Abstract
Changes in gene expression of the brain serotonin (5-HT) 1A receptors may be important for the development and ameliorating depression, however identification of specific stimuli that activate or reduce the receptor transcriptional activity is far from complete. In the present study, the forced swim test (FST) exposure, the first stress session of which is already sufficient to induce behavioral despair in rats, significantly increased 5-HT1A receptor mRNA levels in the brainstem, frontal cortex, and hippocampus at 24 h. In the brainstem and frontal cortex, the elevation in the receptor gene expression after the second forced swim session was not affected following chronic administration of fluoxetine, while in the cortex, both control and FST values were significantly reduced in fluoxetine-treated rats. In contrast to untreated rats, no increase in hippocampal 5-HT1A receptor mRNA was observed in response to FST in rats chronically treated with fluoxetine. Metabolism of 5-HT (5-HIAA/5-HT) in the brainstem was significantly decreased by fluoxetine and further reduced by swim stress, showing a certain degree of independence of these changes on 5-HT1A receptor gene expression that was increased in this brain region only after the FST, but not after fluoxetine. FST exposure also decreased the brainstem dopamine metabolism, which was unexpectedly positively correlated with 5-HT1A receptor mRNA levels in the frontal cortex. Together, these data suggest that the effects of the forced swim stress as well as fluoxetine involve brain region-dependent alterations in 5-HT1A receptor gene transcription, some of which may be interrelated with concomitant changes in catecholamine metabolism.
Keywords: Serotonin 1A receptors, Monoamines, Brain, Swim stress, Behavioral despair, Fluoxetine
Introduction
The onset of depression has been associated with reduced serotonin (5-hydroxytryptamine, 5-HT) neurotransmission, whereas antidepressant drug actions were attributed to an increase in the synaptic 5-HT level (Blier and de Montigny 1994; Millan 2006; Wong et al. 2005). Among multiple receptors, through which released 5-HT acts, 5-HT1A subtype has received much attention in depression research (Cryan and Leonard 2000; Blier and Ward 2003; Lanfumey and Hamon 2004; Savitz et al. 2009). These receptors are widely distributed in brain regions, including the frontal cortex and hippocampus (Vergé et al. 1986), and have a unique ability to modulate total 5-HT system activity by feedback inhibition (Gardier et al. 1996) as well as local responses to 5-HT. As was revealed in animal studies, both somatodendritic 5-HT1A autoreceptors and postsynaptic 5-HT1A receptors are implicated in modulation of depressive-related behavior. It has, for example, been shown that mice with higher autoreceptor gene expression exhibit increased behavioral despair as compared to animals with lower autoreceptor level (Richardson-Jones et al. 2010). In contrast, male mice over-expressing the postsynaptic 5-HT1A receptors demonstrated an antidepressant-like behavior in the forced swim test (Günther et al. 2011). However, specific factors affecting receptor expression in relation to psychopathology are not fully identified.
Stress increases the risk for depression (Caspi et al. 2003; Kendler et al. 1999). Stressful events also change 5-HT extracellular levels, an effect that is regionally specific (Adell et al. 1997; Kirby et al. 1995; Linthorst et al. 2002). Modulation of 5-HT neurotransmission by stress as well as its subsequent normalization might be due to alterations in 5-HT1A receptor gene expression. Dysregulation of these receptors may lead to changes in total 5-HT system activity and by this to the development of depression (Albert and François 2010; Albert et al. 2011). Besides 5-HT, other monoaminergic, noradrenergic (NA) and dopaminergic (DA), pathways have been implicated in stress responses, and they are also targets of antidepressants. On the basis of clinical observations, the hypothesis was put forward that monoaminergic (5-HT, DA and NA) neurotransmission in general is involved in the pathogenesis of depression and treatment of mood disorders (Blier 2001; Brühl et al. 2010; Guiard et al. 2011; Haenisch et al. 2009). However, it is not clear if there is any association between indices of monoamine turnover and expression of 5-HT1A receptors in the brain in conditions that provoke or ameliorate depression. With the aim to get insight in this problem, in the present study, we investigated whether the forced swim test (FST) exposure, the first stress session of which already induces depressive-like state in rats (Porsolt et al. 1978) and chronic antidepressant fluoxetine administration have an impact on 5-HT1A receptor gene expression and the content of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the brainstem, where 5-HT body cells are located, and in the frontal cortex and hippocampus receiving 5-HT innervation as well as on DA, its metabolite, 3,4-dihydroxy-phenylacetic acid (DOPAC) and NA concentrations in these brain regions.
Materials and Methods
Forty adult male Wistar rats, initially weighing about 200 g were used. The animals were caged singly with free access to food and water. All animal use procedures conformed to international European ethical standards (86/609-EEC) and to guide for the care and use of laboratory animals approved by Ministry of Public Health of Russia (Supplement to order N 267 of June 19, 2003). All efforts were made to minimize animal suffering and to reduce the number of animals used.
The animals were divided into four experimental groups (Water, Control; Water, FST; Fluoxetine, Control; Fluoxetine, FST; n = 10 rats per group). Fluoxetine was administered with drinking water at a dose of approximately 7.5 (7.26–7.70) mg/kg/day for 8 weeks as was described previously (Shishkina et al. 2007, 2012). Water groups received distilled water. Half of the animals in Fluoxetine and Water groups were forced to swim according to published FST protocol (Porsolt et al. 1978). In brief, rats were subjected to pre-test session of 15 min followed 24 h later by a 5-min test session. Both swim sessions were carried out between 1000 and 1200 h by placing rats in swim tanks (46 × 20 cm glass cylinders) that were filled with water at 25 °C to a depth of 30 cm. After swim, the rats were dried with towels and returned to their home cages.
Twenty-four hours following the second swim session, the animals were rapidly decapitated together with unstressed control rats. In order to reveal any monoaminergic changes related to stress-induced depression-like state development as well as to the antidepressant-like effect of fluoxetine, this time point for investigations was chosen. First, a state of behavior despair is already evident in test session (Porsolt et al. 1978), however immobility duration continues to increase in subsequent retests (Dal-Zotto et al. 2000; Hitoshi et al. 2007). Second, the antidepressant-like effect of fluoxetine was shown to be facilitated along retest sessions (Mezadri et al. 2011). And finally, monoaminergic neurotransmission affected by acute stress is normalized.
Immediately after animal decapitation, the brain was removed, and brainstem (pons+rostral part of medulla oblongata to −10.5 mm, relative to bregma), frontal cortex, and hippocampus were rapidly dissected, according to the atlas of Paxinos and Watson (1998). Two samples for each dissected brain region, one for gene expression and the other for neurochemical investigations, were separated by a midline cut. Tissue samples were frozen in liquid nitrogen until determination of mRNA levels, monoamine, and their metabolite concentrations.
Semi-quantitative RT-PCR described previously (Shishkina et al. 2007, 2008, 2012) was used to determine mRNA levels. In brief, PCR reactions were conducted using rat-specific primers for 5-HT1A receptors—forward (5′-ctctacgggcgcatcttcaga-3′) and reverse (5′-cccagagtcttcaccgtcttc-3′), beta-actin—forward (5′-cgtgaaaagatgacccagat-3′) and reverse (5′-attgccgatagtgatgacct-3′). The PCR products were quantified relatively to beta-actin mRNA by scanning densitometry (Biodoc II Video Documentation System, Biometra GmbH, Gottingen, Germany). The PCR parameters and detection procedures were estimated to provide a linear relationship between the amount of an input template and the amount of PCR product for all tested mRNAs.
5-HT, 5-HIAA, NA, DA, and DOPAC were determined by high-performance liquid chromatography with electrochemical detection after separation on C4 column (MEB4-5-100, 4.6 × 150 mm, 5 μm particle size, LabAlliance, USA). The dissected brain samples were homogenized in 0.4 ml ice-cold solution of 0.4 perchloric acid containing 3,4-dihydroxy-benzylamine as an internal standard. Homogenates were centrifuged at 15,000×g for 15 min at 4 °C. Then supernatants were filtrated and 20 μl of each was injected into the HPLC system (Waters, USA). The mobile phase consisted of 0.1 M KH2PO4, 0.1 Na2EDTA, octane sulfonic acid (300 mg/l), and 4 % methanol. The flow rate through the system was 1.0 ml/min. Detections were made using an electrochemical detector (Model 105, LabAlliance, USA) set at a potential of +0.75 V versus an Ag/AgCl reference electrode. Peak heights were measured by MultiChrome (Ampersand Ltd, Russia), and sample values were compared with those of standards (1 ng/20 μl; Sigma-Aldrich Corp., St. Louis, MO, USA). The assay limits of detection (signal/noise ratio 3:1) were 5–10 pg on column.
Two-way (factor 1: fluoxetine treatment, factor 2: FST exposure) ANOVA followed by Fisher’s least significant difference (LSD) post hoc test were used. Pearson’s correlation analysis was used to evaluate relationships between distinct parameters. The results were considered significant at probability level <0.05.
Results
5-HT1A Receptor mRNA
Figure 1 illustrates the effects of FST and chronic fluoxetine administration on 5-HT1A receptor gene expression. In untreated rats, exposure to FST increased 5-HT1A receptor mRNA levels in all brain regions investigated when compared with appropriate control animals. Fluoxetine pretreatment did not affect this response of 5-HT1A receptors to FST in the brainstem [main effect of FST: F(1,18) = 8.767, p < 0.01] and frontal cortex [main effect of FST: F(1,16) = 9.843, p < 0.01], although for the cortex, both control and FST receptor mRNA values were reduced in fluoxetine-treated rats [effect of fluoxetine: F(1,16) = 7.951, p < 0.05]. In contrast to these brain regions, no significant FST-induced change in the receptor gene expression was observed in the hippocampus of fluoxetine-treated rats [interaction between FST and fluoxetine: F(1,20) = 8.998, p < 0.01].
Fig. 1.
Effects of FST and chronic fluoxetine on 5-HT1A receptor mRNA levels in the rat brainstem, frontal cortex, and hippocampus. Representative examples of RT-PCR bands corresponding to 5-HT1A and beta-actin mRNAs of each group for all brain regions are shown on the upper parts. *p < 0.05 versus appropriate control group, # p < 0.05 versus water FST group. Results are presented as the mean + SEM. (n = 5–6 per group)
Monoamines
There were no FST or fluoxetine effects on concentrations of 5-HT, NA or DA in brain regions investigated (Table 1).
Table 1.
Effects of the forced swim test and fluoxetine on monoamines and their metabolite concentrations (ng/g tissue) in brain regions
| Brain region, parameter | Water | Fluoxetine | Water versus fluoxetine, p | |||||
|---|---|---|---|---|---|---|---|---|
| Control | FST | p | Control | FST | p | Control | FST | |
| Brainstem | ||||||||
| NA | 431.5 ± 20.6 (9) | 453.9 ± 22.4 (9) | 0.494 | 442.7 ± 17.4 (8) | 428.4 ± 27.2 (9) | 0.663 | 0.738 | 0.425 |
| DA | 60.0 ± 7.7 (9) | 59.0 ± 10.0 (9) | 0.942 | 69.6 ± 11.4 (8) | 68.1 ± 10.0 (9) | 0.912 | 0.496 | 0.509 |
| DOPAC | 28.5 ± 2.1 (9) | 24.8 ± 3.5 (9) | 0.307 | 30.6 ± 2.6 (8) | 23.1 ± 1.9 (9) | 0.051 | 0.590 | 0.654 |
| 5-HT | 530.9 ± 35.5 (9) | 590.2 ± 37.3 (8) | 0.252 | 567.2 ± 47.1 (8) | 521.0 ± 22.1 (9) | 0.370 | 0.480 | 0.183 |
| 5-HIAA | 569.9 ± 41.0 (9) | 618.1 ± 38.4 (8) | 0.337 | 490.6 ± 21.2 (8) | 427.9 ± 33.9 (9) | 0.244 | 0.143 | <0.001 |
| Frontal cortex | ||||||||
| NA | 368.5 ± 27.8 (6) | 321.5 ± 28.7 (6) | 0.303 | 385.9 ± 42.6 (6) | 373.6 ± 23.3 (6) | 0.784 | 0.698 | 0.255 |
| DA | 50.9 ± 7.1 (6) | 43.8 ± 5.3 (6) | 0.465 | 50.0 ± 8.4 (6) | 49.4 ± 5.8 (6) | 0.954 | 0.928 | 0.559 |
| DOPAC | 22.1 ± 3.2 (6) | 17.7 ± 1.2 (6) | 0.376 | 21.7 ± 5.0 (6) | 20.3 ± 2.6 (6) | 0.759 | 0.931 | 0.590 |
| 5-HT | 550.0 ± 41.5 (6) | 515.6 ± 78.3 (6) | 0.688 | 522.5 ± 84.6 (6) | 625.9 ± 59.1 (6) | 0.305 | 0.747 | 0.276 |
| 5-HIAA | 309.7 ± 40.4 (6) | 238.3 ± 36.7 (6) | 0.309 | 317.2 ± 54.3 (6) | 280.6 ± 58.6 (6) | 0.599 | 0.914 | 0.543 |
| Hippocampus | ||||||||
| NA | 285.8 ± 11.4 (7) | 289.2 ± 15.9 (8) | 0.904 | 310.6 ± 16.2 (8) | 297.1 ± 18.7 (8) | 0.597 | 0.428 | 0.721 |
| DA | 57.4 ± 10.7 (7) | 64.4 ± 16.8 (8) | 0.783 | 95.6 ± 20.4 (8) | 64.8 ± 19.0 (8) | 0.251 | 0.189 | 0.987 |
| 5-HT | 637.6 ± 99.3 (7) | 647.0 ± 34.3 (8) | 0.901 | 618.1 ± 35.4 (8) | 720.4 ± 44.9 (8) | 0.216 | 0.817 | 0.313 |
| 5-HIAA | 617.7 ± 50.3 (7) | 580.4 ± 31.0 (8) | 0.433 | 438.4 ± 28.8 (8) | 431.0 ± 22.0 (8) | 0.878 | <0.010 | <0.001 |
Data are presented as mean ± SEM
In contrast to neurotransmitter concentrations, several changes in their metabolism were found in the brainstem and hippocampus. Thus, content of 5-HT metabolite, 5-HIAA, was reduced in the brainstem, especially after FST exposure, and in the hippocampus of both control and FST groups in fluoxetine-treated animals as compared with untreated rats (Table 1). The same profile was seen for 5-HT turnover (Fig. 2). Expressed as the 5-HIAA/5-HT ratio, 5-HT turnover was significantly reduced in the brainstem [F(1,30) = 18.844, p < 0.001] and hippocampus [F(1,27) = 16.170, p < 0.00], but not in the frontal cortex of animals administered with fluoxetine. There was also a trend for FST to decrease the 5-HT turnover in the brainstem [F(1,30) = 3.713, p = 0.0635], however, as detected by direct comparisons, this effect was significant (p < 0.05) only in fluoxetine-treated rats. 5-HT turnover in the frontal cortex and hippocampus at 24 h after the FST exposure did not differ from the appropriate control values.
Fig. 2.
Effects of FST and chronic fluoxetine on 5-HIAA/5-HT ratios in the rat brainstem, frontal cortex, and hippocampus. *p < 0.05 versus fluoxetine control group, # p < 0.05 versus appropriate water group
There were significant effects of FST on the content of DA metabolite, DOPAC, [F(1,31) = 4.702, p < 0.05] (Table 1) and turnover (DOPAC/DA) [F(1,31) = 10.126, p < 0.01] (Fig. 3) in the brainstem.
Fig. 3.
Effects of FST and chronic fluoxetine on DOPAC and DOPAC/DA ratios in the rat brainstem. *p < 0.05 versus fluoxetine control group
Immobility
Long-term fluoxetine administration significantly reduced duration of immobility during the test session compared with untreated animals (water—216.8 ± 9.5 s, fluoxetine—175.6 ± 8.5 s, p < 0.01; n = 10 in each group).
Correlations
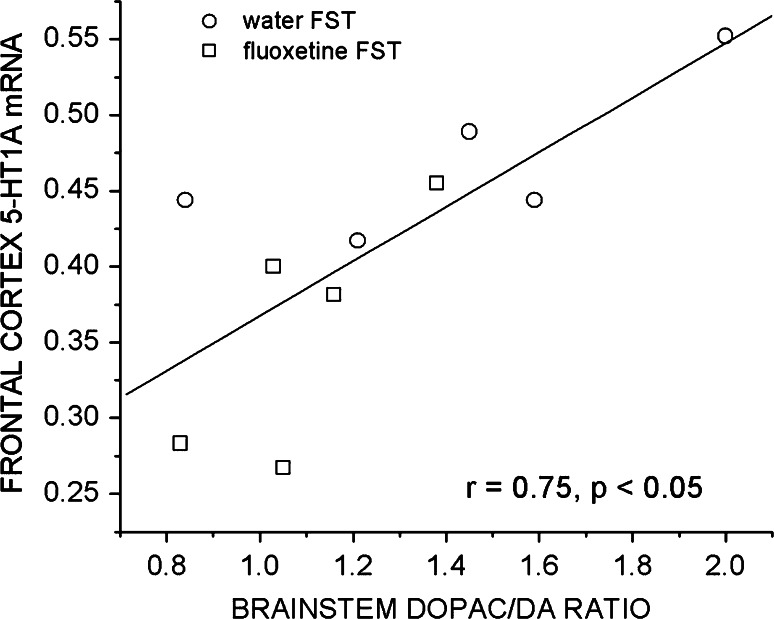
Correlation analysis revealed a positive correlation between the FST values of 5-HT1A receptor mRNA in the frontal cortex and DOPAC/DA ratios (r = 0.75, p < 0.05) in the brainstem (Fig. 4). Significant negative correlation was revealed between the FST values of 5-HT1A receptor mRNA and 5-HT concentrations in the brainstem (r = −0.82, p < 0.05). Both these correlations were insignificant in unstressed rats (cortical 5-HT1A receptor mRNA–brainstem DOPAC/DA, r = 0.07, N.S.; and 5-HT1A receptor mRNA–5HT concentrations in the brainstem, r = −0.23, N.S.).
Fig. 4.
Correlation between FST values of 5-HT1A receptor mRNA in the frontal cortex and DOPAC/DA ratios in the brainstem
Discussion
In the present study we addressed several questions: (1) whether 5-HT1A receptor gene expression in brain regions is affected by stress that induces behavior despair? (2) Does antidepressant treatment influence the response of the receptor mRNA to stress? (3) Are there any relationships between monoamine concentration or metabolism and receptor expression in stressed animals?
The results of this investigation show that exposure of adult rats to the FST, swim procedures of which are stressful for rats, produced an increase in 5-HT1A receptor mRNA level in all brain regions (the brainstem, frontal cortex, and hippocampus) assessed 24 h after the second swim session. The increase in 5-HT1A receptor gene expression in the brainstem after the FST is in agreement with that reported in our previous study (Shishkina et al. 2012) as well as with the increase in gene and protein receptor expression observed in the rat dorsal raphe nucleus after a single-prolonged stress (Luo et al. 2011). An elevation in 5-HT1A receptor antagonist binding was also found in the dorsal raphe 24 h following long (30 min) swim stress (Raghupathi and McGonigle 1997). In the present study, prolonged fluoxetine treatment had no effect on the brainstem 5-HT1A receptor mRNA levels in both control and stressed animals. Consistent with our results, neither the number of 5-HT1A receptor-labeled dendrites in the nucleus raphe dorsalis nor the density of somatodendritic 5-HT receptor labeling on the plasma membrane of these dendrites were affected by chronic treatment with this antidepressant (Riad et al. 2008). Taking into account the involvement of 5-HT1A autoreceptors in the feedback regulation of 5-HT release, it is likely that the increase in the brainstem receptor expression observed after two stress swim sessions may be an adaptive response to limit enhanced 5-HT release within the raphe nuclei and in target areas under acute swim stress (Adell et al. 1997). Activation of 5-HT neurons is confirmed by an increase in expression of Fos in these neurons located in the rat caudal dorsal nucleus after the forced swim for 15 min (Commons 2008). The significance of the brainstem 5-HT1A receptor response to FST in the feedback regulation of these neurons may be supported by negative correlation revealed between stress values of the receptor mRNA and 5-HT turnover found in our study, which is in line with the time course of 5-HT metabolism after FST reported by Herring et al. (2008). In this study, concentration of 5-HT metabolite, 5-HIAA, in such terminal region as hippocampus was transiently increased at 7 h after an acute swim stress and then returned to physiological baseline at 24 h. In our experiments, the increase in the brainstem receptor gene expression 24 h after the second forced swim was accompanied by a moderate decrease of 5-HT turnover in this brain region.
In the frontal cortex, like in the brainstem, FST exposure induced significant increases in 5-HT1A receptor gene expression in rats chronically treated with fluoxetine and untreated animals, while both control and FST values were reduced in fluoxetine-treated rats. In agreement with our data, a significant increase in 5-HT1A receptor mRNA was also observed in the prefrontal cortex of male rats exposed to chronic restraint stress (Iyo et al. 2009). The role of postsynaptic 5-HT1A receptors, especially in the cortex, in the pathophysiology of depression and in the antidepressant drug action is widely discussed. The relevance of the enhancement of 5-HT1A receptor mRNA expression in the frontal cortex for stress-induced behavior despair may be supported by observations on the prenatally stressed rats. These animals, showing increased immobility in the forced swim test, have increased level of cortical 5-HT1A mRNA, and both changes, behavioral and gene expression, were reversed by chronic imipramine treatment (Morley-Fletcher et al. 2004). In our study, chronic fluoxetine administration, in addition to the antidepressant-like effect, also led to a decrease in 5-HT1A receptor gene expression in the frontal cortex. However, using transgenic mice, the antidepressant-like phenotype was linked with an over-expression of the cortical 5-HT1A receptor protein (Günther et al. 2011). Also, pharmacological studies have shown that specific activation of 5-HT1A receptors in the rat frontal cortex significantly decreased immobility time in the FST (Assié et al. 2010). Hence, 5-HT1A receptor protein levels may not match mRNA levels. This assumption is supported, for example, by the observation that in the prefrontal cortex, chronically stressed rats had increased 5-HT1A receptor mRNA levels, although its protein was reduced (Iyo et al. 2009).
As for the hippocampus, there are marked discrepancies in data concerning the modulation of hippocampal 5-HT1A receptor gene expression by a short-term stress. The observed inconsistencies can be explained by differences between the nature and length of the stressor used as well as the number of stress procedures. Thus, rats showed a significant decrease in 5-HT1A mRNA levels in the hippocampus 24 h after exposure to prolonged acute stressor consisted of 2 h restrain, 20 min of swim, and ether vapor (López et al. 1999). No changes in the receptor gene expression were revealed 24 h after exposure to a single swim lasting 15 or 30 min as well as after restraint for 2 h (Raghupathi and McGonigle 1997). And finally, as in our study, a significant increase in the level of hippocampal 5-HT1A receptor mRNA levels was observed in male rats 24 h after the second session of the forced swim test (Dalla et al. 2010). We also found that the increase in hippocampal 5-HT1A receptor mRNA in response to FST evident in untreated animals was absent following chronic treatment with fluoxetine. In contrast to short-term stress, chronic stress in general down-regulated 5-HT1A receptor mRNA in the hippocampus, and the suppressed expression of 5-HT1A receptor gene was shown to be reversed by chronic administration of citalopram (Wang et al. 2009).
Mechanisms of the effects of stress and fluoxetine on 5-HT1A receptor gene expression remain to be elucidated. It has been reported that 5-HT1A receptor density and mRNA expression in terminal regions, including the frontal cortex and hippocampus, are insensitive to both reduced and elevated 5-HT neurotransmission (Krystal and Neumeister 2009) that are often observed after stress, including swim (Adell et al. 1997; Kirby et al. 1995; Linthorst et al. 2002), and fluoxetine treatment (Wong et al. 2005). Corticosterone, the level of which increases under stress, was demonstrated to be implicated in the regulation of 5-HT1A receptor mRNA expression at least in the hippocampus (Neumaier et al. 2000), however, corticosteroids were shown to repress 5-HT1A receptor gene through the negative glucocorticoid response element in its promoter (Ou et al. 2001). Among different transcriptional factors involved in the control of 5-HT1A receptor gene, interesting results were recently reported for Freud-1 (Iyo et al. 2009) that negatively regulates basal 5-HT1A receptor expression in neurons (Ou et al. 2003). In this study, an increase in 5-HT1A mRNA levels in the prefrontal cortex of chronically stressed rats was accompanied by the down-regulation of Freud-1 mRNA and protein levels (Iyo et al. 2009).
There are numerous data supporting the existence of reciprocal relationships between the NA and 5-HT systems (Blier 2001). It is also possible that 5-HT1A receptor function may be affected by NA. In agreement with such hypothesis, neonatal lesion of the central noradrenergic system has been reported to modify the reactivity of this receptor in adult rats (Dabrowska et al. 2008). Concentration of DA metabolite, DOPAC, as well as DOPAC/DA ratio in the brainstem are used as an index of the firing activity of NA neurons and rate of NA release (Buda et al. 1994; Lin et al. 2008). Significant positive correlation revealed between FST values of the 5-HT1A receptor mRNA in the frontal cortex and DOPAC/DA ratios in the brainstem may provide the first indirect evidence for functional association of the receptor expression and NA activity. Thus, stress-induced activation of NA system and subsequent stimulation of the NA release in target areas including frontal cortex (Kvetnansky et al. 2009) may lead to the increase in 5-HT1A receptor gene expression in this brain region, whereas long-term fluoxetine administration, decreasing the activity of NA neurons, effect that was observed in the locus coeruleus after prolonged SSRI administration (Nestler et al. 1990; Szabo et al. 2000), suppresses the cortical receptor expression. However, future analysis and experiments are needed to evaluate this hypothesis.
In conclusion, our data demonstrated up-regulation of the brain 5-HT1A receptor gene expression by the FST. The increases in the receptor expression are likely a part of the neural mechanisms of stress-coping responses. The level of 5-HT1A receptor gene expression in the frontal cortex and the receptor response to swim stress in the hippocampus may be modulated by chronic fluoxetine administration. Taken together, the present findings suggest that the mechanisms of the forced swim and fluoxetine action involve alterations in the brain 5-HT1A receptor gene expression, some of which may be interrelated with concomitant changes in catecholamine metabolism.
Acknowledgments
This study was supported by RFBR grants N 09-04-00284 and N 12-04-01102.
References
- Adell A, Casanovas JM, Artigas F (1997) Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology 36:735–741 [DOI] [PubMed] [Google Scholar]
- Albert PR, François BL (2010) Modifying 5-HT1A receptor gene expression as a new target for antidepressant therapy. Front Neurosci 4:35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Le François B, Millar AM (2011) Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain 4:21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié MB, Bardin L, Auclair AL, Carilla-Durand E, Depoortère R, Koek W, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A (2010) F15599, a highly selective post-synaptic 5-HT(1A) receptor agonist: in vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol 13:1285–1298 [DOI] [PubMed] [Google Scholar]
- Blier P (2001) Crosstalk between the norepinephrine and serotonin systems and its role in the antidepressant response. J Psychiatry Neurosci 26(Suppl):S3–10 [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C (1994) Current advances and trends in the treatment of depression. Trends Pharmacol Sci 15:220–226 [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM (2003) Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry 53:193–203 [DOI] [PubMed] [Google Scholar]
- Brühl AB, Kaffenberger T, Herwig U (2010) Serotonergic and noradrenergic modulation of emotion processing by single dose antidepressants. Neuropsychopharmacology 35:521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda M, Lachuer J, Devauges V, Barbagli B, Blizard D, Sara SJ (1994) Central noradrenergic reactivity to stress in Maudsley rat strains. Neurosci Lett 167:33–36 [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389 [DOI] [PubMed] [Google Scholar]
- Commons KG (2008) Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system. Eur J Neurosci 27:2611–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Leonard BE (2000) 5-HT1A and beyond: the role of serotonin and its receptors in depression and the antidepressant response. Hum Psychopharmacol 15:113–135 [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Nowak P, Brus R (2008) Reactivity of 5-HT1A receptor in adult rats after neonatal noradrenergic neurons’ lesion—implications for antidepressant-like action. Brain Res 1239:66–76 [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z (2010) Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol 106:226–233 [DOI] [PubMed] [Google Scholar]
- Dal-Zotto S, Martí O, Armario A (2000) Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav Brain Res 114:175–181 [DOI] [PubMed] [Google Scholar]
- Gardier AM, Malagié I, Trillat AC, Jacquot C, Artigas F (1996) Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam Clin Pharmacol 10:16–27 [DOI] [PubMed] [Google Scholar]
- Guiard BP, Chenu F, El Mansari M, Blier P (2011) Characterization of the electrophysiological properties of triple reuptake inhibitors on monoaminergic neurons. Int J Neuropsychopharmacol 14:211–223 [DOI] [PubMed] [Google Scholar]
- Günther L, Rothe J, Rex A, Voigt JP, Millan MJ, Fink H, Bert B (2011) 5-HT(1A)-receptor over-expressing mice: genotype and sex dependent responses to antidepressants in the forced swim-test. Neuropharmacology 61:433–441 [DOI] [PubMed] [Google Scholar]
- Haenisch B, Linsel K, Brüss M, Gilsbach R, Propping P, Nöthen MM, Rietschel M, Fimmers R, Maier W, Zobel A, Höfels S, Guttenthaler V, Göthert M, Bönisch H (2009) Association of major depression with rare functional variants in norepinephrine transporter and serotonin1A receptor genes. Am J Med Genet B Neuropsychiatr Genet 150B:1013–1016 [DOI] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Tang PH, Skelton MR, Lucot JP, Gudelsky GA, Vorhees CV, Williams MT (2008) Comparison of time-dependent effects of (+)-methamphetamine or forced swim on monoamines, corticosterone, glucose, creatine, and creatinine in rats. BMC Neurosci 9:49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K (2007) Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res 85:3574–3585 [DOI] [PubMed] [Google Scholar]
- Iyo AH, Kieran N, Chandran A, Albert PR, Wicks I, Bissette G, Austin MC (2009) Differential regulation of the serotonin 1A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience 163:1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841 [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I (1995) Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res 682:189–196 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A (2009) Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res 1293:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M (2009) Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89:535–606 [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M (2004) 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord 3:1–10 [DOI] [PubMed] [Google Scholar]
- Lin Y, Quartermain D, Dunn AJ, Weinshenker D, Stone EA (2008) Possible dopaminergic stimulation of locus coeruleus alpha1-adrenoceptors involved in behavioral activation. Synapse 62:516–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst AC, Peñalva RG, Flachskamm C, Holsboer F, Reul JM (2002) Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci 16:2441–2452 [DOI] [PubMed] [Google Scholar]
- López JF, Liberzon I, Vázquez DM, Young EA, Watson SJ (1999) Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol Psychiatry 45:934–937 [DOI] [PubMed] [Google Scholar]
- Luo FF, Han F, Shi YX (2011) Changes in 5-HT1A receptor in the dorsal raphe nucleus in a rat model of post-traumatic stress disorder. Mol Med Report 4:843–847 [DOI] [PubMed] [Google Scholar]
- Mezadri TJ, Batista GM, Portes AC, Marino-Neto J, Lino-de-Oliveira C (2011) Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J Neurosci Methods 195:200–205 [DOI] [PubMed] [Google Scholar]
- Millan MJ (2006) Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 110:135–370 [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudéry M, Mocaer E, Froger N, Lanfumey L, Laviola G, Casolini P, Zuena AR, Marzano L, Hamon M, Maccari S (2004) Chronic treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT(1A) receptor mRNA in prenatally stressed rats. Neuropharmacology 47:841–847 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, McMahon A, Sabban EL, Tallman JF, Duman RS (1990) Chronic antidepressant administration decreases the expression of tyrosine hydroxylase in the rat locus coeruleus. Proc Natl Acad Sci USA 87:7522–7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Sexton TJ, Hamblin MW, Beck SG (2000) Corticosteroids regulate 5-HT(1A) but not 5-HT(1B) receptor mRNA in rat hippocampus. Brain Res Mol Brain Res 82:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR (2001) Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem 276:14299–14307 [DOI] [PubMed] [Google Scholar]
- Ou XM, Lemonde S, Jafar-Nejad H, Bown CD, Goto A, Rogaeva A, Albert PR (2003) Freud-1: A neuronal calcium-regulated repressor of the 5-HT1A receptor gene. J Neurosci 3:7415–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, London [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391 [DOI] [PubMed] [Google Scholar]
- Raghupathi RK, McGonigle P (1997) Differential effects of three acute stressors on the serotonin 5-HT1A receptor system in rat brain. Neuroendocrinology 65:246–258 [DOI] [PubMed] [Google Scholar]
- Riad M, Rbah L, Verdurand M, Aznavour N, Zimmer L, Descarries L (2008) Unchanged density of 5-HT(1A) autoreceptors on the plasma membrane of nucleus raphe dorsalis neurons in rats chronically treated with fluoxetine. Neuroscience 151:692–700 [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED (2010) 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65:40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC (2009) 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol 88:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN (2007) Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience 150:404–412 [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN (2008) Serotonergic changes produced by repeated exposure to forced swimming: correlation with behavior. Ann NY Acad Sci 1148:148–153 [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Berezova IV, Dygalo NN (2012) Stress-induced activation of the brainstem Bcl-xL gene expression in rats treated with fluoxetine: correlations with serotonin metabolism and depressive-like behavior. Neuropharmacology 62:177–183 [DOI] [PubMed] [Google Scholar]
- Szabo ST, de Montigny C, Blier P (2000) Progressive attenuation of the firing activity of locus coeruleus noradrenergic neurons by sustained administration of selective serotonin reuptake inhibitors. Int J Neuropsychopharmacol 3:1–11 [DOI] [PubMed] [Google Scholar]
- Vergé D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M (1986) Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci 6:3474–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA (2009) Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Res 170:245–251 [DOI] [PubMed] [Google Scholar]
- Wong DT, Perry KW, Bymaster FP (2005) Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov 4:764–774 [DOI] [PubMed] [Google Scholar]