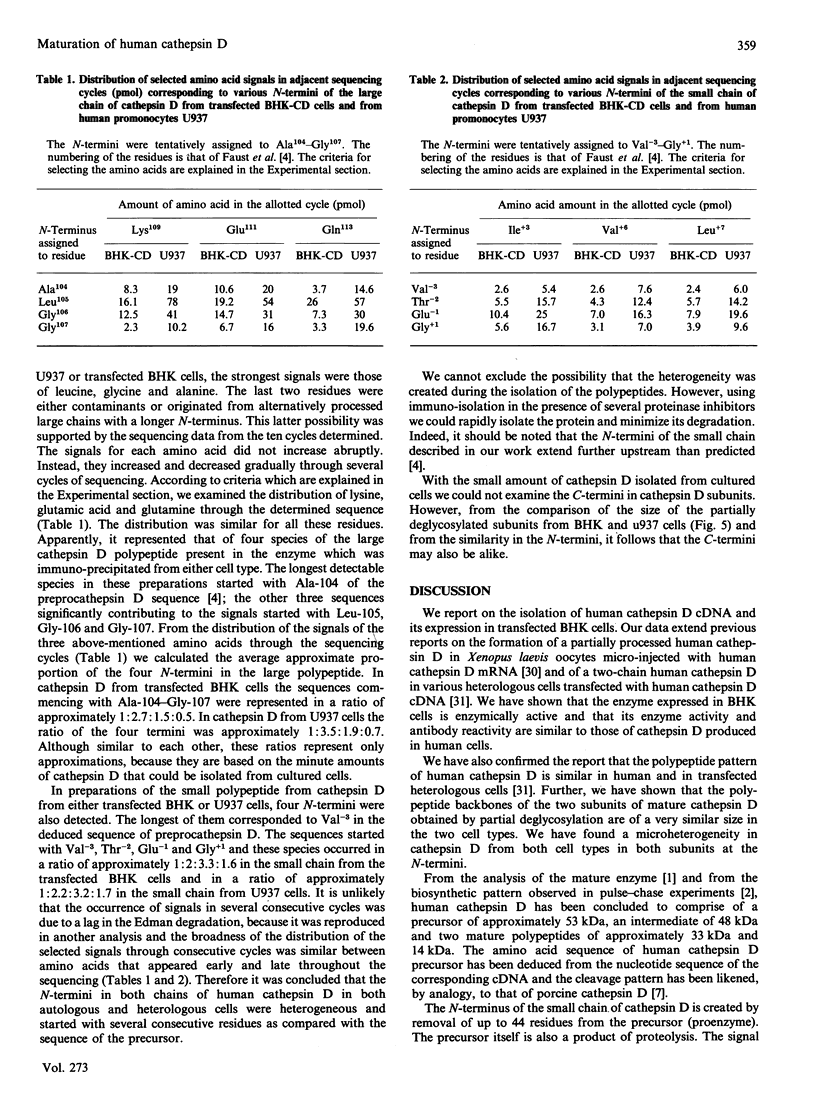
In medium and in homogenates from baby-hamster kidney cells (BHK) transfected with human cathepsin D cDNA, an elevated activity of cathepsin D was found as compared to non-transfected cells. The elevated activity was removed by titrating the homogenates with an anti-(human cathepsin D) antibody. Metabolic labelling and immunoprecipitation revealed that, in the transfected cells, human cathepsin D was synthesized as a 53-kDa precursor indistinguishable from that found in human cells. A portion of the precursor was secreted and the remainder was processed to intermediate and mature chains within a few hours of synthesis. The precursor that was released from the transfected cells had a slightly smaller apparent size than that from cultured human fibroblasts. This difference was abrogated when the precursors were treated with glycopeptidase F. In the intracellular small chain a difference was observed in the size of carbohydrate chains that were cleavable with endo-beta-N-acetylglucosaminidase H. Sequence analysis of the N-termini of mature intracellular cathepsin D indicated a N-terminal trimming in both large and small chains from both human and transfected hamster cells. The proteolytic maturation of human cathepsin D in BHK cells closely resembles that in human cells, whereas a portion of the carbohydrate side chains is processed differently. The trimming of the N-termini in mature cathepsin D is proposed to be a part of the maturation and aging of this protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artelt P., Morelle C., Ausmeier M., Fitzek M., Hauser H. Vectors for efficient expression in mammalian fibroblastoid, myeloid and lymphoid cells via transfection or infection. Gene. 1988 Sep 7;68(2):213–219. doi: 10.1016/0378-1119(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Augereau P., Garcia M., Mattei M. G., Cavailles V., Depadova F., Derocq D., Capony F., Ferrara P., Rochefort H. Cloning and sequencing of the 52K cathepsin D complementary deoxyribonucleic acid of MCF7 breast cancer cells and mapping on chromosome 11. Mol Endocrinol. 1988 Feb;2(2):186–192. doi: 10.1210/mend-2-2-186. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin D. Adv Exp Med Biol. 1977;95:291–300. doi: 10.1007/978-1-4757-0719-9_17. [DOI] [PubMed] [Google Scholar]
- Braulke T., Geuze H. J., Slot J. W., Hasilik A., von Figura K. On the effects of weak bases and monensin on sorting and processing of lysosomal enzymes in human cells. Eur J Cell Biol. 1987 Jun;43(3):316–321. [PubMed] [Google Scholar]
- Conner G. E. Isolation of procathepsin D from mature cathepsin D by pepstatin affinity chromatography. Autocatalytic proteolysis of the zymogen form of the enzyme. Biochem J. 1989 Oct 15;263(2):601–604. doi: 10.1042/bj2630601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner G. E., Udey J. A., Pinto C., Sola J. Nonhuman cells correctly sort and process the human lysosomal enzyme cathepsin D. Biochemistry. 1989 Apr 18;28(8):3530–3533. doi: 10.1021/bi00434a057. [DOI] [PubMed] [Google Scholar]
- Cully J., Harrach B., Hauser H., Harth N., Robenek H., Nagata S., Hasilik A. Synthesis and localization of myeloperoxidase protein in transfected BHK cells. Exp Cell Res. 1989 Feb;180(2):440–450. doi: 10.1016/0014-4827(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Erickson A. H. Biosynthesis of lysosomal endopeptidases. J Cell Biochem. 1989 May;40(1):31–41. doi: 10.1002/jcb.240400104. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Conner G. E., Blobel G. Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem. 1981 Nov 10;256(21):11224–11231. [PubMed] [Google Scholar]
- Faust P. L., Kornfeld S., Chirgwin J. M. Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4910–4914. doi: 10.1073/pnas.82.15.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust P. L., Wall D. A., Perara E., Lingappa V. R., Kornfeld S. Expression of human cathepsin D in Xenopus oocytes: phosphorylation and intracellular targeting. J Cell Biol. 1987 Nov;105(5):1937–1945. doi: 10.1083/jcb.105.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORHAM L. W., WAYMOUTH C. DIFFERENTIATION IN VITRO OF EMBRYONIC CARTILAGE AND BONE IN A CHEMICALLY-DEFINED MEDIUM. Proc Soc Exp Biol Med. 1965 May;119:287–290. doi: 10.3181/00379727-119-30160. [DOI] [PubMed] [Google Scholar]
- Gieselmann V., Hasilik A., von Figura K. Processing of human cathepsin D in lysosomes in vitro. J Biol Chem. 1985 Mar 10;260(5):3215–3220. [PubMed] [Google Scholar]
- Grässel S., Röling A., Hasilik A. Immunoprecipitation of labeled antigens with Eupergit C1Z. Anal Biochem. 1989 Jul;180(1):72–78. doi: 10.1016/0003-2697(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Von Figura K. Oligosaccharides in lysosomal enzymes. Distribution of high-mannose and complex oligosaccharides in cathepsin D and beta-hexosaminidase. Eur J Biochem. 1981 Dec;121(1):125–129. doi: 10.1111/j.1432-1033.1981.tb06440.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., von Figura K., Conzelmann E., Nehrkorn H., Sandhoff K. Lysosomal enzyme precursors in human fibroblasts. Activation of cathepsin D precursor in vitro and activity of beta-hexosaminidase A precursor towards ganglioside GM2. Eur J Biochem. 1982 Jul;125(2):317–321. doi: 10.1111/j.1432-1033.1982.tb06685.x. [DOI] [PubMed] [Google Scholar]
- Hentze M., Hasilik A., von Figura K. Enhanced degradation of cathepsin D synthesized in the presence of the threonine analog beta-hydroxynorvaline. Arch Biochem Biophys. 1984 Apr;230(1):375–382. doi: 10.1016/0003-9861(84)90120-6. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Shewale J. G., Tang J. Amino acid sequence of porcine spleen cathepsin D. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3703–3707. doi: 10.1073/pnas.81.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M., Braulke T., von Figura K., Hasilik A. Effects of differentiation-inducing agents on synthesis, maturation and secretion of cathepsin D in U937 and HL-60 cells. Biol Chem Hoppe Seyler. 1987 Apr;368(4):413–418. doi: 10.1515/bchm3.1987.368.1.413. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tang J., Wong R. N. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987 Jan;33(1):53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- Vara J. A., Portela A., Ortín J., Jiménez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986 Jun 11;14(11):4617–4624. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. Enhanced breakdown of arylsulfatase A in multiple sulfatase deficiency. Eur J Biochem. 1982 Apr 1;123(2):317–321. doi: 10.1111/j.1432-1033.1982.tb19770.x. [DOI] [PubMed] [Google Scholar]
- Westley B. R., May F. E. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res. 1987 May 11;15(9):3773–3786. doi: 10.1093/nar/15.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Takahashi T., Wang X. J., Wong R. N., Hartsuck J. A., Tang J. Structures at the proteolytic processing region of cathepsin D. J Biol Chem. 1988 Nov 5;263(31):16504–16511. [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]