Abstract
Hypobaric hypoxia (HH) induced neurodegeneration has been attributed to several factors including increased oxidative stress, glutamate excitotoxicity, decreased growth factors, apoptosis, etc. Though enriched environment (EE) has been known to have beneficial effects in various neurological disorders, its effect on HH mediated neurodegeneration remains to be studied. Therefore, the present study was conducted to explore the effect of EE on HH induced neurodegeneration. Male Sprague–Dawley rats were placed in enriched and standard conditions during exposure to HH (7 days) equivalent to an altitude of 25,000 ft. The effect of EE on oxidative stress markers, apoptosis, and corticosterone level in hippocampus was investigated. EE during exposure to HH was found to decrease neurodegeneration as evident from decreased caspase 3 expression and LDH leakage. However, no significant changes were observed in ROS, MDA, and antioxidant status of hippocampus. HH elevates corticosterone level and affected the diurnal corticoid rhythm which may contribute to neurodegeneration, whereas EE ameliorate this effect. Because of the association of neurotrophins and stress and/or corticosterone the BDNF and NGF levels were also examined and it was found that HH decreases their level but concurrent exposure to EE maintains their level. Moreover, inhibition of Tyrosine kinase receptor (Trk) with K252a nullifies the protective effect of EE, whereas Trk activation with agonist, amitriptyline showed protective effect similar to EE. Taken together, we conclude that EE has a potential to ameliorate HH mediated neuronal degeneration which may act through antioxidant independent pathway by modulation of neurotrophins.
Keywords: Enriched environment, Hypobaric Hypoxia, Hippocampus, Corticosterone, Neurotrophins, Trk
Introduction
High altitude (HA) exposure is considered as an extreme physiological stress inducing wide range of deleterious effects at cellular level. Altered neurotransmitter synthesis, uptake and release (Benveniste et al. 1984; Rossi et al. 2000), free radical generation (Askew, 2002), and changes in gene expression and protein functions (Pellegrini-Giampietro et al. 1992; Gorter et al. 1997; Chandel et al. 1998) are characteristically associated with hypoxia and ischemia (Hartman et al. 2005). These alterations lead to hypobaric hypoxia (HH) mediated memory impairment and cognitive dysfunctions (Kramer et al. 1993; Shukitt-Hale et al. 1998). Recent findings suggested that severe HA hypoxia could cause increased cellular oxidative stress with consequent damage to lipids, proteins, and DNA. The oxidative stress may be one of the causative factors in HA induced memory deficit (Hota et al. 2008).
Antioxidants (enzymatic and/or non-enzymatic) can protect the brain against oxidative damage (Ozturk et al. 2005) in several ways including: (a) removal of ROS/RNS, (b) inhibition of ROS/RNS formation, and (c) binding with metal ions needed for catalysis of ROS generation. Glutathione peroxidase (GPx) and glutathione reductase (GR) are well known intracellular antioxidant enzymes. GPx convert peroxides into nontoxic forms, with the concomitant oxidation of glutathione (GSH) into the glutathione disulfide (GSSG) and GR recycles GSSG to GSH. Other enzymes and pathways are also involved in the management of cellular defense against oxidative/nitrosative stress. Glutathione-s-transferase (GST) detoxifies deleterious substances and glucose-6-phosphate dehydrogenase (G-6PD) provides electron donors for antioxidant defense system. Enzymes that remove both superoxide and hydrogen peroxide (H2O2) protect the cells from oxidative stress, but when the production of O2 − and H2O2 crosses the normal threshold, the system becomes compromised. Catalase and GPx, and superoxide dismutase (SOD), constitute the major defense system against superoxide radicals (Dekosky et al. 2004; Dringen et al. 2005). Analysis of these antioxidants, products of Lipid peroxidation, and protein damage, may yield a snapshot of oxidative stress.
Environmental enrichment has numerous beneficial effects on brain and behavior. Several studies have investigated its effect on functional recovery following experimental models of stroke and traumatic brain injury. Environmental enrichment refers to housing conditions, either home cages or exploratory chambers that facilitate enhanced sensory, cognitive, and motor stimulation relative to standard housing conditions. The exact nature of the environmental enrichment protocols used also varies widely between laboratories, and is often not fully described in published experimental methods.
As regards the cellular and molecular pathways related to neuroprotection, it has been reported that enriched environment (EE) induces members of the neurotrophin family, especially brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), a possible modulator of neuronal survival and plasticity (Rossi et al. 2006; Lee and Paffenbarger 1998; Gelfo et al. 2010). Almli and colleagues in their study demonstrated that intracerebroventricular BDNF pretreatment resulted in significant protection against hypoxia-ischemia (HI) induced histological injury and spatial memory impairments (Almli et al. 2001). In addition, it has been proposed that oxidative stress induced pathway may be involved in EE mediated cognitive and motor changes (Fernández et al. 2004). Moreover, it was found that there is a significant increase in the activity of antioxidant enzymes GST and SOD after the combined treatment, EE and antioxidant diet (Opii et al. 2008).
In view of above findings, present study aimed at investigating the effect of HH on neuronal degeneration and the potential beneficial effect of EE during exposure to HH and the possible mechanism involved.
Material and Methods
Chemicals
Rabbit polyclonal caspase 3, BDNF, and NGF were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-conjugated anti-rabbit secondary antibody and all other chemicals and reagents used were purchased from Sigma (St Louis, MO, USA). Electrophoresis materials and chemicals were purchased from Bio-Rad (Hercules, CA, USA). Kits used for biochemical estimation was purchased from RANDOX (RANDOX Laboratory Ltd.).
Animals
Three month-old male Sprague–Dawley rats (230 g) were used in this study. They were maintained in a temperature-controlled room (25 ± 2°C at 65 ± 5% humidity) on a 12/12 h light/dark cycle with food and water available ad libitum. The experimental protocol was approved by the ethics committee of our institute in accordance with the guidelines of “Committee for the Purpose of Control and Supervision of Experiments on Animals” of Govt. of India. Rats were tested for anxiety levels in a plus maze and categorized into anxious and normal individuals basing on their preference for closed and open arms. The normal individuals were then taken for further experimental purposes. Rats (n = 133) were divided into seven experimental groups as shown in Table 1 and further investigations were carried out.
Table 1.
Experimental design describing groups, no. of individuals, exposure duration, and interventions
| Group | No. of animals (n) | Hypobarichypaxia exposure | Standard cage (SC)/enriched cage (EC) | Antagonist/agonist |
|---|---|---|---|---|
| I | (30) | Nil | SC | Nil |
| II | (30) | 7 days | SC | Nil |
| II | (25) | 7 days | EC | Nil |
| IV | (24) | Nil | EC | Nil |
| IV | (06) | 7 days | EC | K252a |
| V | (06) | Nil | EC | K252a |
| VI | (06) | 7 days | SC | Amitriptyline |
| VII | (06) | Nil | SC | Amitriptyline |
Note: EE refers to groups kept in enriched cage during HH exposure
EE Condition
Rats were randomly assigned to the enriched or the standard environmental condition. EE cages consisted of clear Plexiglas cages (35 in. × 20 in. × 25 in.) with two horizontal platform (20 × 15 in.) dividing the cage into three floors. On the ground floor, there was a plastic running wheel, nesting material, and an assortment of differently colored and textured plastic toys (balls, tubes, boxes, and bells) that were changed every alternate day. Steel ladders allowed rats to reach the upper floors, where they had access to food and water. Standard cages were clear Plexiglas laboratory cages (18 × 25 × 13 in.). Rats were caged in groups of three. Enriched and standard cages were placed in a temperature-controlled room (22°C) with a light–dark 12:12 cycle (light on 0700–1900 h). Food and water were given ad libitum. Rats were housed in each experimental condition for 7 days.
Exposure to HH
The rats were divided into different groups. The HH condition was created in a specially designed animal decompression chamber in which rats were exposed to altitude of 7,600 m (25,000 ft) maintained by reducing the ambient barometric pressure. The exposure was for a period of 7 days with 15 min interval each day for replenishment of food and water and changing the cages housing the animals. Body weight was measured every day during this period. Fresh air was continuously flushed at a rate of 8 l/min to prevent accumulation of carbon dioxide within the chamber. The temperature and humidity in the chamber were maintained at 28 ± 2°C and 60 ± 3%, respectively. The rate of ascent and descent was maintained at 300 m/min. Depending upon the groups rats were kept in two different cages during exposure i.e., EE group were kept in enriched cage while other groups kept in standard cage.
Administration of Inhibitors
To determine role of neurotrophins, intracerebroventricular (ICV) injection of either vehicle (VEH; PBS, pH 7.4) or tyrosine kinase (Trk) inhibitor i.e., K252a was administered (250 μM, 5 μl) in adult rat brain into the left hemisphere before exposure, as prescribed by Yung (Yun et al. 2009). Injections were performed with a Hamilton syringe with a 27 gauge needle. The location of each injection was 2-mm rostral, 1.5-mm lateral to bregma, and 2-mm deep to the skull surface. After exposure rats were anesthetized with 150 mg/kg pentobarbital intraperitoneally, perfused through the left ventricle with PBS, pH 7.4 and fixed with 4% paraformaldehyde for immunohistochemistry. Amitriptyline, an agonist for Trk was administered intraperitoneally (i.p.) before HH exposure at a dose of 14 μM.
Tissue Processing
Rats were sacrificed after HH exposure by decapitation and hippocampus was dissected out. Samples from 12–15 animals per group were collected and instantaneously placed in liquid nitrogen and stored at −80°C until biochemical assays. For biochemical estimation samples were homogenized in 10 volume of 0.1 M KCl in a teflon-glass homogenizer. The homogenate were centrifuged at 3,000×g for 10 min to remove nuclei and cell debris; the supernatant of mixed and preserved organelles, was used for the assays. All procedure was performed at temperature of 4°C. For western blot analysis 10% tissue homogenate was prepared in ice cold lysis buffer (10 mM Tris. HCl, 100 mM NaCl, 0.1 mM Dithiothreitol, 1 mM EDTA, 0.1% NaN3, 100 μg/ml PMSF, protease inhibitor cocktail, pH 7.6) followed by centrifugation of the homogenate at 10,000×g for 10 min at 4°C. The supernatant was then collected and stored at −80°C for further studies.
Oxidative Status
Reactive Oxygen Species (ROS)
The free radicals were estimated spectrofluorimetrically taking 25 μl of the sample using 2′,7′-dichlorofluorescin-diacetate (DCFHDA) by the method of Robinson (Robinson et al. 1998). In brief, 1.494 ml 0.1 M PBS (pH 7.4) was added to 25 μl of the sample followed by addition of 6 μl of DCFHDA (1.25 mM). The sample was then incubated for 15 min at 37°C in dark and the fluorescence was measured at 488 nm excitation and 525 nm emissions. The readings thus obtained were converted to fluorescent units/mg protein by calculating the protein present in 25 μl in each sample from a standard curve.
Lipid Peroxidation
The lipid peroxidation was determined spectrophotometrically by measuring the malondialdehyde (MDA) that is produced as an end product as described by Utley et al. (1967). Each molecule of MDA reacts with two molecules of thiobarbituric acid (TBA) to form a colored MDA–TBA complex that can be quantified spectrophotometrically at 531 nm. In brief, to 250 μl of supernatant, 750 μl of 20% TCA and 750 μl of 0.67% TBA was added. The samples were incubated in a water bath at 85°C for 45 min. The samples were then kept at room temperature followed by centrifugation at 2,000×g for 5 min. About 200 μl of the supernatant was taken and the absorbance was measured at 531 nm using spectrophotometer. The MDA formed was estimated using the molar extinction coefficient of MDA–TBA complex i.e., 1.56 × 105 cm2 mmol−1 and the value thus obtained was expressed in μmol/ml of homogenate.
LDH Activity
Lactate dehydrogenase (LDH) (EC 1.1.1.2) is known to be a neuronal stress marker. Increased activity of LDH indicates stress conditions in neuronal cells. The LDH activity in neurons was estimated using LDH assay kit from RANDOX (RANDOX Laboratory Ltd., UK) and the enzyme activity was expressed in U/mg of protein.
Antioxidant Status
GSH and GSSG levels
The GSH and GSSG levels were measured fluorimetrically from the crude homogenate according to the procedure followed by Hissin and Hilf (Hissin and Hilf 1976). In brief, 250 μl of the crude homogenate was taken to which equal volume of 10% metaphosphoric acid was added followed by centrifugation at 10,000×g for 30 min at 4°C. The supernatant thus obtained was then used for estimation of GSH and GSSG spectrofluorimetrically at 350 nm excitation and 420 nm emission.
SOD, GPx, and GST activities were determined in tissues. In brief, tissue homogenates were centrifuged at 600×g for 10 min at 4°C to remove crude fractions. Then, SOD activity was assayed by its ability to increase the effect of riboflavin sensitized photooxidation of o-dianisidine (Mylorie et al. 1986). GPx (Lawrence and Burk 1976), and GST (Habig and Jacoby 1981) activities were measured using cumene hydroperoxide and 1-chloro-2,4-dinitrobenzene as substrates, respectively.
Estimation of Corticosterone by HPLC
After completion of HH exposure plasma from six individuals of each group was taken. Time of sample collection was at 10:00 AM and kept same for all groups within a time window of 30 min. Levels of corticosterone was estimated in plasma using high performance liquid chromatography (Waters, Milford, MS, USA). The extraction of corticosterone from plasma was done by method followed by Wong and colleague, with some modifications using diethyl ether (Wong et al. 1994) Samples were resolved using C18 RP column with acetonitrile:water:glacial acetic acid (35:65:05 v/v) as solvent phase in isocratic condition. 20 μl of the extracted sample was injected with the help of an autosampler (Waters) to the HPLC system. The flow rate of the mobile phase was maintained at 1 ml/min and detection of corticosterone fraction was done at 254 nm with a UV detector. The pressure in the column was maintained at 1,800 psi and the samples were run for 30 min. A standard plot was prepared using the endogenous steroid free plasma in the range 20–2,000 ng by serial dilution. The standards were tested individually at different concentrations to record detection limit, retention time, and peak area under isocratic conditions. Concentration of corticosterone was calculated from a standard plot of peak area of corticosterone vs. concentration of corticosterone.
Estimation of Neurotrophins Level (BDNF and NGF) by Immunoblotting
The effect of EE on expression of active Caspase 3, BDNF, and NGF were studied by immunoblotting. Animals were anesthetized and sacrificed immediately after the exposure and the hippocampi were dissected out at 4°C. 10% tissue homogenate was then prepared in ice cold lysis buffer (10 mM Tris. HCl, 100 mM NaCl, 0.1 mM Dithiothreitol, 1 mM EDTA, 0.1% NaN3, 100 μg/ml PMSF, protease inhibitor cocktail, pH 7.6) followed by centrifugation of the homogenate at 10,000×g for 10 min at 4°C. The supernatant was then collected and aliquot for further studies. The total protein content in 10 μl of the homogenate was estimated by the method suggested by Bradford (Bradford 1976) using BSA (1 mg/ml) as standard. 50 μg of sample protein was then resolved by SDS-PAGE and transferred to nitrocellulose membranes pre-soaked in transfer buffer (20% methanol, 0.3% Tris, 1.44% glycine) using a semidry transblot module (BIORAD). The membranes were stained with Ponceau S to control for equal loading and transfer of samples. The membranes were blocked with 5% non fat milk, washed with PBST (1 l 0.01 M phosphate buffer saline, pH 7.4, 1 ml Tween 20) and probed separately with primary antibodies at a dilution of 1:1,000 prepared in 5% non fat milk. The membranes were then washed with PBST and incubated in HRP-conjugated goat anti-rabbit IgG at a dilution of 1:5,000 in 5% non fat milk. Membranes were finally washed with PBST and the bands were developed on X-ray films using chemiluminescent substrate (Sigma Chemicals). The bands thus obtained on the films were quantified by densitometry to determine the expression of the protein. Actin expression was considered as loading control for all the immunoblotting experiments.
Caspase 3 Expression by Immunohistochemistry
Every sixth coronal section containing hippocampus was mounted on positively charged glass slides with two sections per slide. Three slides per animal, containing sections with identical z-axes, were selected per brain region for each immunostaining. Slides containing the frozen sections were equilibrated at RT and hydrated with 1× PBS for 30 min. Antigen retrieval was performed by incubating the sections in 10 mM citrate buffer (pH 6.0) at 80°C for 30 min followed by cooling down to RT. After rehydration with 1× PBS, sections were permeabilized with 0.5% Triton X-100 in PBS for 1 h and blocked in 1× PBS containing 5% normal goat serum (NGS), 5% BSA, 0.1% sodium azide, and 0.5% Triton X-100 for 1 h. The same solution minus NGS was used to dilute caspase 3 primary antibody. Sections were incubated with the primary antibodies (1:100) overnight at 4°C. After washing with 1× PBS, they were incubated with biotinylated secondary antibodies (1:200) for 3 h. Sections were washed three times for 5 min in PBS, then incubated with avidin–biotin-peroxidase complex (ABC; Vector, Burlingame, CA) for 1 h. Specific binding was visualized with 0.07% diaminobenzidine (DAB; Sigma, St Louis, MO), 0.02% H2O2 in PBS. Sections then cover slipped with mounting medium (Vectashield, Vector, Burlingame, CA) and visualized in bright field microscope (Olympus CX41, Olympus, Tokyo, Japan). Counting of caspase 3 positive cells was done by Stereo investigator (MBF Biosciences, Williston, USA). In brief, CA1 region in hippocampus of every sixth coronal section was visualized and caspase 3 positive cells were counted in all these sections. Average of caspase 3 positive cells was taken from all these sections in each group and represented graphically.
Estimation of Caspase 3 Activity (DEVD-AMC Cleavage Assay)
After HH exposure rats were dissected out and hippocampus was removed and frozen in dry ice. The Asp-Glu-Val-Asp-(7-amino-4-methylcoumarin) (DEVD-AMC) cleavage assay was performed as described by Han et al. (Han and Holtzman 2000). Tissue samples were homogenized in lysis buffer (10 mM HEPES, pH 7.4, 5 mM MgCl2, 1 mM DTT, 1% TritonX-100, 2 mM EGTA, 2 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail) and centrifuged at 12,000×g for 10 min at 4°C. Tissue lysates (10 μl) were incubated in a 96-well plate with 90 ml of assay buffer (10 mM HEPES, pH 7.4, 42 mM KCl, 5 mM MgCl2, 1 mM DTT, and 10% sucrose) containing 30 mM acetyl-DEVD-AMC (Calbiochem). The emitted fluorescence was measured every 5 min for 30 min at room temperature at an excitation wavelength of 360 nm and an emission wavelength of 460 nm using a microplate fluorescence reader. DEVD-AMC cleavage activity was obtained from the slope by plotting fluorescence units against time. Acetyl-AMC (Calbiochem) was used to obtain a standard curve, and the enzyme activity was calculated as picomoles of AMC per milligram of protein per minute.
Protein Assay
The total protein content in 10 μl of the homogenate was estimated by the method suggested by Bradford (Bradford 1976) using BSA (1 mg/ml) as standard.
Statistical Analysis
Results of all estimations are representations of six individual observations and statistical analysis for multiple comparisons was done using two-way ANOVA. Data are presented as mean ± SEM unless otherwise stated. Pair wise comparisons were used to evaluate the difference between different experimental groups. All analysis was followed by post hoc student’s Newman’s Keul’s test, when necessary. Probability values less than 5% (p < 0.05) were considered significant for all statistical analysis.
Results
Effect of EE on Body Weight and Food Intake
Body weight of rats from all groups was measured every day during exposure. Results shows that HH causes a significant fall in the body weight (Table 2a), whereas on exposure to EE during hypoxia weight loss is significantly reduced (p < 0.001) when compared to hypoxic group. Food intake was also measured during HH which shows correlation with above result (Table 2b). There was a gradual decrease in food intake with time during HH, whereas group exposed to hypoxia in enriched cage does not show any decline in food intake.
Table 2.
Represents changes in weight (a) and food intake (b) in all groups
| (a) | |||
|---|---|---|---|
| Groups | n | Initial weight | Final weight |
| Control | 11 | 235 ± 15 | 245 ± 18 |
| Control + EE | 10 | 241 ± 16 | 240 ± 14 |
| Hypoxia | 8 | 239 ± 13 | 186 ± 16* |
| Hypoxia + EE | 12 | 228 ± 11 | 225 ± 15** |
| (b) | ||||
|---|---|---|---|---|
| Day | Groups | |||
| Food intake (gm/animal) | ||||
| Control | Control + EE | Hypoxia | Hypoxia + EE | |
| 1st | 9.08 ± 0.78 | 8.90 ± 0.12 | 8.45 ± 0.15 | 9.02 ± 0.21 |
| 2nd | 10.18 ± 0.23 | 10.12 ± 0.34 | 8.34 ± 0.20 | 8.67 ± 0.36 |
| 3rd | 10.04 ± 1.20 | 10.11 ± 1.12 | 7.45 ± 1.22 | 7.12 ± 1.14 |
| 4th | 9.12 ± 0.89 | 10.01 ± 1.08 | 6.02 ± 0.45 | 6.99 ± 0.64 |
| 5th | 10.07 ± 0.66 | 10.13 ± 0.11 | 5.45 ± 0.98 | 6.67 ± 0.43 |
| 6th | 9.25 ± 0.24 | 10.22 ± 0.85 | 5.00 ± 0.79 | 6.65 ± 0.98 |
| 7th | 10.34 ± 0.89 | 9.22 ± 0.69 | 5.36 ± 0.82 | 6.77 ± 0.48 |
HH leads to decrease in food intake and hence weight loss significantly, whereas on exposing them with EE increases food intake and reduces weight loss, whereas no significant difference was found in case of control group exposed to EE
* Represents p < 0.001 when compared to control group, whereas ** represents p < 0.05 when compared to hypoxia group
Effect of EE on Oxidative Stress Indicators
Free Radical
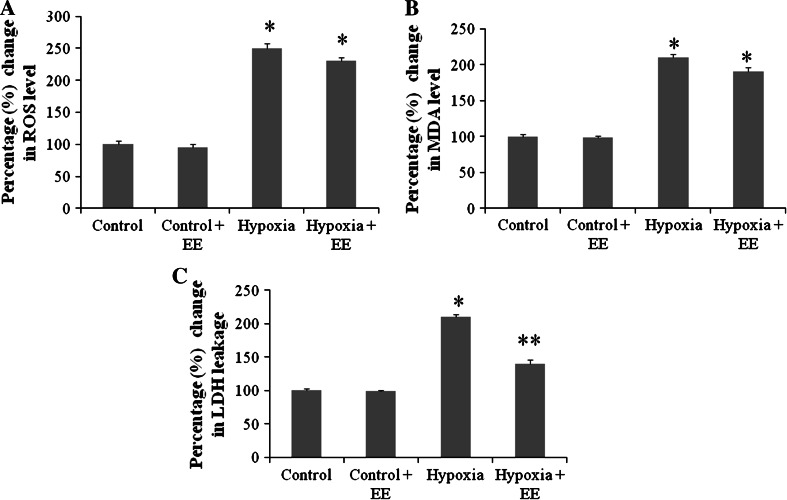
Free radical measurement was performed spectrofluorimetrically by the DCFHDA method in rat hippocampus. The percentage change of free radical level is depicted in Fig. 1a. The free radical level significantly increased after 7 days (p < 0.01) of HH exposure as compared to control group, and rats housed in EE cage during HH also showed a significant increase in free radical generation.
Fig. 1.
Represents percentage change in ROS (a), lipid peroxidation (b), and LDH release (c) in hippocampus. Data represents mean ± SEM with n = 6. EE exposure fails to ameliorate HH mediated upregulation in ROS and MDA level, whereas it shows promising effect in reducing LDH leakage during hypobaric hypoxic exposure. * Represents p < 0.001 when compared to control group, whereas ** represents p < 0.05 when compared to hypoxia group
Lipid Peroxidation
The result presented in Fig. 1b shows that exposure of rat to HH for 7 days resulted in significant increase (p < 0.001) of MDA production in rat hippocampus when compared to control group. Rats housed in EE also showed significant increase in MDA level.
LDH activity
The activity of LDH was measured by UV method using RANDOX kit (RANDOX Laboratory Ltd.). The result (Fig. 1c) indicates that the LDH activity was considerably increased (p < 0.01) on 7 days of HH exposure in comparison to control group. On the contrary, EE decreases (p < 0.001) the LDH activity during hypoxic exposure when compared to hypoxic group.
Effect of EE on Antioxidant Status of Hippocampus
The GSH system was measured spectrofluorimetrically by the method of Hissin and Hilf (1976). Table 3 shows the changes in GSH system. The results indicated that GSH level was significantly decreased (p < 0.01) in 7 days exposure group, when compared to control group. Similar to the results of free radicals generation, here also the rats housed in EE cage during exposure to HH showed decrease in reduced GSH level. Whereas level of GSSG significantly increased after hypoxic exposure for 7 days and similar to GSH no significant changes was observed in EE group when compared to hypoxic group as shown in Table 3.
Table 3.
Shows level of GSH and antioxidant enzymes activity in hippocampus of all groups
| Control | Control + EE | Hypoxia | Hypoxia + EE | |
|---|---|---|---|---|
| GSH (μmol/g tissue) | 2.23 ± 0.18 | 2.10 ± 0.12 | 1.04 ± 0.09* | 1.02 ± 0.06* |
| GSSG (μmol/g tissue) | 1.18 ± 0.12 | 1.09 ± 0.07 | 2.29 ± 0.15* | 2.25 ± 0.13* |
| GSH-Px (nmol/min/mg protein) | 48.89 ± 5.89 | 45.48 ± 5.50 | 28.98 ± 4.12* | 27.45 ± 5.19* |
| GST (nmol/min/mg protein) | 92.54 ± 9.23 | 94.13 ± 7.49 | 70.09 ± 4.12** | 75.89 ± 6.76** |
| SOD (U/mg protein) | 9.14 ± 1.54 | 9.23 ± 1.23 | 8.79 ± 1.07 | 8.39 ± 0.96 |
Data represents mean ± SEM with n = 6
* Represents p < 0.001 when compared to control group, whereas ** represents p < 0.05 when compared to hypoxia group
Activity of different antioxidant enzymes i.e., GPx, GST, and SOD were also assessed in all groups. Exposure to HH reduces GPx and GST activity and concurrent exposure with EE during HH fails to ameliorate this change. In contrast to these results no significant changes was observed in SOD activity in all groups (Table 3).
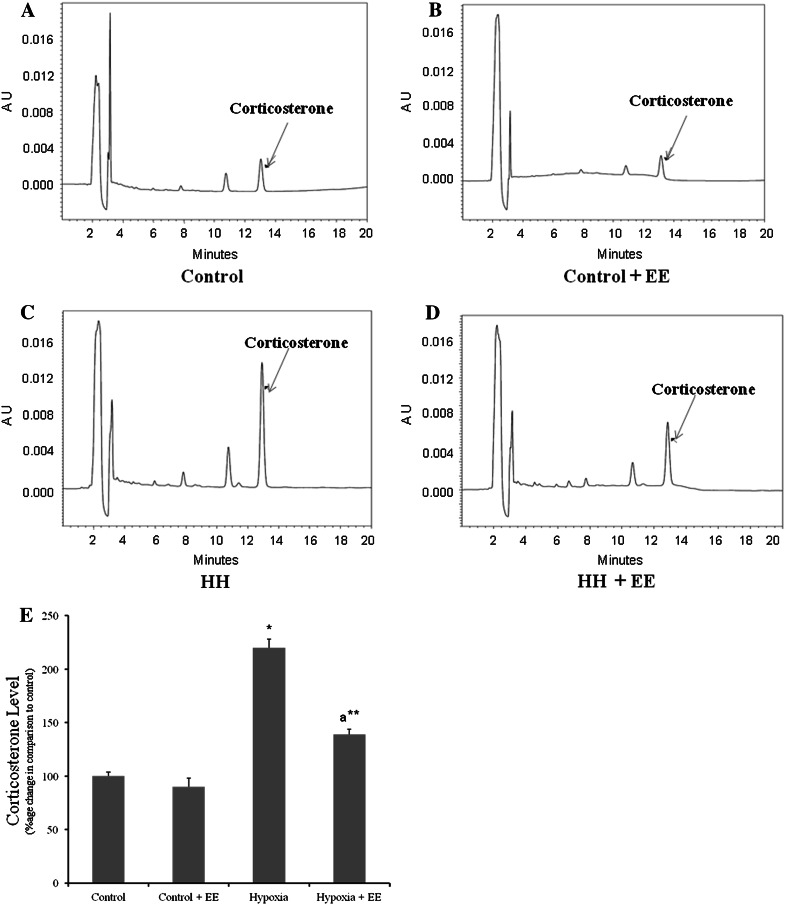
EE Down Regulates HH Mediated Corticosterone Elevation
Figure 2 shows the level of corticosterone in different groups and it was observed that exposure to HH for 7 days leads to elevation in corticosterone level, whereas EE during exposure to HH significantly (p < 0.001) prevents the elevation of the level of corticosterone and the values remains normal.
Fig. 2.
Corticosterone level averaged across group exposed to hypoxia with or without EE. Hypoxia leads to elevation in corticosterone level in plasma (c) when compared to control (a), whereas in presence of EE hypoxia fails to upregulate corticosterone level (d). No significant changes was found in control group housed in EE (b) and percentage change in corticosterone level in all groups (e). * Represents p < 0.001 when compared to control group, whereas ** represents p < 0.01 when compared to hypoxia group
Effect of EE on HH Mediated Neurodegeneration
Figure 3 depicts the caspase 3 expression in CA1 region of hippocampus of rats from all groups. HH leads to robust increase in caspase 3 expression in hippocampus which is in confirming previous studies from our lab (Maiti et al. 2006; Hota et al. 2007). Surprisingly group exposed to EE during hypoxia showed two fold declines in the caspase 3 expression when compared to group kept in standard cage (Fig. 3). This shows that EE protects hippocampus against HH mediated cell death.
Fig. 3.
Western blot analysis of caspase 3. Lanes from left to right shows Normoxia, Hypoxia, Hypoxia + EE, and Normoxia + EE. Corresponding graphs denote mean ± SEM of percentage change in optical density of three individual blots. * Represents p < 0.001 when compared to control group, whereas ** represents p < 0.05 when compared to hypoxia group
EE Mediate Neuroprotection via Trk Activation
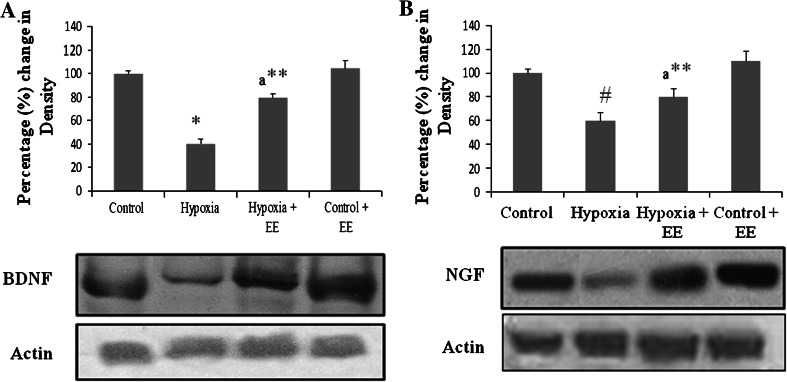
Results showed that HH significantly reduces the level of BDNF (Fig. 4a) and NGF (Fig. 4b), whereas no significant difference was found in case of other neurotrophins i.e., NT3 and NT4 (data not shown). On exposing them to EE during HH there is significant elevation in level of BDNF as well NGF as evident from western blot analysis. Therefore, the neuroprotection provided by EE may be regulated via neurotrophins.
Fig. 4.
Western blot analysis of BDNF (a) and NGF (b). Lanes from left to right shows Normoxia, Hypoxia, Hypoxia + EE, and Normoxia + EE. Corresponding graphs denote mean ± SEM of percentage change in optical density of three individual blots. * Represents p < 0.001 when compared to control group whereas ** represents p < 0.01 when compared to hypoxia group
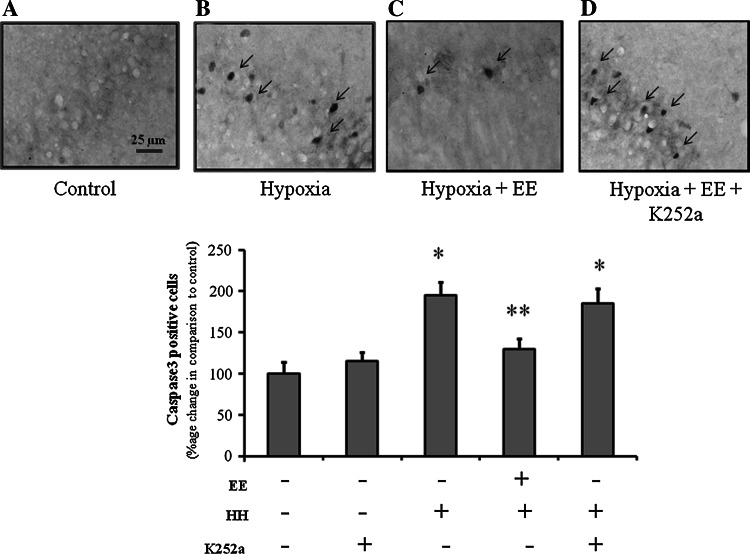
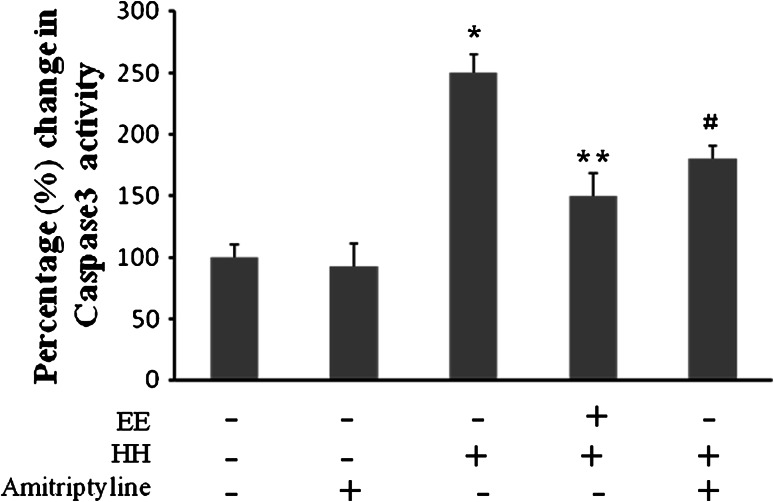
To explore the role of BDNF and NGF in EE mediated neuroprotection against HH we inhibit their respective receptors with K252a, a Trk inhibitor. Results showed that inhibition of TrK with K252a increases caspase 3 expression in CA1 region of hippocampus (Fig. 5), which establishes that BDNF and NGF play a major role in neuroprotection against HH mediated apoptosis. However, no changes were seen on administration of K252a in control group. To further substantiate our finding, we activated Trk using amitriptyline, tyrosine kinase agonist. The results showed that Trk activation with amitriptyline significantly decreases caspase 3 activation during HH which is equivalent to group kept in EE during HH exposure (Fig. 6).
Fig. 5.
EE mediated neuroprotection against HH induced neuronal degeneration in CA1 regions of hippocampus, is neurotrophins dependent as evident from increase in caspase 3 immunoreactivity on inhibiting Trk by K252a. a Normoxia, b Hypoxia, c Hypoxia + EE, d Hypoxia + EE + K252a. Total magnification 400×, bar indicate 25 μm. Graph showing percentage increase in caspase 3 positive neurons. Bars represent mean ± SEM of average of six fields in six individuals of each group. *p < 0.001 and **p < 0.05 when compared to normoxic group
Fig. 6.
Percentage change in caspase 3 activity using DEVD-AMC cleavage assay in different groups. EE decreased caspase 3 activity equivalent to positive control i.e., Trk agonist (Amitriptyline). This shows that EE work through neurotrophins regulated Trk activation. Bars represent mean ± SEM of six individuals. Asterisk and equal and parallel to symbol denotes p < 0.001 and p < 0.005, respectively, when compared to control group, whereas double asterisk represents p < 0.005 when compared to hypoxic group
Discussion
EE is well known for its diverse beneficial effect on the brain physiology. The present study shows that EE provides significant protection against HH mediated neurodegeneration but fails to ameliorate oxidative status of cells in hippocampus. Such an effect probably involves an increase in expression of neurotrophic factors (e.g., BDNF and NGF as shown here) and down regulation of the expression of caspase 3, a well known marker of apoptosis.
The experimental paradigm in present investigation is a psychophysiological stress i.e., HH as compared to other chemically induced methods or ligation methods as in ischemia and it is more closer to field conditions existing at HA in comparison to any other chemically induced stress. Present study revealed that chronic exposure to HH leads to loss in body weight (Table 2a). Probable causes for the weight loss are higher metabolic rate, different energy output, reduced perception of hunger feeling together with decreased food intake (Table 2b) etc. In subjects with normal Basal metabolic index (BMI), loss of body weight during HH has been described in several studies (Tschöp et al. 1998; Westerterp 2001). Surprisingly, our result showed that EE prevents loss in body weight during HH. To our knowledge this is first study demonstrating the effect of EE on body weight during HH condition although the mechanism is not fully understood. This prevention of loss in body weight may be due to increased perception in feeling of hunger and thirst which maintains an optimal level of food intake and body water in rats.
Occurrence of oxidative stress and depletion of the antioxidant status has also been shown to be invariably associated with hypoxic stress through both in vitro and in vivo models. In concordance with previous studies (Maiti et al. 2006; Hota et al. 2007), our result showed accentuated free radical generation and lipid peroxidation in the hippocampus during chronic exposure to HH. The results from present study showed that provision of EE during HH exposure did not have any effect on oxidative stress parameters (Fig. 1a, b). Pereira et al. (2009) previously in his report demonstrated that EE has no significant effect on oxidative status of brain following HI. In our study too, we observed no significant effect of EE on oxidative stress parameters during exposure to HH.
Antioxidant status of hippocampus was also studied and found that HH reduced total thiol level of cells which can be explained as a protective response against oxidative stress due to elevated free radical generation which confirms our previous findings (Maiti et al. 2006). Activation of endogenous antioxidants including SOD, GST, GPx, and GR is one of the main defense mechanisms of cells against oxidative stress induced by hypoxia (Stroev et al. 2005). Antioxidant enzymes activity was also studied. It has been reported that EE has no significant effect in augmentation of reduced antioxidant status of cells during HH. Antioxidant enzymes activity also remains unchanged on EE exposure during HH (Table 3). EE exposure has been previously reported to have no significant effect on antioxidant enzyme activity following HI (Pereira et al. 2009). Some studies on the other hand stated that EE stimulation attenuated pro-oxidative processes and triggered anti-oxidative defense mechanisms (Herring et al. 2009). This difference may be due to different duration of exposure to EE, as it has been observed that EE during recovery after stress may trigger antioxidant defense mechanism but its effect during concurrent exposure to stress has been less studied. EE provides scope for locomotory as well as exploratory activities and has voluntary exercise option too; all these conditions have no or little effect on oxidative status of cells, which shows that although these conditions individually proved beneficial in many neurological disorders but they may work through different mechanisms as they fail to improve oxidative status of cells in our case.
In contrast to the above results, it is observed that EE lowers down the HH mediated elevation in LDH which shows that EE provides protection against cell death. To further validate this we checked expression of caspase 3 level as our previous work showed that HH leads to apoptosis as evident from upregulated caspase 3 expression (Maiti et al. 2006; Hota et al. 2008) and we again found that EE significantly reduces HH mediated apoptosis. Likely, reason for this finding could be downregulation of stress load in group exposed to EE during HH. Corticosterone level was assessed to study the stress level in all groups. Chronic elevation of corticosterone level has been associated with restraint stress, social stress and prolonged moderate stress induced memory impairment (Sgoifo and Papi 1995; Nur Azlina and Nafeeza 2008). In agreement to above findings our results also showed that HH elevated corticosterone level which may disturb diurnal corticoid rhythm. The increased levels of corticosterone in animals exposed to HH raise the possibility that the hippocampus may have been adversely affected since high levels of glucocorticoids are known to be neurotoxic (Sapolsky, 1996). Bakos in his study showed that EE influences HPA axis activity and plasma level of stress hormone (Bakos et al. 2009). Our study also showed that EE reduces the HH mediated stress level as evident from reduced corticosterone level in rats and hence provides protection against HH mediated neurodegeneration. This hypothesis does not rule out contributions from other factors as well.
Neurotrophins were identified as promoters of neuronal survival. In addition, they regulate many aspects of neuronal development and function, including synapse formation and synaptic plasticity (Louis 2006). Neurotrophins can also be affected by stressors or corticosterone administration. Because of the association that has been demonstrated between the neurotrophins and stress and/or corticosterone, as evident from finding of Skelton (Skelton et al. 2007), NGF and BDNF levels during exposure were also studied to determine if alterations in these neurotrophins occurred. The results of the present study showed significant decline in the level of neurotrophins, primarily BDNF and NGF on chronic exposure to HH. The group of rats housed in EE during HH showed that the BDNF and NGF level were maintained as in control group.
EE exposure proves to be beneficial in various neurological disorders. These beneficial effects of EE in the presence of brain damage have been partially attributed to up regulation of neurotrophins, proteins involved in neuronal survival and in activity-dependent plasticity. Several studies showed that EE improves motor functions and increases levels of different neurotrophins after neurological disorders like hemicerebellar lesion, stroke, ischemia, etc., (Gelfo et al. 2010; Söderström et al. 2009). The findings of this study are in agreement with these observations.
A study by Nitta showed that exogenous administration of neurotrophins attenuated corticosterone induced neural death. But in the presence of Trk inhibitor (K252a) the effect was nullified. (Nitta et al. 1999). Also in another study, BDNF and NGF were found to be neuroprotective in many stress models like Staurosporine (STS) and Etoposide (ETS) induced apoptotic cell death as demonstrated by inhibiting Trk receptor with K252a (Nguyen et al. 2010). K252a is a well known Trk inhibitor which inhibits BDNF and NGF receptors i.e., TrkA and TrkB, respectively, at different concentrations. K252a is very sensitive to TrkB as it inhibits TrkB at lower dose, whereas to inhibit TrkA dose need to be increased. The present study also showed that at 250 μM, K252a inhibits both receptors significantly. In the present study to explore the role of neurotrophins in EE mediated neuroprotection against HH, we inhibited Trk receptors and showed that inhibition of Trk (TrkA and TrkB) with K252a increases caspase 3 expression in hippocampus. Glucocorticoid receptors (GR) are known to interact with TrkB and that the TrkB–GR interaction and the BDNF-dependent binding of PLC-γ to TrkB decreased following corticosterone exposure. (Tadahiro et al. 2008). These reports are in agreement with our findings that Trk plays a pivotal role in EE mediated neuroprotection during HH.
To further validate the role of tyrosine kinase in EE mediated neuroprotection against HH induced apoptosis, we utilized agonist for Trk, amitriptyline which elicits potent TrkA and TrkB activation in mice with a similar temporal pattern and acts as a novel agonist for both TrkA and TrkB. (Sung et al. 2009). Supplementation of amitriptyline during HH significantly decreases caspase 3 activity which confirms the role of Trk activation in EE mediated neuroprotection. This neuroprotective capability of amitriptyline may be attributed to its parallel activation of TrkA and TrkB. Reports suggested that Amitriptyline exhibits potent neurotrophic activities including neurite outgrowth in PC12 cells, neuronal survival in primary neurons and neuroprotection in mice, which mimic the primary neurotrophic effects of NGF. Amitriptyline has been found to be effective in treating chronic neuropathic pain (Ho et al. 2008; Watson, 2000).
Taken together present study not only explored the protective effect of EE on HH induced neurodegeneration but also revealed the interaction between corticosterone and neurotrophins. Present study also brings to light that EE mediated neuroprotection is independent of antioxidant signaling as it fails to prevent HH induced oxidative stress. Interestingly it has been observed that Trk activation is required for EE mediated neuroprotection which proves the role of neurotrophins mainly BDNF and NGF. These findings lead us to suggest that, diurnal corticoid rhythm and neurotrophins levels are important for neuronal protection and survival during HH. It is unclear whether elevated corticosterone level regulates level of neurotrophins or vice versa during HH. It may be postulated that decreased levels of neurotrophins are involved in HH mediated neurodegeneration and corticosterone may be involved in mediating some part of this effect. In future studies, we will determine how changes in corticosterone and neurotrophins are related to the learning and memory deficits observed during HH.
Acknowledgment
The study was fully supported by Defence Research and Development Organization (DRDO), Ministry of Defence India.
Abbreviations
- HA
High altitude
- HH
Hypobaric hypoxia
- HI
Hypoxia-ischemia
- EE
Enriched Environment
- BDNF
Brain derived neurotrophic factor
- NGF
Nerve growth factor
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- MDA
Malondialdehyde
- LDH
Lactate dehydrogenase
- GSH
Glutathione
- GSSG
Glutathione disulfide
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GST
Glutathione s-transferase
- Trk
Tyrosine kinase
References
- Almli LM, Hamrick SE, Koshy AA, Tauber MG, Ferriero DM (2001) Multiple pathways of neuroprotection against oxidative stress and excitotoxic injury in immature primary hippocampal neurons. Brain Res Dev Brain Res 132:121–129 [DOI] [PubMed] [Google Scholar]
- Askew EW (2002) Work at high altitude and oxidative stress: antioxidant nutrients. Toxicology 180:107–119 [DOI] [PubMed] [Google Scholar]
- Bakos J, Hlavacova N, Rajman M, Ondicova K, Koros C, Kitraki E, Steinbusch HW, Jezova D (2009) Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner. Neuroscience 164(2):788–797 [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH (1984) Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 43(5):1369–1374 [DOI] [PubMed] [Google Scholar]
- Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Ann Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia induced transcription. Proc Natl Acad Sci USA 95(20):11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Taffe KM, Abrahamson EE, Dixon CE, Kochanek PM, Ikonomovic MD (2004) Time course analysis of hippocampal nerve growth factor and antioxidant enzyme activity following lateral controlled cortical impact brain injury in the rat. J Neurotrauma 21:491–500 [DOI] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J (2005) Peroxide detoxification by brain cells. J Neurosci Res 79:157–165 [DOI] [PubMed] [Google Scholar]
- Fernández CI, Collazo J, Bauza Y, Castellanos MR, López O (2004) Environmental enrichment-behavior-oxidative stress interactions in the aged rat: issues for a therapeutic approach in human aging. Ann N Y Acad Sci 1019:53–57 [DOI] [PubMed] [Google Scholar]
- Gelfo F, Cutuli D, Foti F, Laricchiuta D, De Bartolo P, Caltagirone C, Petrosini L, Angelucci F (2010) Enriched environment improves motor function and increases neurotrophins in hemicerebellar lesioned rats. Neurorehabil Neural Repair 25(3):243–252 [DOI] [PubMed] [Google Scholar]
- Gorter JA, Petrozzino JJ, Aronica EM, Rosenbaum DM, Opitz T, Bennett MV, Connor JA, Zukin RS (1997) Global ischemia induces downregulation of GluR2 mRNA and increases AMPA receptor mediated Ca2 + influx in hippocampal CA1 neuron of gerbil. J Neurosci 17:6179–6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig WH, Jacoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405 [DOI] [PubMed] [Google Scholar]
- Han BH, Holtzman DM (2000) BDNF protects the neonatal brain from hypoxic–ischemic injury in vivo via the ERK pathway. J Neurosci 20(15):5775–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Lee JM, Zipfel GJ, Wozniak DF (2005) Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res 10(1043(1-2)):48–56 [DOI] [PubMed] [Google Scholar]
- Herring A, Blome M, Ambrée O, Sachser N, Paulus W, Keyvani K (2009) Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathol 20(1):66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissin PJ, Hilf RA (1976) Fluorimetric method for determination of oxidized and reduced glutathione in tissue. Anal Biochem 74:214–226 [DOI] [PubMed] [Google Scholar]
- Ho KY, Huh BK, White WD, Yeh CC, Miller EJ (2008) Topical amitriptyline versus lidocaine in the treatment of neuropathic pain. Clin J Pain 24:51–55 [DOI] [PubMed] [Google Scholar]
- Hota SK, Barhwal K, Singh SB, Ilavazhagan G (2007) Differential temporal response of hippocampus, cortex and cerebellum to hypobaric hypoxia: a biochemical approach. Neurochem Int 51:384–390 [DOI] [PubMed] [Google Scholar]
- Hota SK, Barhwal Kalpana, Singh SB, Ilavazhagan G (2008) Chronic hypobaric hypoxia induced apoptosis in CA1 region of hippocampus: a possible role of NMDAR mediated p75NTR upregulation. Exp Neurol 212:5–13 [DOI] [PubMed] [Google Scholar]
- Kramer AF, Coyne JT, Strayer DL (1993) Cognitive function at high altitude. Hum Factors 35:329–344 [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958 [DOI] [PubMed] [Google Scholar]
- Lee IM, Paffenbarger RS Jr (1998) Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke 29:2049–2054 [DOI] [PubMed] [Google Scholar]
- Louis FR (2006) Neurotrophin-regulated signalling pathways. Phil Trans R Soc B 361:1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti Panchanan, Singh SB, Sharma AK, Muthuraju S, Banerjee PK, Ilavazhagan G (2006) Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem Int 49:709–716 [DOI] [PubMed] [Google Scholar]
- Mylorie AA, Collins H, Umbles C, Kyle J (1986) Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetate. Toxicol Appl Pharmacol 82:512–520 [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Kim CK, Cho JH, Lee KH, Ahn JY (2010) Neuroprotection signaling pathway of nerve growth factor and brain-derived neurotrophic factor against staurosporine induced apoptosis in hippocampal H19–7/IGF-IR. Exp Mol Med 42(8):583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta A, Ohmiya M, Sometani A, Itoh M, Nomoto H, Furukawa Y, Furukawa S (1999) Brain-derived neurotrophic factor prevents neuronal cell death induced by corticosterone. J Neurosci Res 57:227–235 [DOI] [PubMed] [Google Scholar]
- Nur Azlina MF, Nafeeza MI (2008) Tocotrienol and alpha-tocopherol reduce corticosterone and noradrenalin levels in rats exposed to restraint stress. Pharmazie 63(12):890–892 [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA (2008) Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging 29:51–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk E, Demirbilek S, Kadir But A, Saricicek V, Gulec M, Akyol O, Ozcan Ersoy M (2005) Antioxidant properties of propofol and erythropoietin after closed head injury in rats. Prog Neuropsychopharmacol Biol Psychiatry 29:922–927 [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Zukin RS, Bennett MV, Cho S, Pulsinelli WA (1992) Switch in glutamate receptor subunit gene expression in CA1 subfield of hippocampus following global ischemia in rats. Proc Natl Acad Sci USA 89:10499–10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LO, Nabinger PM, Strapasson AC, Nardin P, Gonçalves CA, Siqueira IR, Netto CA (2009) Long-term effects of environmental stimulation following hypoxia-ischemia on the oxidative state and BDNF levels in rat hippocampus and frontal cortex. Brain Res 1247:188–195 [DOI] [PubMed] [Google Scholar]
- Robinson JP, Bruner LH, Basoe CF, Hudson JL, Ward PA, Phan SH (1998) Measurement of intracellular fluorescence of human monocytes relative to oxidative metabolism. J Leukoc Biol 43:304–310 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D (2000) Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403:316–321 [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M (2006) Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 24:1850–1856 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (1996) Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1:1–19 [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Papi F (1995) Effects of social and non-social acute stressors on plasma levels of cate-cholamines and corticosterone in wild rats. Rendiconti Lincei 6:289–298 [Google Scholar]
- Shukitt-Hale B, Banderet LE, Lieberman HR (1998) Elevation-dependent symptom, mood, and performance changes produced by exposure to hypobaric hypoxia. Int J Aviat Psychol 8(4):319–334 [DOI] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV (2007) Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology 32:734–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström I, Strand M, Ingridsson AC, Nasic S, Olsson T (2009) 17 beta-estradiol and enriched environment accelerate cognitive recovery after focal brain ischemia. Eur J Neurosci 29(6):1215–1224 [DOI] [PubMed] [Google Scholar]
- Stroev SA, Gluschenko TS, Tjulkova EI, Rybnikova EA, Samoilov MO, Pelto-Huikko M (2005) The effect of preconditioning on the Cu, Zn superoxide dismutase expression and enzyme activity in rat brain at the early period after severe hypobaric hypoxia. Neurosci Res 53(1):39–47 [DOI] [PubMed] [Google Scholar]
- Sung WukJang, Liu Xia, Chan Chi-Bun, Weinshenker David, Hall RA, Xiao Ge, Yel Keqiang (2009) The antidepressant amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem Biol 16(6):644–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadahiro Numakawal, Kumamaru Emi, Adachi Naoki, Yagasaki Yuki, Izumi Aiko, Kunugi Hiroshi (2008) Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-_ signaling for glutamate release via a glutamate transporter. PNAS 106:647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöp M, Strasburger CJ, Hartmann G, Biollaz J, Bärtsch P (1998) Raised leptin concentrations at high altitude associated with loss of appetite. Lancet 352:1119–1120 [DOI] [PubMed] [Google Scholar]
- Utley HG, Bernheim F, Hochstein P (1967) Effect of sulphydryl reagents on per oxidation of microsomes. Arch Biochem Biophys 118:29–32 [Google Scholar]
- Watson CP (2000) The treatment of neuropathic pain: antidepressants and opioids. Clin J Pain 16:S49–S55 [DOI] [PubMed] [Google Scholar]
- Westerterp KR (2001) Energy and water balance at high altitude. News Physiol Sci 16:134–137 [DOI] [PubMed] [Google Scholar]
- Wong YN, Chien BM, D’mello AP (1994) Analysis of corticosterone in rat plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl 661(2):211–218 [DOI] [PubMed] [Google Scholar]
- Yun Wang, Qi Jin-Shun, Kong Shuzhen, Sun Yajie, Fan Jing, Jiang Min, Chen Gong (2009) BDNF-TrkB signaling pathway mediates the induction of epileptiform activity induced by a convulsant drug cyclothiazide. Neuropharmacology 57(1):49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]