Abstract
Huntington’s disease (HD) is a neurodegenerative disorder characterized by motor, cognitive, and psychiatric symptoms. The most characteristic structural feature of this disease is neurodegeneration accompanied by gliosis in the striatum. BDNF has been proposed to protect striatal neurons from degeneration, because it is an important survival factor for these neurons from development to adulthood. Considering the extensive gliosis and the survival effects of BDNF, we constructed an adenovirus to express a BDNF cDNA in astrocyte cells using a promoter of the glial fibrillary acidic protein gene. Cells stably transfected in vitro with a BDNF cDNA driven by this promoter expressed BDNF and responded to external stimuli increasing BDNF production. When the vector was applied into the striata of mice transgenic for HD, long-term expression of the transgene was observed, associated with a delay of onset of the motor phenotype of the R6/2 HD transgenic mice. The present data indicate that the striatal expression of BDNF is a potential adjuvant for the treatment of HD.
Keywords: Brain-derived neurotrophic factor, Huntington’s disease, Glial fibrillary acidic protein promoter, Adenovirus, Gene therapy
Introduction
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder caused by an expansion of the CAG codon repeat in exon 1 of the huntingtin (htt) gene, which is translated into an extended polyglutamine tract (>35) in the huntingtin protein (Htt) (The Huntington’s Disease Collaborative Research Group 1993). HD is a fatal disorder characterized by progressive motor, cognitive, and psychiatric symptoms, with gross atrophy of the caudate nucleus and putamen accompanied by neuronal loss and astrogliosis as the most striking neuropathological changes (Graveland et al. 1985; Reiner et al. 1988). There are several proposed pathways implied in HD neurodegeneration (Segovia and Arregui 2007), and among them, decreased neurotrophic support by brain-derived neurotrophic factor (BDNF) is one of the most relevant, because expression profiles in some HD models suggest BDNF depletion plays a role in striatal degeneration (Strand et al. 2007); in addition BDNF is crucial for the survival and function of striatal neurons (Baquet et al. 2004; Canals et al. 2004), and it is reduced in the striatum of HD patients; furthermore in some HD models BDNF over-expression ameliorates the HD phenotype (Gharami et al. 2008; Lynch et al. 2007; Pineda et al. 2005; Xie et al. 2010).
BDNF is provided to the striatum by anterograde transport from the cortex, and to a lesser extent from the substantia nigra (Altar et al. 1997) moreover, its transcription and vesicle transport is stimulated by wild type Htt, consequently, in HD patients this leads to decreased BDNF trophic support (Ferrer et al. 2000; Zuccato et al. 2008). This neurotrophin is also decreased in some transgenic mice models for HD (Zuccato and Cattaneo 2007). The medium spiny neurons, the most affected population of neurons in HD, depend on BDNF for their differentiation during development (Ivkovic and Ehrlich 1999; Mizuno et al. 1994), which also protects these neurons from different kinds of insults (Bemelmans et al. 1999; Gratacos et al. 2001; Nakao et al. 1995; Saudou et al. 1998).
Several strategies to increase BDNF in brain have been proposed, to compensate for the deficit that occurs in patients, including indirect methods like environmental enrichment (Spires et al. 2004) and drugs (Apostol et al. 2008), or directly using engineered cells (Dey et al. 2010) and gene therapy (Bemelmans et al. 1999; Kells et al. 2004).
Since HD is a chronic neurodegenerative disease it is necessary to design specific methods to deliver bioactive molecules into the brain for extended periods. To accomplish this goal, we decided to take advantage of astrogliosis, one of the pathological features of this disease which consists of a reaction of astrocytes to brain injury with hypertrophy and up-regulation of the glial fibrillary acidic protein (GFAP) (Buffo et al. 2008), to express BDNF in brain. Astrocytes have many properties that make them excellent candidates for gene therapy, for example: they secrete many different substances including neurotrophic factors (Abiru et al. 1998; Bergami et al. 2008); outnumber neurons by over fivefold; are normal residents of the central nervous system (Sofroniew and Vinters 2010); have a long life span; and are more resistant to different insults when compared with neurons. Thus, astrocytes can be both a sensor of brain damage and a platform to express protective transgenes. Since GFAP is a protein that increases its expression during reactive gliosis, which occurs during brain injury or neurodegenerative diseases such as HD (Selkoe et al. 1982), we decided to use a promoter of this gene (gfa2), that responds to injury and extracellular signals (Brenner et al. 1994; Segovia et al. 1998) to drive the expression of a BDNF cDNA exclusively in astrocytes. In this manner, astrocytes will specifically express the BDNF transgene and generate a feedback mechanism by which increased damage associated with disease progression will up-regulate the transcription of BDNF, the therapeutic protein, for long periods of time.
This gene therapy strategy avoids the need for continuous administration of neurotrophins, because the direct transference of the transgene allows the in situ production of BDNF directly into the striatum. To obtain the long-term expression of the transgenes, cell type specific promoters have been used (Benitez and Segovia 2003). The gfa2 promoter transcriptionally targets the expression of transgenes to astrocytes (Brenner et al. 1994), regulates gene expression in response to injury, and is active for prolonged periods (Brenner et al. 1994). Furthermore, we have already developed transfection and viral transfer systems based on this promoter, and used them in experimental models of Parkinson’s disease and in different glioma models, thus showing its efficacy (Benitez et al. 2007; Cortez et al. 2000; Trejo et al. 1999).
For this work, we constructed an adenovirus that expresses BDNF under the transcriptional control of the gfa2 promoter. First, we demonstrate that these vectors can transduce glial cells in vitro and appropriately express the transgene, thus increasing the production and release of BDNF. We also transferred the adenoviral vector directly to the striatum of R6/2 mice by stereotaxic injection. The in vivo expression of the transgene was corroborated 3 days after the intrastriatal injection and at the end of the behavioral tests (8 weeks after the injection). We assessed the effect of the transgene on several behavioral tests, and found significant delays in the impairment of several tests, including open field and feet-clasping. These data support the use of BDNF as a potential adjuvant for the treatment of HD.
Results
Transgenes Respond to External Stimuli
To increase BDNF levels in HD striatum, we designed a gene therapy strategy that transcriptionally targets BDNF expression to astrocytes. Astrocytes are part of the neural tissue, a long lived cell population, more resistant to different kinds of damage compared to neurons and posses an efficient secretory system (Cortez et al. 2000). Thus, to transcriptionally target the expression of BDNF to astrocytes, we subcloned a rat BDNF cDNA containing the full open reading frame into the multiple cloning site of the pgfa2aMCS plasmid which posseses a human promoter of the GFAP (gfa2), an intron and the polyadenylation signal of the mouse protamine 1 (mp-1). A schematic representation of the pgfa2BDNF vector is presented in Fig. 1a. Then, using the previous expression cassette, we produced replication-defective recombinant adenovirus using the AdEasy System (gfa2-BDNF adenovirus, AdB) (He et al. 1998). As a control, we used an adenovirus that expresses LacZ (gfa2-LacZ adenovirus, AdZ) which also has the gfa2 promoter and mp-1 fragment as previously reported (Benitez et al. 2007).
Fig. 1.
Glial-specific expression of transgenic BDNF and its response to external stimuli. a Schematic representation of the gfa2-BDNF-mp1 cassette shows a human promoter of the GFAP (gfa2), a rat cDNA of brain-derived neurotrophic factor and the polyadenylation signal and intron of mouse protamine-1 (mp-1). Arrows show where the oligonucleotides align to identify the specific expression of the transgene. b Top panel, analysis by RT-PCR of transgenic BDNF mRNA expression in C6 (rat astrocytoma) U373 (human glioblastoma) cells and COS-1 cells (monkey kidney) after adenoviral transference. Bottom panel, β-actin expression as control. − negative control, no DNA added; + pgfa2BDNF positive control, using plasmid; fsk, forskolin; u/t, untreated. c Immunocytochemistry to determine BDNF expression after adenoviral transduction with gfa2-BDNF adenovirus (AdB), and gfa2-LacZ adenovirus (AdZ) in glial cells (C6 and U373) and in COS-1 cells; bar represents 100 μm. d Determination of BDNF released, as assessed by ELISA, from glial (C6 cells) stably expressing pGfa2BNF or pGfa2LacZ with high K+ (50 mM) or in control, not-stimulating conditions (u/t); bar represents mean ± SEM. n = 3 ANOVA followed by Newman Keuls ★★ P < 0.001
In order to determine the glial-specific expression of the AdB vector, we infected C6 (murine glioma), U373 (human astrocytoma) glial cell lines and as negative control COS-1 cells (monkey kidney); then, the specific glial expression of the transgene was determined by RT-PCR. We used primers that specifically recognized transgenic mRNA; amplifying a chimeric fragment composed of a BDNF fragment together with mp-1 from the expression vector, as is indicated with arrowheads in Fig. 1a. We only observed expression of transgenic BDNF in transduced glioma derived cells (C6 and U373) (Fig. 1b, top panel), but not in COS-1 cells, which indicates the specific expression of the vector in cells of glial origin. We stimulated cells with forskolin, an adenilate cyclase activator previously reported to increase the activity of the gfa2 promoter (Segovia et al. 1994), and observed an increased expression of BDNF mRNA in stimulated glial cells. Also, the RT-PCR fragment amplified from transduced cells is smaller than the product amplified from the positive control (pgfa2BDNF plasmid), an effect caused by the excision of the intron of protamine-1, this is an evidence of the appropriate processing of the transgenic mRNA. We also amplified by RT-PCR a fragment of β-actin as a control of the integrity of the RNA (Fig. 1b, bottom panel). To ascertain the appropriate processing and translation of the transgenic mRNA, we observed increased levels of BDNF protein, as determined by immunocytochemistry, and only observed increased BDNF expression in glial cells transduced with AdB, but not in COS-1 cells or cells transduced with AdZ (Fig. 1c).
We also determined whether glial cells were capable of releasing transgenic BDNF to the extracellular medium, thus we stably transfected C6 cells with the pgfa2BDNF or pgfa2LacZ vector and measured by ELISA the levels of BDNF released to the medium by cells stimulated or not stimulated with high potassium (Abiru et al. 1998). We found a statistically significant increase of BDNF in the media from cells transfected with BDNF (C6BDNF) compared with C6LacZ cells, which was significantly higher when the cells were stimulated with high potassium to increase exocytosis (Fig. 1d). These results demonstrate that glial cells not only produce transgenic BDNF, but can also release it to the extracellular medium in basal conditions and increase both its production and secretion in response to external stimuli like high potassium and forskolin.
Increased BDNF Levels in Primary Cultures of Astrocytes After Adenoviral Transduction with AdB
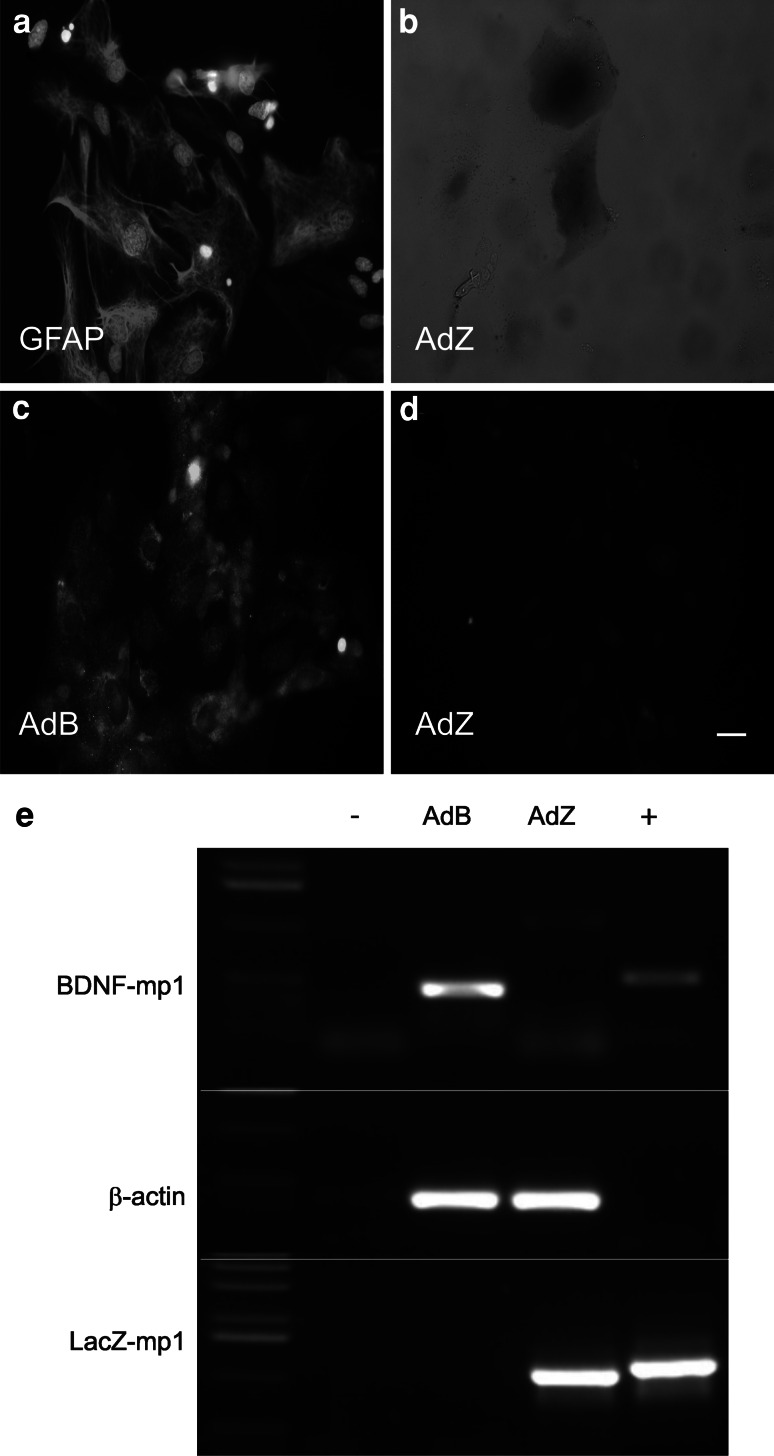
To further determine whether we could increase BDNF levels in astrocytes, we used primary cultures of mouse astrocytes. We characterized primary cultures of astrocytes by immunofluorescence for GFAP, and found that the cultures were highly enriched with astrocytes (Fig. 2a). Then we transduced these cultures with the AdZ vector, and by cytochemistry identified the expression of the lacZ gene (blue precipitate). This shows that the AdZ virus is functional and that the cultures could be infected with adenoviral vectors and once transduced, astrocytes expressed the transgenes driven by the gfa2 glial-specific promoter (Fig. 2b). Next, we transduced the primary cultures with AdB, and evaluated the expression of BDNF by immunocytochemistry. We found an increased expression of BDNF exclusively in astrocytes infected with AdB (Fig. 2c) but not in AdZ infected cells (Fig. 2d). To insure that the expression of the transgenes was due to the adenoviral vectors, we amplified by RT-PCR a specific fragment of the transgenes. We found both the expression of BDNF and lacZ transgenes only in astrocytes transduced with the corresponding virus (Fig. 2e, top and bottom panels). As a positive control of each PCR of the transgenes, we used as template the plasmid that contains each transgene. After transgene transcription, there is again the excision of the intron of protamine-1, resulting in a difference in size between the product amplified from the transduced cells and the plasmids. These results confirm that primary cultures were infected with adenovirus and expressed the transgenic mRNAs which were also correctly processed by astrocytes. We also amplified a fragment of β-actin as a control of the integrity of the RNA (Fig. 2e, central panel).
Fig. 2.
Transgene expression in primary astrocyte cultures transduced with adenoviral vectors. a Characterization of primary astrocyte-enriched primary cultures by immunocytochemistry against GFAP, nuclei counterstained with DAPI. b Detection of LacZ expression by cytochemistry. c, d Expression of BDNF revealed by immunocytochemistry in cells transduced with AdB (c) compared with AdZ transduced cells (d). a–d Bar represents 100 μm. e RT-PCR assays to detect the specific expression of the transgenes in primary cultures of astrocytes. Top panel, expression of transgenic BDNF mRNA in primary astrocyte cultures. Central panel, expression of β-actin as control. Bottom panel, expression of LacZ transgenic mRNA. − negative control, no DNA added; + pgfa2BDNF or pgfa2LacZ as positive controls
Adenoviral Transduction of BDNF into the Striatum of R6/2 Mice Delays the Onset of the Motor Phenotype of R6/2 Mice
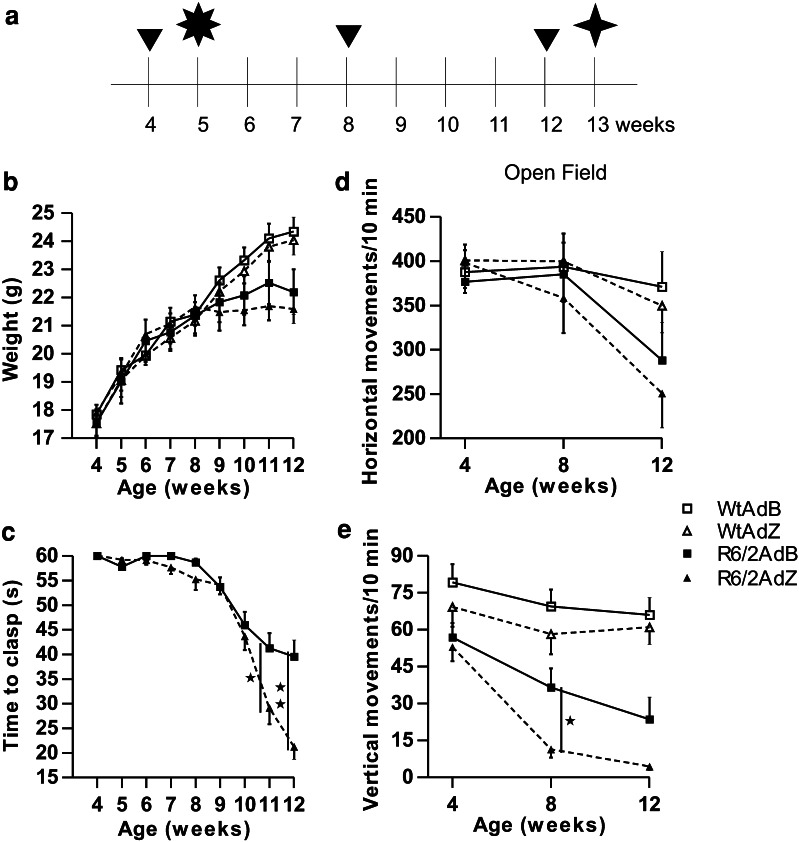
Transgenic R6/2 mice were obtained employing a DNA genomic fragment containing a mutated exon 1 of the human HD gene, these mice develop a progressive neurological phenotype which includes feet-clasping, failure to gain weight at a normal rate, and reduced mobility at advanced stages of phenotypic behavioral expression (Mangiarini et al. 1996). To examine whether the adenoviral transfer of BDNF would prevent the progression of the behavioral phenotype, we followed the protocol shown in Fig. 3a. Mice were weaned and genotyped at 3.5 weeks of age, were then divided into two groups according to genotype, wild type (Wt) and transgenic (R6/2) mice. At week 4, the following parameters: weight, feet-clasping, hand wire endurance, open field activity, climbing, and rotarod endurance were registered to record the basal behavior. At week 5, both groups of mice (Wt and R6/2) were injected bilaterally in the striata with the adenoviral vectors AdB or AdZ, the last one as control. After that, we measured feet-clasping, hand wire endurance and balance on the rotarod every week until week 12. To avoid habituation, climbing and open field test were only performed at weeks 4, 8, and 12. To explore the general health status of the mice, their weight was registered every week, and data are shown in Fig. 3b. AdZ-treated R6/2 mice presented a decrease in weight gain that was statistically different at week 11 compared with both groups of wild type mice (P < 0.05). At week 12, both groups of R6/2 mice (AdB and AdZ) were statistically different compared with both groups of wild type mice (P < 0.05). Between both groups of R6/2 mice there were no statistical differences at any time registered. This indicates that AdB adenoviral transduction does not have an effect on weight decrease.
Fig. 3.
Effect of intrastriatal transgene administraton in wild type and transgenic mice. a Schematic timeline showing when the behavioral tests were performed. Arrowheads indicate when open field tests, and climbing tests were performed. Weight, feet-clasping, grip strength, and rotarod tests were recorded every week from 4 to 12 weeks of age. The asterisk indicates adenoviral administration, and  indicates when mice were euthanized. b Weight of wild type and R6/2 mice treated with AdB or AdZ. c Time to present complete feet-clasping behavior of R6/2 mice. d Horizontal movements of mice in the open field behavioral test. e Vertical movements of mice in the open field test. Data are means ± SEM (n = 12–18 mice per group). ANOVA followed by Newman Keuls. ★
P < 0.05; ★★
P < 0.001. Symbols represent: R6/2 mice treated with AdZ filled triangle; Wt mice treated with AdZ open triangle; R6/2 mice treated with AdB filled square; and Wt mice treated with AdB open square
indicates when mice were euthanized. b Weight of wild type and R6/2 mice treated with AdB or AdZ. c Time to present complete feet-clasping behavior of R6/2 mice. d Horizontal movements of mice in the open field behavioral test. e Vertical movements of mice in the open field test. Data are means ± SEM (n = 12–18 mice per group). ANOVA followed by Newman Keuls. ★
P < 0.05; ★★
P < 0.001. Symbols represent: R6/2 mice treated with AdZ filled triangle; Wt mice treated with AdZ open triangle; R6/2 mice treated with AdB filled square; and Wt mice treated with AdB open square
As previously indicated feet-clasping is a characteristic behavior of R6/2 mice that appears after tail suspension. Wt mice did not show this behavior, so we present only the data from R6/2 mice. AdB-treated mice presented a longer latency to clasp since week 11 compared with AdZ mice, and it was of 41% (41.28 ± 3.03 and 29.12 ± 3.29 s, respectively) at week 12 (P < 0.001) (Fig. 3c). This shows that AdB treatment of R6/2 mice reduces feet-clasping behavior.
Decreased mobility has been reported for R6/2 mice, so we explored this behavior using the open field test. We found no statistical differences between genotype or treatment groups in the horizontal movements at any age, although the reduction in AdZ R6/2 mobility was noticeable (Fig. 3d). However, in the vertical movements, there was a statistical difference at 8 weeks between the AdB and AdZ R6/2 mice (Fig. 3e). At this age, AdZ R6/2 mice were also different from both groups of control mice (P < 0.001), and AdB R6/2 mice were only different from AdB Wt mice (P < 0.001) but not from AdZ Wt mice. At week 12, there was no difference between AdB and AdZ R6/2 mice, but both were different from AdB and AdZ Wt mice groups (P < 0.001). Thus, AdB treatment delays the onset of the impaired mobility that occurs in R6/2 mice.
AdB Reduces the Deficits on Climbing Behavior of R6/2 Mice
Climbing behavior was evaluated because it is a normal spontaneous activity of mice. In all the climbing parameters that we explored, R6/2 mice presented a deficit at 8 and 12 weeks, as was previously reported, when compared with wild type mice, however, we did not find statistical differences at 4 weeks between transgenic and control wild type animals, perhaps because of differences in mice colony or the detection methods (Hickey et al. 2005). The administration of adenovirus had no effect on Wt mice treated with either AdB or AdZ. In the latency to climb 8-week-old AdB-treated R6/2 mice began to climb in less time than AdZ R6/2 mice (P < 0.05). But the AdB R6/2 mice were also different from both groups of Wt mice, Wt AdB (P < 0.001), and Wt AdZ (P < 0.05). By week 12, there were no differences between R6/2 treated mice and both were different from the controls (P < 0.001) (Fig. 4a). We found no statistical difference in the time spent climbing by R6/2 mice treated both with AdB or AdZ virus (Fig. 4b), neither in the climbing events (Fig. 4c). Although the baseline of R6/2 mice treated with AdZ at week 4 in Fig. 4b appears already lower, it is not statistical different from the wild type mice. For both parameters we found a statistical difference between Wt mice and R6/2 mice at 8 and 12 weeks of age with and no difference between the two transgenic groups (P < 0.001). Finally, there was a statistical difference between AdB and AdZ-treated R6/2 mice in the number of rearing events during the climbing test at 12 weeks old (Fig. 4d). Also there were a statistical difference between Wt and R6/2 mice at 8 and 12 weeks old in both treatments (P < 0.001).
Fig. 4.
Mitigation of climbing deficiencies in R6/2 mice after adenoviral glial-specific transference of BDNF. a Latency to climb, determined as the time for the first climbing event. b Number of climbing events. c Time spent climbing, total time that mice spend climbing. d Rearing, number of times the mice stood on their rear feet but did not climb. Symbols represent: R6/2 mice treated with AdZ filled triangle; Wt mice treated with AdZ open triangle; R6/2 mice treated with AdB filled square; and Wt mice treated with AdB open square. Data are mean ± SEM (n = 12–18 mice per group). ANOVA followed by Newman Keuls. ★ P < 0.05
AdB-Treated R6/2 Mice Remain for Longer Periods Hanging Under the Grid and on the Accelerating Rotarod
To determine whether the adenoviral transference of AdB in R6/2 mice had an effect on grip force, we placed the mice at the center of a grid that was then inverted, so the mice stay hanging from the grid. During the time the mice remain hanging, we also measured the number of movements they performed, determined as the number of crosses over marks drawn on the grid. We only report R6/2 mice grip force, because Wt mice did not fall from the grid at any age during the time tested. We present the grip force of R6/2 mice as the time the mice remained suspended (Fig. 5a). Since week 9, AdB-treated mice remained more time than AdZ-treated mice, but only at week 12 the difference was statistically different (P < 0.001). The number of movements presented by R6/2 mice treated with AdB compared with AdZ mice was statistically significant at week 5 (P < 0.001) and at week 6 the R6/2 AdB-treated mice continued to show more movements than R6/2 AdZ mice (P < 0.05) (Fig. 5b). AdB treatment delays the time of movement decrease under the grid in R6/2 mice. The last behavioral test evaluated was the balance of mice on an accelerating rotarod (2–40 rpm in 5 min). Since week 5, both Wt mice groups were statistically different from both groups of R6/2 mice (P < 0.001). We found that R6/2 mice treated with AdB, remained more time on the rod at week 5 of age compared with AdZ mice (P < 0.05) (Fig. 5c). For the remaining tests mice treated with AdB continued more time in average on the rotarod, but there were no statistical differences between the two groups. We also analyzed the velocity at which mice fall from the rod, and in this parameter, we did not find a statistical difference between the R6/2 mice groups, but again there were different from both treated Wt mice (P < 0.001) (Fig. 5d).
Fig. 5.
Adenoviral transduction of BDNF decreases deficits on grip force and rotarod of R6/2 mice. a Time R6/2 mice hold under the grid. b Number of crosses over the grid of R6/2 mice, while clinging to the grid. c Time mice remained on the accelerating rotarod. d Velocity at which mice falls from the accelerating rotarod. Symbols represent: R6/2 mice treated with AdZ filled triangle; Wt mice treated with AdZ open triangle; R6/2 mice treated with AdB filled square; and Wt mice treated with AdB open square. Data are mean ± SEM (n = 12–18 mice per group). ANOVA followed by Newman Keuls. ★ P < 0.05; ★★ P < 0.001
Transgenic BDNF Expression Persists for 8 Weeks in the Striata of R6/2 Mice
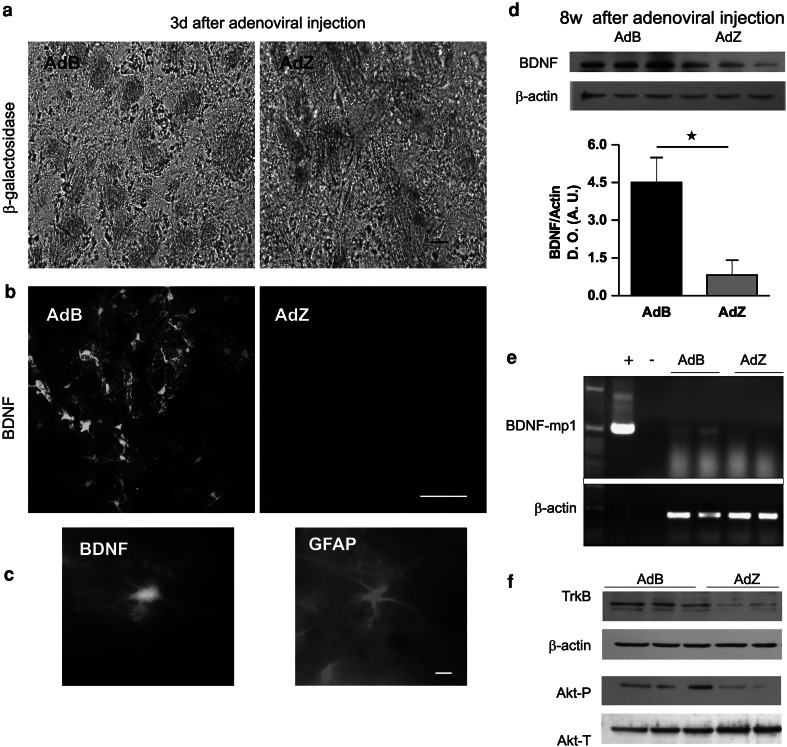
First, we wanted to confirm the transduction of AdB and AdZ virus in vivo, so we injected the adenovirus in the striata of mice, and analyzed the expression of the transgenes 3 days after administration. For AdZ we performed histochemistry for X-gal, and found the blue precipitate only in AdZ transduced striata, but not in the AdB infected striata (Fig. 6a). To detect BDNF expression after AdB transduction, we carried out immunohistochemistry assays (Fig. 6b). As shown, we observed an important increase of BDNF in AdB transduced striatum compared with AdZ infected striatum. The glial-specific expression of BDNF was confirmed by the co-localization of BDNF and GFAP as shown by a representative image (Fig. 6c). To determine whether increased BDNF levels persisted for longer periods western blot assays were used to detect the active form of BDNF (Mowla et al. 2001) in the striatum of mice injected with AdB at the end of the behavioral tests (13-week-old mice). As a control we also assayed for the expression for β-actin in the same blots (Fig. 6d, top panel). The increase of BDNF persists for at least 8 weeks after the intrastriatal injection of AdB, and was statistical different when compared with AdZ-treated mice (Fig. 6d, bottom panel). Western blot analysis showed a 53% increase of BDNF in AdB-treated mice when compared with AdZ mice, 3 days after administration (data not shown), and a difference of 426% in 13-week-old animals (Fig. 6d, bottom panel). These data show a sustained and strong expression of the BDNF transgene. To confirm that the increased BDNF levels are caused by the transfer of transgenic BDNF, we detected by RT-PCR the specific expression of the BDNF transgene only in the striatum of mice injected with AdB but not with AdZ (Fig. 6e, top panel). We also amplified a fragment of β-actin as a control of the integrity of the RNA (Fig. 6e, bottom panel). These results confirm the glial-specific transduction of AdB into the striatum of R6/2 mice increased BDNF levels that persisted for at least for 8 weeks, and reduced some motor behavioral deficits. Furthermore, to determine the biological activity of transgenic BDNF, we ascertained the levels of TrkB, the BDNF receptor, and of phosphorylated Akt, as indicators of the BDNF-induced intracellular signaling, and determined, by western blot analysis, that both parameters are elevated in the striata of R6/2 mice that received BDNF, compared with the lacZ-expressing controls (Fig. 6e, f). TrkB levels, determined by the densitometry of western blot assays, were determined as absorbance arbitrary units of AdB-treated mice (0.664) and AdZ-treated mice (0.03), thus indicating a robust stimulation induced by transgenic BDNF, n = 3.
Fig. 6.
In vivo expression of BDNF in the striatum of R6/2 mice after AdB transduction. a Striatal expression of β-galactosidase after transduction with AdZ but not with AdB, revealed by histochemistry with X-gal. Bar represents 100 μm. b BDNF expression 3 days after of adenoviral administration determined by immunohistochemistry. Bar represents 40 μm. c Astrocyte-specific expression of BDNF after AdB transference revealed by double immunohistochemistry against BDNF (left) and GFAP (right). Bar represents 10 μm. a–c Results obtained 3 days after intrastriatal adenoviral injection. d Sustained increased expression of BDNF in the striatum of R6/2 mice transduced with AdB measured by western blot for BDNF, and β-actin as control (top panel). Bottom panel, densitometry analysis of BDNF levels in the striata of R6/2 mice transduced with AdB or AdZ. Optic density (O.D.), arbitrary units (A.U.). Bars are mean ± SEM (n = 3 mice per group). Unpaired t test ★P < 0.05. eTop panel, specific expression of BDNF-mp1 transgene determined by RT-PCR in the striata of AdB transduced mice, but not in the AdZ-transduced mice. Bottom panel, β-actin as control. f TrkB expression and Akt activation in the striata of mice transduced with AdB compared with AdZ transduced mice. Top panel shows a representative figure of TrkB expression, β-actin is shown as control. Bottom panel, Akt-P (phosphorilated Akt), and Akt-T (total Akt) expression in mice striata. d–f Data were obtained after 8 weeks of adenoviral injection
Discussion
In HD, reactive gliosis is a very important feature considering that astrocyte–neuron ratio can be, in the last stage of the disease, as high as 65 reactive astrocytes per every 2 neurons in the HD striatum (Vonsattel et al. 1985). During reactive gliosis, astrocytes suffer modifications like hypertrophy and up-regulation of GFAP (Selkoe et al. 1982). The AdB vector was designed to specifically drive the expression of BDNF in astrocytes using the gfa2 promoter. Thus, the promoter of this protein could act as a sensor of brain damage, and together with BDNF, can act as a positive feedback system that after sensing damage can increase the production of BDNF in order to protect the affected neurons.
The GFAP promoter has been previously used in therapeutic strategies for HD expressing NGF (Kordower et al. 1997) or BDNF (Giralt et al. 2010). In the latter experiments transgenic mice expressing the neurotrophin under the control of a GFAP promoter were developed, and then stem cells or astrocytes were transplanted from these mice to the striata of rats or nude mice where it was found the grafts protected neurons from quinolate administration. YAC128 transgenic mice have also been generated that express BDNF under the transcriptional control of α subunit of Ca2+/calmodulin-dependent protein kinase II, and behavioral recovery was observed (Xie et al. 2010). Instead of using cell therapy, which clinical outcomes have been controversial (Cicchetti et al. 2009), we used in vivo gene therapy to transfer the BDNF transgene, because it can be placed directly in the region of interest to transduce cells without using donor cells, thus avoiding several of the problems associated with cellular implants from a external donor (Lim et al. 2010).
The in vitro assays were performed to confirm that cells of glial origin were capable of producing and secreting transgenic BDNF and respond to external stimuli; cells were stimulated with high potassium and we detected higher levels of BDNF in the culture media when compared with control cells. Astrocytes have a very well developed machinery for exocytosis normally employed during gliotransmission, and it has been demonstrated that cortical astrocytes secreted pro-BDNF when induced by theta burst stimulation (Bergami et al. 2008). The mechanisms involved in astrocyte BDNF secretion we observed, could be via a SNARE-dependent mechanism homologous to that reported for cortical astrocytes used to recycle pro-BDNF (Bergami et al. 2008).
Then, to test the potential beneficial effects of the adenoviral glial-specific gene therapy system to overcome the striatal BDNF deficit, we used the direct adenoviral intracerebral transduction of the striatum of R6/2 mice, a frequently used model of HD (Arregui and Segovia 2009). These mice exhibit a progressive neurological phenotype, which includes involuntary stereotypic movements that we can test for behavioral improvements (Mangiarini et al. 1996).
Even though, we only observed a delay on the onset of the motor phenotype, or in certain tests a partial recovery of the HD phenotype, it should be considered, that the expression of the transgene was limited to a region of the striatum, and not to all the brain areas affected by the disease, and obtained after a single adenoviral injection. The AdB treatment, did not affect mice body weight of either genotype, but there is a small trend to increase weight in AdB-treated mice compared with controls, possibly because of an improved motor status, which allows mice to eat more compared with AdZ-treated mice. Weight is not modified by other experimental therapies for HD like CEP-1347, an inhibitor of mixed lineage kinases (Apostol et al. 2008) or by ceftriaxone a β-lactam antibiotic which increase GLT-1 expression (Miller et al. 2008), although those treatments improved the motor performance of R6/2 mice as our treatment. The AdB-treated R6/2 mice presented a delay to show clasping behavior compared with AdZ-treated R6/2 mice, a typical neurological symptom of these mice (Mangiarini et al. 1996) which is a parameter frequently used to evaluate different HD therapies like CEP-1347, a kinase inhibitor that increased the delay of clasping behavior by 60% and restored to normal levels BDNF in the cortex of 10-week-old R6/2 mice (Apostol et al. 2008). The AdB treatment of R6/2 mice caused a significant delay in feet-clasping behavior since week 11 and the delay was of 41% in 12-week-old mice compared with AdZ-treated mice.
Another group evaluated the open field behavior of R6/2 mice receiving BDNF using an adenoviral vector with a CMV promoter, and found no improvement on horizontal movements compared with controls, similar to our report (Cho et al. 2007). These investigators only found a delay in motor impairment after the simultaneous adenoviral transduction of BDNF together with Noggin (a soluble inhibitor of bone morphogenetic proteins) which in concert increased neostriatal neurogenesis (Cho et al. 2007). However, we found differences in the vertical movements on open field of 8-week-old transgenic mice compared with controls, a behavior not analyzed by Cho et al. Since in R6/1 mice rearing is increased in young animals, and is reduced in older animals (Perez-Severiano et al. 2000), we consider that AdB, moderates the worsening of this behavior.
Climbing behavior permits the detection of early deficits during the normal spontaneous behavior of mice (Hickey et al. 2005). We found that the administration of AdB decreased the latency to climb of 8-week-old R6/2 mice, and slowed the decline of rearing during this test compared with AdZ mice in 12-week-old animals, a behavior which diminished at this age (Steele et al. 2007). These results confirm that the AdB treatment improves some of the parameters recorded during the climbing test, and they coincide with the improvement in vertical movements or rearing observed during the open field test.
Grip force control is the ability to skillfully manipulate objects, and depends on the force adjusted to the task. The basal ganglia are involved in the control of precision grip force, and both are disturbed in HD patients (Prodoehl et al. 2009). We found a significant improved performance on the grid force of R6/2 treated with AdB which could be explained by a recovery of striatal functioning compared with AdZ control mice. The last behavioral test that we explored was the rotarod, this is a widely used motor behavioral test, but the protocols employed vary considerably. The sensitivity to detect motor deficits depend on the task and the protocol employed, being the accelerating rotarod more sensitive than fixed speed rotarod to detect early motor deficits (Pallier et al. 2009). We report differences in the accelerating rotarod test, between 5-week-old AdB and AdZ-treated R6/2 mice. The study of Cho et al. 2007, did not found a behavioral improvement in this test after adenoviral transduction of the striatum, the difference could be due the different promoter and the rotarod protocol employed, since we used an accelerating protocol whereas the previous work used a constant speed protocol (Cho et al. 2007).
The adenoviral glial-specific transference of a BDNF cDNA was confirmed at both short (3 days) and long periods of time (8 weeks) after intracerebral administration. The latter period is considered an extended time of expression for a viral vector, for example transgene expression using baculovirus with an improved GFAP promoter that contains a CMV enhancer and ITR from adenovirus lasts for 90 days, whereas with GFAP and CMV promoters the expression was undetectable at this time (Wang and Wang 2006). Transgenes driven by the gfa2 promoter can be transcribed for extended periods, because it remains active throughout postnatal life (Brenner et al. 1994).
The increased levels of striatal BDNF accompanied by augmentation of both TrkB and Akt-P suggests that the Akt pathway was stimulated and participated in the behavioral improvement. Neurotrophin signaling is very complex, and for BDNF it is mediated through two receptors: TrkB and p75. We examined TrkB signaling because this pathway leads to cell survival and/or differentiation. The TrkB receptor is associated with three principal signaling pathways; the extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K), and phospholipase Cγ pathways (Chao 2003). We decided to analyze the pro-survival AKT pathway because it has been shown to be altered in HD patients and in a transgenic model (Colin et al. 2005), and this pathway also mediates BDNF induction of dopamine and cyclic AMP-regulated phosphoprotein 32 kDa (DARP-32) in medium size spiny neurons (Bogush et al. 2007).
The concept of using growth factors to protect neurons in neurodegenerative diseases, including HD, has been extensively studied (Levy et al. 2005). Although there are sound bases to support their potential therapeutic approach, contradictory reports have appeared. For instance the expression of CNTF induces deleterious effects in an HD experimental model (Denovan-Wright et al. 2008) which can mean that in some cases, an excess of neurotrophins can be harmful, thus their expression needs to be carefully regulated. Also BDNF expression by itself has not been associated with behavioral recovery in an HD model, but it was necessary the simultaneous expression of BDNF and Nogging for behavioral recovery to occur, a protocol that appears to increase neurogenesis in the striatum (Cho et al. 2007). However, another study concludes that BDNF does not induce adult neurogenesis (Galvao et al. 2008). The difference between these studies could be the context because the study that concludes that BDNF and Noggin induced neostriatal neurogenesis was developed in an HD model and the other on wild type mice, and it has also been proposed that there is increased neurogenesis in HD patients (Curtis et al. 2003). In this work, we have not determined the effect of transgenic BDNF on striatal adult neurogenesis; thus we cannot, at this point, assert whether the behavioral improvements we observed are caused by increased neurogenesis or by protecting post-mitotic neurons. However, based on the study of Cho et al. we expected that neurogenesis could be increased with BDNF expression, but they also showed that is not enough to induce behavioral recovery (Cho et al. 2007). So we attribute the delay of onset or partial recovery to a better performance or survival of the adult cells, both neurons and glia, because we found that transgenic BDNF induces the activation the Akt, an antiapoptotic kinase, which improves locomotor performance in a Drosophila HD model when was co-expressed with mutant Htt in glial cells (Lievens et al. 2008). And also BDNF expression has been linked to behavioral recovery in different HD models (Gharami et al. 2008; Lynch et al. 2007; Pineda et al. 2005; Xie et al. 2010).
Many experimental trials in HD mice models have demonstrated that BDNF administration can be beneficial to striatal neurons, but delivery strategies and BDNF bioavailability need to be further improved (Zuccato and Cattaneo 2007). Thus, to clinically use a gene therapy approach based on BDNF, great care regarding doses, regulation of gene expression, as well as a precise anatomical location and time of administration should be observed. Also, cellular responses to BDNF are very different depending on how it is delivered, acute or gradual administration, which leads to different responses and morphological effects in neurons (Ji et al. 2010). In general the continuous infusion of a drug reduces its toxicity compared with the methods of large doses applied in brief periods, and this is what probably happened with BDNF negative effects like weight loss and seizures (Cunha et al. 2009). For this reasons, we present a method to express BDNF in a regulated and specific manner, in a restricted area, that avoids the problems associated with the grafting of tissue or genetically modified cells, and which delays the onset or induces partial behavioral recovery in an HD model. Therefore, we consider that this is a potentially relevant strategy that may serve as an adjuvant for the treatment of HD.
Materials and Methods
Vector Production
The pgfa2BDNF vector was designed to express a rat BDNF cDNA under the transcriptional control of a human promoter of the GFAP (gfa2). The vector was obtained by subcloning a rat BDNF cDNA (generously donated by Dr. M. Mouradian National Institutes of Health, Bethesda, MD) into the pgfa2aMCS plasmid using the enzymes NotI and SpeI. This plasmid also contains an intron and the polyadenylation site of the mouse protamine-1 (Trejo et al. 1999). The resulting pgfa2BDNF plasmid was analyzed by restriction mapping and then sequenced.
The adenoviral vectors were produced according to the pAdEasy System (http://www.conloncancer.org) (He et al. 1998). Briefly, the gfa2-BDNF-mp1 fragment was excised from the pgfa2BDNF plasmid using BglII, subcloned into the pShuttle vector to produce the pSgfa2BDNF vector and the orientation of the cDNA was determined by restriction mapping. The recombinant virus AdB was expanded in HEK293 cells and purified by ultracentrifugation in a cessium chloride gradient. Viral titers were determined by end point cytopathic effect assay on HEK293 cells and are expressed as plaque-forming units per milliliter (pfu/ml). As a control, we employed the AdSLacZ virus previously reported, but here renamed as AdZ (Benitez et al. 2007).
Cell Culture and Cell Transduction
COS1, C6, HEK 293, and U373 cells were grown as previously described (Benitez et al. 2007). Enriched primary cultures of rat astrocytes were prepared and maintained according to Cortez et al. (2000). All cells were grown at 37°C in a 5% CO2 humidified atmosphere. COS1, C6, and U373 cells were transduced with a MOI of 100 with AdB or AdZ, 24 h after transduction the expression of the transgenes was analyzed.
Transfection and ELISA
C6 cells were stably co-transfected with either pgfa2BDNF or pgfa2LacZ together with the pBABEpuro plasmid, which was used to select transfected cells using a 10:1 ratio, with Lipofectamine as indicated by the manufacturer (Invitrogen, Carlsbad, CA). Two days after lipofection, cells were selected with puromicin (5 μg/ml). Then, cells were subcloned and maintained in selection medium. C6BDNF and C6LacZ cells were seeded in medium without serum for 24 h with or without high potassium (50 mM), the medium was recovered, and BDNF levels were measured by ELISA as indicated by the manufacturer (Promega Corporation, Madison WI).
RT-PCR Assays
RNA was extracted using the TriPure reagent (Roche, Indianapolis, IN). Five micrograms of total RNA were reverse-transcribed with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA), and 5 μl of the resulting cDNA amplified by PCR. The primers used to amplify BDNF were: sense 5′-CTGGCGGTTCATAAGGATAGAC-3′ and mp1 antisense 5′-GACTTGCTATTCTGTGCATC-3′ (Trejo et al. 1999), comprising a fragment of the BDNF open reading frame, and the mp-1 fragment of the pGfa2BDNF plasmid, thus specifically allowing to determine the expression of transgenic BDNF. We amplified a β-actin fragment using primers previously reported (Choi-Lundberg and Bohn 1995). The primers for LacZ gene were: sense 5′-GCATCGAGCTGGGTAATAAGCG-3′ and the previously indicated mp1 primer.
Immunofluorescence and X-gal Analysis
Cells were fixed in 4% p-formaldehyde in PBS (pH 7.4), and then processed. Thirteen week-old mice were euthanized with an overdose of pentobarbital. Mice were perfused with 0.9% saline solution followed by 4% p-formaldehyde in PBS (pH 7.4) and brains were obtained. For histochemical or immunofluorescence detection, 40-μm tissue sections were processed. An anti-BDNF polyclonal antibody (1:100, Santa Cruz Biotechnology Santa Cruz, CA) was revealed with a fluorescein-tagged secondary antibody. For GFAP detection we used a monoclonal antibody (1:500, Chemicon, Temecula, CA) and then revealed it with a Texas red-labeled secondary antibody, cells were counterstained with DAPI. The co-localization of BDNF and GFAP was determined as previously described, but sequential staining for each protein was performed. To detect the expression of the lacZ gene we used the X-gal detection kit (Mirus, Madison, WI). All images were obtained using a fluorescent microscope (Olympus BX-51, Tokyo, Japan) and processed using the Image Pro-Plus program, Version 4.5 (Media Cybernetics, Bethesda, MD), with the exception of the determination of BDNF over-expression in mice striatum which was obtained by confocal-laser microscopy images acquired with a Leica TCS SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Western Blot Analysis
Total protein was isolated using the TriPure reagent (Roche) according to the manufacturer’s instructions. Twenty-five micrograms of total protein were separated on 10% SDS-PAGE gels and transferred into PVDF membranes (BioRad, Hercules, CA). Membranes were blocked for 1 h at room temperature in 5% nonfat milk/TBST (0.05% Tween-20, TBS) and incubated with the primary antibodies overnight at 4°C. Blots were then incubated with a peroxidase-coupled secondary antibody followed by enhanced chemiluminescence detection (Perkin Elmer, Boston, MA), according to the manufacturer’s instructions. The primary antibodies were anti-BDNF 1:100, anti-TrkB 1:500, anti-Akt-T 1:500, anti-Akt-P 1:500 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β actin 1:500 (Garcia-Tovar et al. 2001).
R6/2 Transgenic Mice and Genotyping
The R6/2 mice were generated by Mangiarini et al. (1996), and purchased from Jackson Laboratories (Bar Harbor, Maine). A colony of R6/2 mice was established in our vivarium by backcrossing them onto CBA × C57BL/6 F1 animals, as previously described (Perez-Severiano et al. 2000). We employed female mice for the experiments, and kept male mice for breeding. Mice were housed together in groups of mixed genotypes the experimenters were blind to the genotype and treatment of the mice. All animal procedures were approved by the Institutional Review Committee are in accordance with current Mexican legislation, NOM-062-ZOO-1999, and in agreement with the National Institutes of Health (NIH, Bethesda, MD) guide for the care and use of laboratory animals. Genotyping was determined by PCR analysis as previously described (Perez-Severiano et al. 2000). Mice were weighed once a week to the nearest 0.1 g.
In Vivo Adenoviral Transduction
Five-week-old mice were anesthetized i.p. with ketamine (100 mg/kg) and xylazine (10 mg/kg). Using a stereotaxic frame (Stoelting, Wood Dale, Il), we injected (1 μl of AdB or AdZ) with a viral titer of 1.26 × 1011/ml on each striatum at a 0.2 μl/min rate (0.8 mm rostral to bregma, ±2 mm lateral to midline, and −2.5 mm ventral to the brain surface). At the beginning of week 5, the adenoviral injection was made, and at the end of the same week the corresponding behavioral tests were performed to evaluate the effect of the expression of the transgenes on the behavioral tests. Viral infection did not affect the survival of the animals, neither wild type nor R6/2 mice.
Behavioral Tests
To measure feet-clasping, mice were suspended by the tail for a maximum of 60 s. The time when the behavior was presented was registered. Open field spontaneous activity was monitored using an automated system (Electronic Motility Meter 40Fc; Motron Products, Sweden). Horizontal and vertical movements were recorded for a single 10 min session in 4, 8, and 12-week-old mice (Perez-Severiano et al. 2000). Climbing activity was recorded by placing the mice underneath a wire cylinder for 5 min. The latency to climb, the total time spent climbing, the number of instances of climbing and rearing were counted (Hickey et al. 2005). Wire-hang endurance was tested every week. For this test, mice were placed at the center of a horizontal circular wire mesh with circles drawn then it was gently inverted. The time that each mouse remained on the wire mesh was recorded (Chopra et al. 2007). The maximum time we allowed mice to stay on the inverted wire was 60 s. The number of times each mouse crossed the circles was also recorded. Motor coordination and balance were measured using rotarod analysis. The axle was covered with rubber tubing to prevent the mice from clinging to the axle (Hockly et al. 2003). Mice were trained three times daily for 3 consecutive days at a different constant speeds (10, 20, and 30 rpm), and were subsequently tested twice a week in accelerated rotarod conditions from 2 to 40 rpm over a period of 300 s. The latency to fall from the rotarod was recorded for each mouse, and the average of two trials was used for statistical analysis.
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). Results were considered statistically significant at P < 0.05. For clarity, in all of the behavioral tests only the statistical differences between AdB and AdZ R6/2 mice is indicated. The administration of the Ad vectors induced no differences in wild type animals.
Acknowledgments
We want to thank Dr. M. Mouradian for the kind gift of the BDNF cDNA, Dr. B. Vogelstein (Johns Hopkins University) for providing us with the reagents (pAdSystem) to construct the adenoviral vectors, and Dr. M. Hernández (Cinvestav) for providing us with the antibody against β-actin. We thank R. Sánchez for technical support. This work was partially supported by Conacyt Grants 42721-M and 54756 (JS).
References
- Abiru Y, Katoh-Semba R, Nishio C, Hatanaka H (1998) High potassium enhances secretion of neurotrophic factors from cultured astrocytes. Brain Res 809:115–126 [DOI] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389:856–860 [DOI] [PubMed] [Google Scholar]
- Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, Cattaneo E, Marsh JL, Thompson LM (2008) CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol Cell Neurosci 39:8–20 [DOI] [PubMed] [Google Scholar]
- Arregui L, Segovia J (2009) Transgenic murine models for Huntington’s disease. In: Lilia Rocha-Arrieta L, Granados-Soto V (eds) Models of neuropharmacology. Research Signpost, Kerala, pp 35–60 [Google Scholar]
- Baquet ZC, Gorski JA, Jones KR (2004) Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci 24:4250–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans AP, Horellou P, Pradier L, Brunet I, Colin P, Mallet J (1999) Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum Gene Ther 10:2987–2997 [DOI] [PubMed] [Google Scholar]
- Benitez JA, Segovia J (2003) Gene therapy targeting in the central nervous system. Curr Gene Ther 3:127–145 [DOI] [PubMed] [Google Scholar]
- Benitez JA, Arregui L, Vergara P, Segovia J (2007) Targeted-simultaneous expression of Gas1 and p53 using a bicistronic adenoviral vector in gliomas. Cancer Gene Ther 14:836–846 [DOI] [PubMed] [Google Scholar]
- Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M, Canossa M (2008) Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol 183:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogush A, Pedrini S, Pelta-Heller J, Chan T, Yang Q, Mao Z, Sluzas E, Gieringer T, Ehrlich ME (2007) AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J Biol Chem 282:7352–7359 [DOI] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A (1994) GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci 14:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M (2008) Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci USA 105:3581–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J (2004) Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J Neurosci 24:7727–7739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309 [DOI] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA (2007) Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest 117:2889–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC (1995) Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res 85:80–88 [DOI] [PubMed] [Google Scholar]
- Chopra V, Fox JH, Lieberman G, Dorsey K, Matson W, Waldmeier P, Housman DE, Kazantsev A, Young AB, Hersch S (2007) A small-molecule therapeutic lead for Huntington’s disease: preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse. Proc Natl Acad Sci USA 104:16685–16689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Saporta S, Hauser RA, Parent M, Saint-Pierre M, Sanberg PR, Li XJ, Parker JR, Chu Y, Mufson EJ, Kordower JH, Freeman TB (2009) Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci USA 106:12483–12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin E, Regulier E, Perrin V, Durr A, Brice A, Aebischer P, Deglon N, Humbert S, Saudou F (2005) Akt is altered in an animal model of Huntington’s disease and in patients. Eur J Neurosci 21:1478–1488 [DOI] [PubMed] [Google Scholar]
- Cortez N, Trejo F, Vergara P, Segovia J (2000) Primary astrocytes retrovirally transduced with a tyrosine hydroxylase transgene driven by a glial-specific promoter elicit behavioral recovery in experimental parkinsonism. J Neurosci Res 59:39–46 [PubMed] [Google Scholar]
- Cunha C, Angelucci A, D’Antoni A, Dobrossy MD, Dunnett SB, Berardi N, Brambilla R (2009) Brain-derived neurotrophic factor (BDNF) overexpression in the forebrain results in learning and memory impairments. Neurobiol Dis 33:358–368 [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL (2003) Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci USA 100:9023–9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denovan-Wright EM, Attis M, Rodriguez-Lebron E, Mandel RJ (2008) Sustained striatal ciliary neurotrophic factor expression negatively affects behavior and gene expression in normal and R6/1 mice. J Neurosci Res 86:1748–1757 [DOI] [PubMed] [Google Scholar]
- Dey ND, Bombard MC, Roland BP, Davidson S, Lu M, Rossignol J, Sandstrom MI, Skeel RL, Lescaudron L, Dunbar GL (2010) Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav Brain Res 214:193–200 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T (2000) Brain-derived neurotrophic factor in Huntington disease. Brain Res 866:257–261 [DOI] [PubMed] [Google Scholar]
- Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A (2008) Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci 28:13368–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tovar CG, Perez A, Luna J, Mena R, Osorio B, Aleman V, Mondragon R, Mornet D, Rendon A, Hernandez JM (2001) Biochemical and histochemical analysis of 71 kDa dystrophin isoform (Dp71f) in rat brain. Acta Histochem 103:209–224 [DOI] [PubMed] [Google Scholar]
- Gharami K, Xie Y, An JJ, Tonegawa S, Xu B (2008) Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington’s disease phenotypes in mice. J Neurochem 105:369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, Friedman HC, Caneda-Ferron B, Urban N, Moreno E, Rubio N, Blanco J, Peterson A, Canals JM, Alberch J (2010) BDNF regulation under GFAP promoter provides engineered astrocytes as a new approach for long-term protection in Huntington’s disease. Gene Ther 17:1294–1308 [DOI] [PubMed] [Google Scholar]
- Gratacos E, Perez-Navarro E, Tolosa E, Arenas E, Alberch J (2001) Neuroprotection of striatal neurons against kainate excitotoxicity by neurotrophins and GDNF family members. J Neurochem 78:1287–1296 [DOI] [PubMed] [Google Scholar]
- Graveland GA, Williams RS, DiFiglia M (1985) Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science 227:770–773 [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95:2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MA, Gallant K, Gross GG, Levine MS, Chesselet MF (2005) Early behavioral deficits in R6/2 mice suitable for use in preclinical drug testing. Neurobiol Dis 20:1–11 [DOI] [PubMed] [Google Scholar]
- Hockly E, Woodman B, Mahal A, Lewis CM, Bates G (2003) Standardization and statistical approaches to therapeutic trials in the R6/2 mouse. Brain Res Bull 61:469–479 [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Ehrlich ME (1999) Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J Neurosci 19:5409–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B (2010) Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci 13:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B (2004) AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther 9:682–688 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Winkler C, Fricker R, Charles V, Messing A, Mufson EJ, Wong SC, Rosenstein JM, Bjorklund A, Emerich DF, Hammang J, Carpenter MK (1997) Grafts of EGF-responsive neural stem cells derived from GFAP-hNGF transgenic mice: trophic and tropic effects in a rodent model of Huntington’s disease. J Comp Neurol 387:96–113 [DOI] [PubMed] [Google Scholar]
- Levy YS, Gilgun-Sherki Y, Melamed E, Offen D (2005) Therapeutic potential of neurotrophic factors in neurodegenerative diseases. BioDrugs 19:97–127 [DOI] [PubMed] [Google Scholar]
- Lievens JC, Iche M, Laval M, Faivre-Sarrailh C, Birman S (2008) AKT-sensitive or insensitive pathways of toxicity in glial cells and neurons in Drosophila models of Huntington’s disease. Hum Mol Genet 17:882–894 [DOI] [PubMed] [Google Scholar]
- Lim ST, Airavaara M, Harvey BK (2010) Viral vectors for neurotrophic factor delivery: a gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol Res 61:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA (2007) Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’s disease. J Neurosci 27:4424–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506 [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV (2008) Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience 153:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H (1994) Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol 165:243–256 [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276:12660–12666 [DOI] [PubMed] [Google Scholar]
- Nakao N, Kokaia Z, Odin P, Lindvall O (1995) Protective effects of BDNF and NT-3 but not PDGF against hypoglycemic injury to cultured striatal neurons. Exp Neurol 131:1–10 [DOI] [PubMed] [Google Scholar]
- Pallier PN, Drew CJ, Morton AJ (2009) The detection and measurement of locomotor deficits in a transgenic mouse model of Huntington’s disease are task- and protocol-dependent: influence of non-motor factors on locomotor function. Brain Res Bull 78:347–355 [DOI] [PubMed] [Google Scholar]
- Perez-Severiano F, Rios C, Segovia J (2000) Striatal oxidative damage parallels the expression of a neurological phenotype in mice transgenic for the mutation of Huntington’s disease. Brain Res 862:234–237 [DOI] [PubMed] [Google Scholar]
- Pineda JR, Canals JM, Bosch M, Adell A, Mengod G, Artigas F, Ernfors P, Alberch J (2005) Brain-derived neurotrophic factor modulates dopaminergic deficits in a transgenic mouse model of Huntington’s disease. J Neurochem 93:1057–1068 [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Vaillancourt DE (2009) Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev 33:900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB (1988) Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA 85:5733–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Finkbeiner S, Devys D, Greenberg ME (1998) Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95:55–66 [DOI] [PubMed] [Google Scholar]
- Segovia J, Arregui L (2007) Mechanisms of neuronal death associated to Huntington’s disease. In: Massieu L, Arias C, Moran J (eds) The neurochemistry of neuronal death. Research Signpost, Kerala, pp 197–222 [Google Scholar]
- Segovia J, Lawless GM, Tillakaratne NJ, Brenner M, Tobin AJ (1994) Cyclic AMP decreases the expression of a neuronal marker (GAD67) and increases the expression of an astroglial marker (GFAP) in C6 cells. J Neurochem 63:1218–1225 [DOI] [PubMed] [Google Scholar]
- Segovia J, Vergara P, Brenner M (1998) Differentiation-dependent expression of transgenes in engineered astrocyte cell lines. Neurosci Lett 242:172–176 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Salazar FJ, Abraham C, Kosik KS (1982) Huntington’s disease: changes in striatal proteins reflect astrocytic gliosis. Brain Res 245:117–125 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, Hannan AJ (2004) Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci 24:2270–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Jackson WS, King OD, Lindquist S (2007) The power of automated high-resolution behavior analysis revealed by its application to mouse models of Huntington’s and prion diseases. Proc Natl Acad Sci USA 104:1983–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, Beal MF, Jones L, Kooperberg C, Olson JM, Jones KR (2007) Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci 27:11758–11768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983 [DOI] [PubMed] [Google Scholar]
- Trejo F, Vergara P, Brenner M, Segovia J (1999) Gene therapy in a rodent model of Parkinson’s disease using differentiated C6 cells expressing a GFAP-tyrosine hydroxylase transgene. Life Sci 65:483–491 [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577 [DOI] [PubMed] [Google Scholar]
- Wang CY, Wang S (2006) Astrocytic expression of transgene in the rat brain mediated by baculovirus vectors containing an astrocyte-specific promoter. Gene Ther 13:1447–1456 [DOI] [PubMed] [Google Scholar]
- Xie Y, Hayden MR, Xu B (2010) BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci 30:14708–14718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E (2007) Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol 81:294–330 [DOI] [PubMed] [Google Scholar]
- Zuccato C, Marullo M, Conforti P, MacDonald ME, Tartari M, Cattaneo E (2008) Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol 18:225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]