Abstract
Extracellular ATP plays a pivotal role as a signaling molecule in physiological and pathological conditions in the CNS. In several glioma cell lines, ATP is a positive factor for one or more characteristics important for the abnormal growth and survival of these cells. This work presents immunofluorescence and biochemical analyses suggesting that an aerobic metabolism, besides mitochondria, is located also on the plasma membrane of C6 glioma cells. An ATP synthesis coupled to oxygen consumption was measured in plasma membrane isolated from C6 cells, sensitive to common inhibitors of respiratory chain complexes, suggesting the involvement of a putative surface ATP synthase complex. Immunofluorescence imaging showed that Cytochrome c oxydase colocalized with WGA, a typical plasma membrane protein, on the plasma membrane of glioma cells. Cytochrome c oxydase staining pattern appeared punctuate, suggesting the intriguing possibility that the redox chains may be expressed in discrete sites on C6 glioma cell membrane. Data suggest that the whole respiratory chain is localized on C6 glioma cell surface. Moreover, when resveratrol, an ATP synthase inhibitor, was added to culture medium, a cytostatic effect was observed, suggesting a correlation among the ectopic ATP synthesis and the tumor growth. So, a potential direction for the design of new targets for future therapies may arise.
Keywords: ATP synthase, C6 glioma, Cytochrome c oxydase, Ectopic ATP production, Electron transfer chain, Plasma membrane, Resveratrol
Introduction
Brain tumours are responsible for approximately 2% of all cancer deaths. Gliomas, and glioblasotmas are the most common form of primary tumors of the central nervous system (CNS) and despite many therapeutic strategies, such as surgery and chemiotherapy, survival rate for patients with malignant gliomas remains low (Avgeropoulos and Batchelor 1999). The progression of low to high grade is characterized by an increase in neovascularization, focal necrosis, and cellular proliferation (Kaye and Hill 1993; Woodburn et al. 1994). Thus, new therapeutic strategies are urgently needed.
Extracellular ATP and other nucleotides and nucleosides were shown to play a pivotal role as signaling molecules in physiological and pathological conditions in the CNS. In fact, in several glioma cell lines, representative models of extremely aggressive cancer cells (Grobben et al. 2002), ATP is a positive factor for one or more characteristics important for the abnormal growth and survival of these cells, including proliferation, survival (blockage of apoptosis), angiogenesis, and invasion. For example, ATP is a mitotic factor for glioma cells (Morrone et al. 2003) and modulates cell proliferation in primarily cultured astrocytes of neonatal rat cortices and rat striatum. Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures (Neary et al. 1994) and stimulates phospholipase C activity in various cells, with the resulting production of inositol phosphate and diacylglycerol (Neary et al. 1994). It was shown that extracellular ATP-induced calcium signalling in healthy cultured rat cortical astrocytes is due to purinergic receptor occupation (Nobile et al. 2003).
An ectopic location of Fo F1-ATP synthase in many mammalian cell membranes has been reviewed (Chi and Pizzo 2006b), even though most authors ascribe to it functions other than ATP synthesis. An extracellular ATP synthesis was also reported by the umbilical vein endothelial cells (Arakaki et al. 2003), and by hepatocyte culture membranes (Mangiullo et al. 2008). Recently, we have reported an extramitochondrial ATP synthesis, demonstrating that the ectopic electron transfer chain (ETC) proteins were functional and could play an energetic role in rod outer segment disks (a subcellular fraction devoid of mitochondria) of the bovine retina (that is part of CNS) (Panfoli et al. 2009) and in isolated myelin vesicles (IMV; Ravera et al. 2009). Moreover, an ectopic localization of ETC proteins was demonstrated by several proteomic studies (Bae et al. 2004; Foster et al. 2003; Bini et al. 2003; Kim et al. 2004; Kim et al. 2006.
Aiming at studying the source of extracellular ATP in glioma cells, we chose the rat C6 glioma cell line, an established model for human glioma (Kaye et al. 1986). The C6 glioma cell line was chemically induced by N-nitrosomethylurea in the rat CNS (Benda et al. 1968). Although the C6 cells were characterized as an astrocytoma (Benda et al. 1971), they express 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte marker (McMorris et al. 1985) along with glial fibrillary acid protein, suggesting that it is heterogeneous. Electrophoretic studies showed that Myelin Basic Protein and proteolipid protein are characteristic of C6 glioma cells (Volpe et al. 1975). So, the C6 cell line appears to have the potential to express features of both astrocytes and oligodendrocytes, while its phenotype may depend on growth stage and environmental factors (Parker et al. 1980).
In this work, we investigated the ectopic presence and the role of Fo F1-ATP synthase and the ETC proteins on C6 plasma membranes by biochemical and immunohystochemical techniques. Data may be relevant to the pathobiology of the energy dependent diseases (such as cancer) to the understanding of the non-energetic role of extracellular ATP production in the tumour growth.
Methods
Cell Lines
Rat C6 glioma cells (CCL 107; American Type Culture Collection, Rockville, MD, USA) were maintained an atmosphere of 5% CO2, at 37°C in Dulbecco’s Modified Eagle’s MEM supplemented with 10% fetal calf serum (FCS) (Celbio, Italy), 1 mM l-glutamine and 1% (v/v) antibiotics (penicillin and streptomycin) and split twice a week.
C6 Membrane Fraction Preparation
C6 cells were homogenised, after tripsinization, by a Potter–Elvehjem system in few volume of homogenizing medium (HM) containing 0.25 M sucrose, 5 mM HEPES buffer, and 1 mM EDTA (pH 7.2). Protease inhibitor cocktail (Sigma-Aldrich) 30 μg/ml 5-Fluorouracil and 20 μg/ml Ampicillin were present throughout isolation procedures. The homogenate was centrifuged at 500g for 10 min to remove the tissue debris and cell nuclei. To obtain the mitochondria-enriched fraction, the supernatant was centrifuged for 20 min in a Heraeus centrifuge at 20,000g, the pellet was collected, resuspended in HM containing 10 μM cyclosporin, and aliquots stored at −80°C. To obtain the plasma-membrane fraction, the supernatant was layered over a step sucrose gradient (1.2 M:1.6 M:1.84 M) and centrifuged at 90,000g for 1 h in a Beckman ultracentrifuge (Beckman, Fullerton, CA, USA), an SW-27 Rotor. The fraction at the interphase between 1.2 and 1.6 M sucrose was collected and dispersed in a total volume of 20 ml of water to a final sucrose concentration of 0.25 M and centrifuged 90,000g for 60 min. The final pellet was stored at −80°C.
Electrophoresis and Western Blot Analysis
Denaturing electrophoresis (SDS-PAGE) was performed using a Laemmli (Laemmli 1970) protocol. Antibodies (Abs) were: anti-Fo F1-ATP synthase β subunit (Sigma-Aldrich, USA); anti-ND4L (a subunit of Redox Complex I) (Santa Cruz, USA); anti-Cythocrome c (Santa Cruz, USA); anti-mitochondrial import inner membrane translocase (TIM), subunit 8A (Santa Cruz, CA, USA) and anti-adenosine nucleotide translocase (ANT) (Santa Cruz, USA). Abs were diluted in PBS (1:200). Secondary fluorescent Abs were from Sigma-Aldrich. Protein molecular weight (MW) markers were from Fermentas (Fermentas Life Sciences, Hanover, MD, USA).
ATP Synthase Assay
ATP synthesis rate was measured by the luciferin/luciferase chemiluminescent method (Roche Applied Science), as reported (Ravera et al. 2009) on purified membrane fractions of C6 glioma cells (0.03 mg protein/sample). The ATP concentration was measured in a luminometer (Lumi-Scint, Bioscan). 0.5 mM Ouabain, 0.2 mM di(adenosine-5′) penta-phosphate and 2 mM EGTA were added to the assay mix, to inhibit the ATP hydrolysis by Na+/K+ ATPase, adenylate kinase and Ca2+ ATPase, respectively.
Assay of Redox Complexes
The mitochondrial redox complexes I to IV were assayed on the purified membrane fractions of C6 glioma cells according to Ravera et al. (2009).
Oxygraphic Measurements
O2 consumption was assayed on the purified membrane fraction of C6 glioma cells by a thermostatically controlled oxygraph apparatus equipped with an amperometric electrode (Unisense-Microrespiration, Unisense A/S, Denmark) and a rapid mixing device. In a typical experiment, sample (0.03 mg of protein) was incubated, at 23°C, in: 120 mM KCl, 2 mM MgCl2, 1 mM KH2PO4, 50 mM Tris–HCl, pH 7.4 and 25 μg/ml ampicillin (final volume 1,7 ml) (Sgobbo et al. 2007). To observe the uncoupled respiration rate, Nigericin (5 μM) and Valinomycin (10 μM) were added to the suspension just after the sample addition. Then substrates and inhibitors of O2 consumption were added (0.7 mM NADH, 20 mM succinate, 10 mM ascorbate, 40 μM rotenone, 50 μM antimycin A and 0,5 mM potassium cyanide). Respiratory rates are expressed in μM O2/min/mg.
Immunocytochemical Reactions
C6 glioma cells were cultured on slides for 24 h, fixed with 0.3% paraformaldehyde. Then slides were incubated overnight in a moist chamber at 4°C with the rabbit polyclonal antibody against Cytochrome c oxydase (COX IV) (1:200, Sigma, Hercules USA) diluted with PBS 0.1% bovine serum albumin. After a PBS wash step, the slides were incubated for 2 h with the Alexa-488-conjugated anti-rabbit antiserum (1:800; Molecular Probes, USA), as secondary antibody. The slides were then incubated with and antibody against the membrane lectin, the wheat germ agglutinin (WGA) conjugated with Texas-Red, for 1 h. After PBS wash, slides were mounted in a glycerol/PBS (1:1) solution. Immunofluorescence CLSM imaging was performed on an inverted Leica TCS SP5 AOBS confocal laser scanning microscope (Leica Microsystems CMS, Mannheim, Germany), equipped with a set of lasers covering the 458, 476, 488, 496, 514, 543, and 633 nm lines. Confocal fluorescence imaging was done on these samples at 23°C.
Images were collected using a Leica 63XPL APO NA 1.40 oil immersion objective (Leica Microsystems CMS, Mannheim, Germany).
Resveratrol Effect on C6 Cell Culture
C6 glioma cells were cultured at 5,000 cells/well in 96-well plates. 10 μM resveratrol was added 24 h after cell plating. The final medium volume of each well was 200 μl. At 0, 3, and 6 h of incubation, crystal violet assay was used to measure cell growth. Culture medium was removed, cells washed with 1 ml of 1× PBS per well. Fifty microliters of crystal violet solution were added per well and incubated for 20 min. Crystal violet solution was then removed, washed with tap water several times. Plates were left to dry overnight. A hundred microliters of 50% ethanol 0.1% CH3COOH solution was added per well and absorbance was then measured at 595 nm with an Anthos ELISA Reader (Life Sciences International, Basingstoke, UK).
Other Procedures
Protein concentration was determined by Bradford method, using BSA as a standard protein (Bradford 1976).
Results
Characterization of C6 Cell Plasma Membrane Fraction
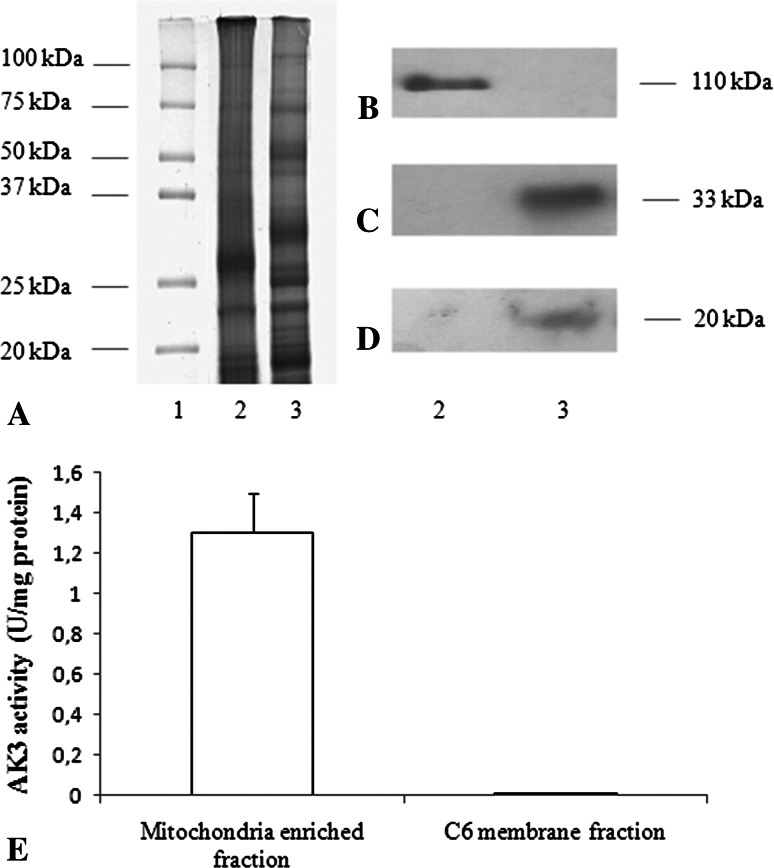
To characterize the membrane-enriched C6 fractions several semiquantitative WB analysis were carried out. Figure 1a shows the electrophoretic protein pattern of the C6 membrane fraction (lane 2) mitochondria-enriched fraction (lane 3, used as positive control), and molecular weight markers (lane 1) as stained with Blue Silver (Candiano et al. 2004). Figure 1b shows the WB with anti-Na+/K+ ATPase, a marker of plasma membrane. The signal was present only in the lane of C6 membrane fraction.
Fig. 1.
The characterization of the C6 membrane fraction is shown. a Shows the protein pattern of the C6 membrane fraction (lane 2) mitochondria-enriched fraction (lane 3) and molecular weight markers (lane 1) as stained with Blue Silver. b–d are a representative semiquantitative WB analysis with anti-Na+/K+ ATPase, anti-ANT, and anti-TOM, respectively. Samples are the same as in a. Each figure is a representative of three experiments. e Reports the AK3 activity in mitochondria-enriched fraction and C6 glioma cell plasma membranes. Each value represents the mean ± SD of five measurements
The procedure to obtain C6 membrane fraction has been carefully designed. Mitochondria were eliminated during the second centrifugation (See “Methods” section). Moreover, the C6 membrane fraction was prepared in absence of cyclosporin A (Crompton et al. 1988) and 2-aminoethoxydiphenyl borate (Chinopoulos et al. 2003), in order to support the formation transition pore in the contaminant mitochondria, if any. Semiquantitative WB, carried out with Abs against ANT and TIM (two typical inner membrane proteins) shows that these proteins were only detectable in mitochondria (Fig. 1c, d, lane 2, C6 membrane fraction; lane 3, mitochondria-enriched fraction). AK3, a typical mitochondrial matrix enzyme was also assayed in both samples. AK3 activity was present in mitochondria-enriched fractions (1.1 ± 0.07 U/mg of total proteins) and was absent in C6 membrane fraction (Fig. 1e).
Fo F1-ATP Synthase in C6 Glioma Cell Surface
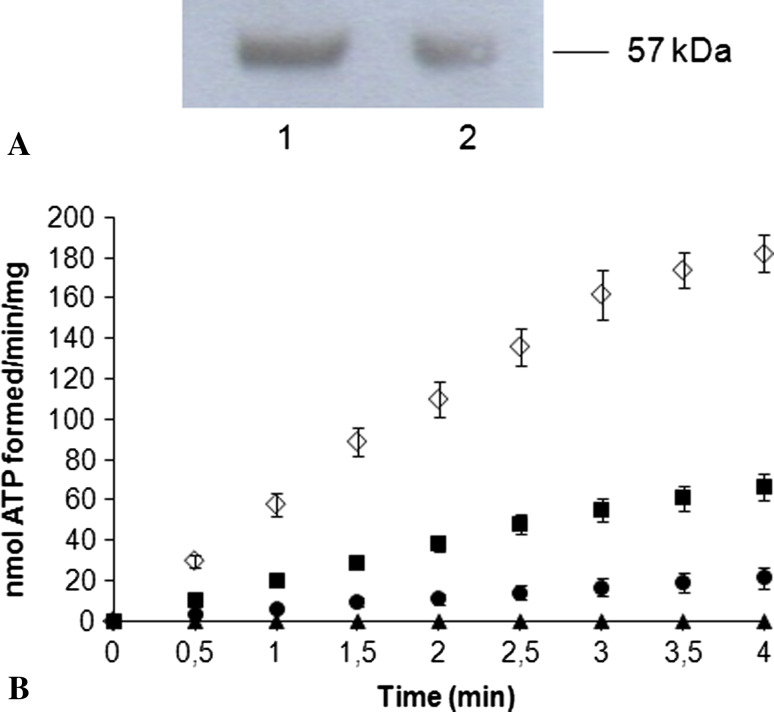
Semiquantitative WB analyses conducted with Abs against β subunits of Fo F1-ATP synthase (Fig. 2a) shows that the protein was present in both samples. The functional implications of these data were studied measuring the rate of ATP synthesis in purified membrane fraction C6 glioma cells, energized with succinate. In order to assess whether ATP synthesis was carried out by Fo F1-ATP synthase, we included in the medium adenylate kinase inhibitors. Exogenous Mg2+ was added, in order to supply saturating Mg-ADP to the ATP synthase complex. Figure 2b reports the ATP synthase activity, which appeared stationary after 3 min, from the assay beginning (white rhombus). The ATP formation was inhibited by oligomycin (89%), the Fo F1-ATPase/H+-pump inhibitor, cyanide (KCN) (65%), used as inhibitor of Complex IV and antimycin A (100%), a specific inhibitor of Complex III.
Fig. 2.
The presence and the functionality of Fo F1-ATP synthase in C6 glioma cell plasma membrane is shown. a is a representative semiquantitative WB analysis with anti-β subunit of Fo F1-ATP synthase. Samples are: C6 glioma cell plasma membrane (lane 1) and mitochondria-enriched fraction (lane 2); (Experiment was repeated thrice). b Reports ATP synthesis activity C6 glioma cell plasma membranes (white rhombus). Activity was inhibited by oligomycin (black circle, 89%), cyanide (KCN) (black square, 65%), and antimycin A (black triangle, 100%). Each value represents the mean ± SD of five measurements
Spectrophotometric Assay of Redox Complexes
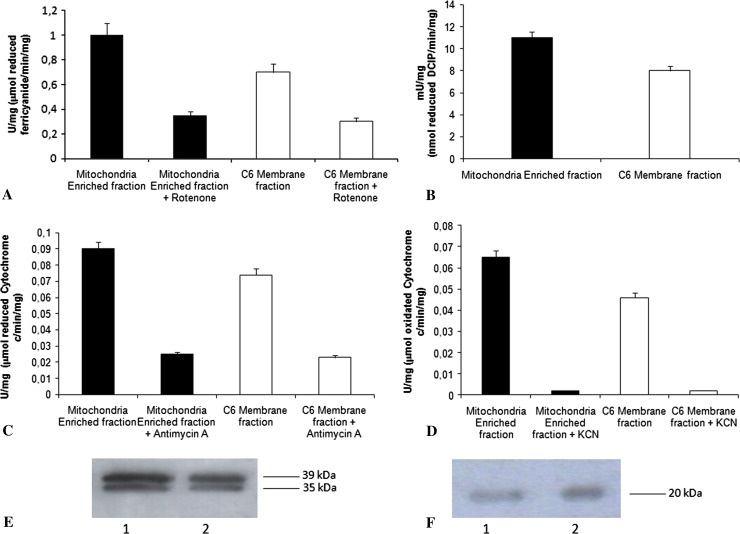
Figure 3 shows the activities of the redox complexes in mitochondrial-enriched fraction and in C6 membrane fractions in the absence or presence of specific inhibitors (rotenone for NADH-ubiquinone oxidoreductase, 50% inhibition, Fig. 3a; antimycin A for cytochrome c reductase, 75% inhibition, Fig. 3c; cyanide for cytochrome c oxidase, 99% inhibition, Fig. 3d). Semiquantitative WB analysis of the same samples is described in Fig. 3e and f (lane 1: C6 membrane fraction; lane 2: mitochondria-enriched fraction). Abs were against NADH ubiquinone oxidoreductase and cytochrome c oxidase, respectively.
Fig. 3.
The activities of the four redox complexes in mitochondria-enriched fraction and in C6 membrane fractions, in the absence or presence of specific inhibitors (a NADH-ubiquinone oxidoreductase, b succinic dehydrogenase, c cytochrome c reductase, d cytochrome c oxidase). Each value represents the mean ± SD of five measurements. e Shows a typical semiquantitative WB analysis, with anti-NADH-ubiquinone oxidoreductase (ND4L). f Shows the same analysis with anti-cytochrome c oxidase. Samples were: C6 membrane fraction, lane 1; mitochondria-enriched fraction, lane 2
Oxygraphic Measurements
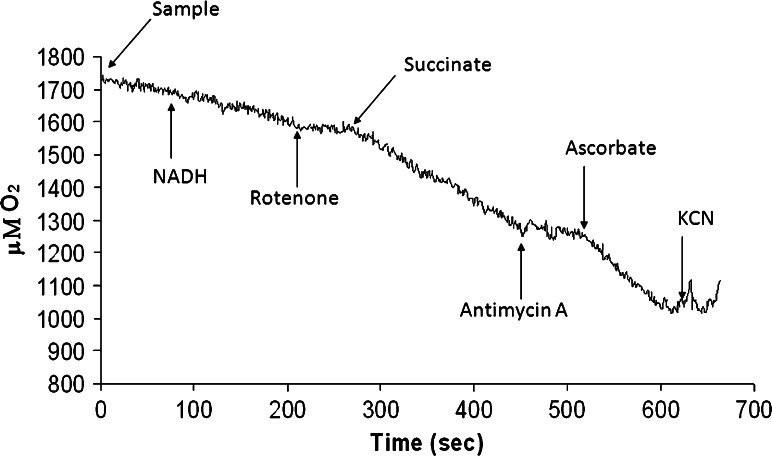
An analysis of respiratory fluxes was also conducted. Figure 4 reports a typical amperometric tracing of Nigericin/Valinomycin uncoupled respiratory rates of C6 cell plasma membrane. The maximal respiratory rates with NADH (Complex I + III + IV), succinate (Complex II + III + IV) and ascorbate (Complex IV) as respiring substrates were sensitive to inhibition by rotenone, antimycin A and cyanide, respectively. Notably, C6 plasma membrane was able to utilize NADH as a respiring substrate (Complex I + III + IV), which suggests a free access of NADH, which mitochondria do not possess.
Fig. 4.
A typical amperometric tracing of a Nigericin (5 μM)/Valinomycin (10 μM) uncoupled respiration rate in C6 cell plasma membrane fraction. The maximal respiratory fluxes by NADH, succinate, and ascorbate are measured as rates sensitive to inhibition by rotenone, antimycin A, and cyanide, respectively. This experiment was repeated four times with identical results
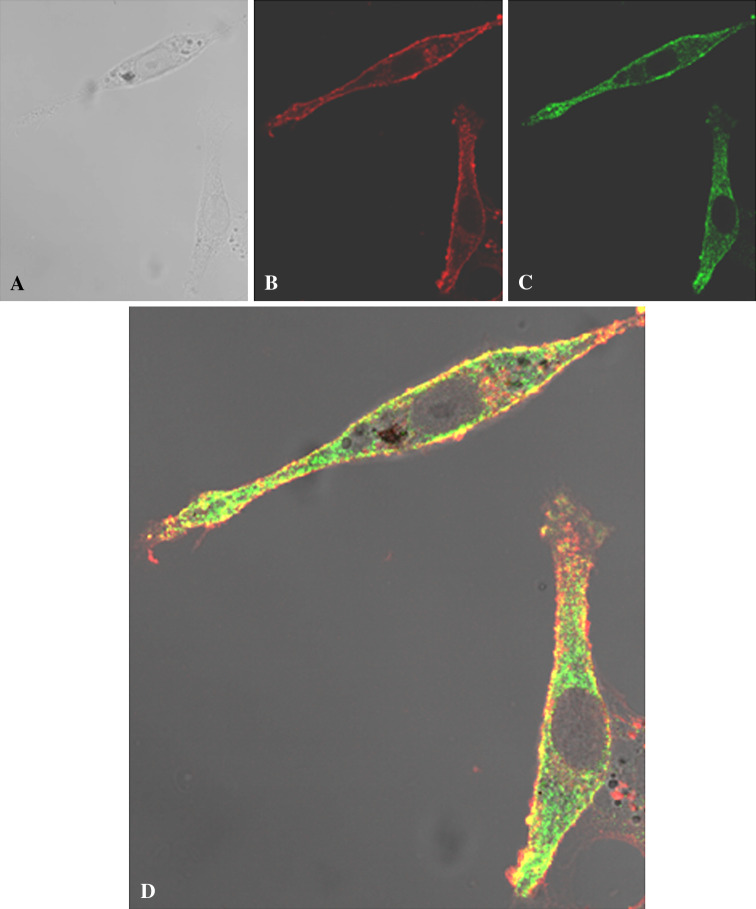
Localization of COX IV and WGA on C6 Cell Surface
Figure 5 shows the colocalization of COX IV and WGA on C6 cell surface. In Fig. 5a, transmission image is reported, while the fluorescent signal of COX IV and WGA are shown in b and c, respectively. Figure 5d shows the image of the merge fluorescence. Interestingly, COX IV fluorescence appeared patched and punctuate, suggesting that ATP synthase is located on discrete sites onto the cellular membrane.
Fig. 5.
Colocalization of COX IV and WGA on C6 plasma membrane surface. a Shows the transmission image, and b, c report the immunofluorescence signal of COX IV and WGA, respectively. The image of the merged fluorescence of a–c is in d. The signal in panel d indicates the colocalization between the signal of COX IV and of WGA. This experiment was performed thrice on different samples
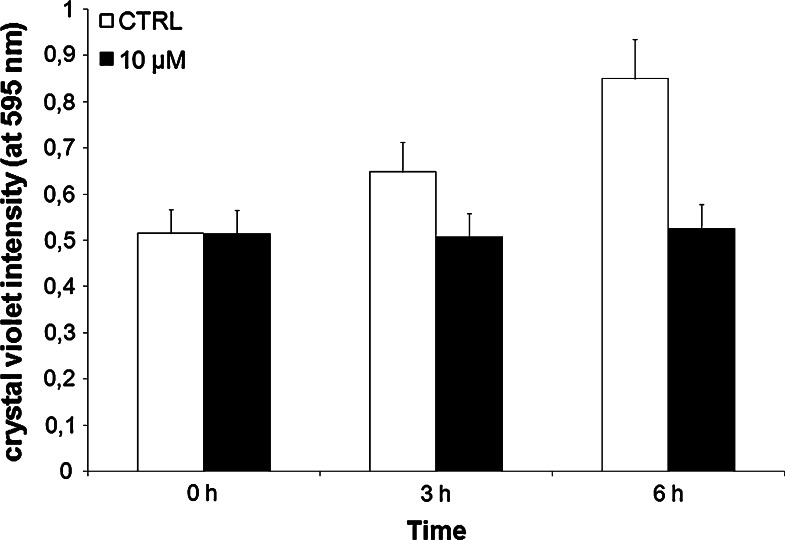
Effect of Resveratrol on C6 Cell Culture
To correlate the ectopic ATP synthesis on plasma membrane with the tumor growth, 10 μM resveratrol, an inhibitor of ATP synthase F1 portion, was added to C6 cells culture medium. In fact it was demonstrated that resveratrol is a Fo F1-ATP synthase inhibitor (Hong and Pedersen 2008) and that it inhibits events associated with tumor initiation and proliferation (Jang et al. 1997). Its effect was studied after 0, 3, and 6 h, by the crystal violet method that evaluates the cell number and viability. This method stains only the viable cells, and its intensity is proportional to the cell number. As shown in Fig. 6, resveratrol induced an cytostatic effect on C6 culture, already after 3 h, with respect to the control, suggesting that the ectopic ATP synthesis is an important factor to tumor growth. Moreover, it is possible to presume that resveratrol could affect both the ectopic ATP synthase, present on plasma membrane and the mitochondrial ATP synthase, altering the intra and extracellular energetic balances.
Fig. 6.
The resveratrol effect on the C6 cells growth at 0, 3, and 6 h after the addition in the culture medium. White columns and black columns represent the control and treated cells, respectively. Each value represents the mean ± SD of six measurements
Discussion
By imaging techniques, oxymetry, and biochemical analyses, we show data suggesting that C6 glioma cell surface may represent a site of ectopic ATP production, thank to the functional presence of the five complexes of respiration ectopically localized therein.
Fo F1-ATP synthase, a mitochondrial enzyme synthesizing ATP from ADP and inorganic phosphate (Pi), has been extensively studied for expression on the cell surface. ATP synthase has been unexpectedly localized in the plasma membrane; by immunofluorescence, and plasma membrane fractionation from various mammalian cell lines (Chi and Pizzo 2006b).
We measured a consistent ATP production (58 nmol/min/106 cells) in purified C6 cell plasma membranes, which was sensitive to olygomicin, a specific inhibitor of F o subunit of ATP synthase, suggesting the involvement of a mitochondrial ATP synthase, as well as to cyanide, an inhibitor of redox complex IV (Fig. 2). Moreover, WB analyses showed that the β subunit of ATP synthase is enriched in C6 membrane fractions (Fig. 2a). A similar ATP synthesis (about 30 nmol/min/mg) by a surface ATP synthase was found in adipocyte 3T3-L1 cells (Kim et al. 2004). In the human umbilical endothelial cells (HUVEC; Arakaki et al. 2003) and hepatome cells (Mangiullo et al. 2008), extracellular ATP synthesis ranged from pico- to nano-moles. Treatment with oligomycin, and other ATP synthase inhibitors decreased the generation of extracellular ATP in HUVEC cells (Arakaki et al. 2003).
We have previously reported the functional ectopic expression of ATP synthase and ETC on mammalian retinal rod outer segments disks and bovine brain IMV coupled to a consistent aerobic ATP production (in the micromolar range) which we hypothesized to supply the energetic demands of these two high energy requiring tissues, that are devoid of mitochondria (Panfoli et al. 2008; Ravera et al. 2009; 2010). In hepatome cells, ATP synthase was hypothesised to control the extracellular ADP/ATP ratio, and intracellular pH homeostasis (Mangiullo et al. 2008).
ATP production by ETC in C6 glioma cell plasma membranes may represent an extracellular signal, an in vivo signal of malignancy progression of the glioma itself. A plasma membrane extracellularly oriented ecto-F1-ATPase may also be involved in the signalling of purinergic receptors, that are membrane cation channels gated by extracellular ATP (Surprenant and North 2008). Occupation by adenine nucleotides or adenosine of purinergic receptors was shown to represent a signal of cellular death in the surroundings of the cell.
The finding of ATP synthase and ETC on C6 cell surface means that ectopic ATP generation is carried out directly into the extracellular fluid.
Inhibition of ATP synthesis on C6 cell plasma membrane by antimycin A, an inhibitor of respiratory complex III (Fig. 2), suggests that ATP synthase is coupled with the respiratory chain. By WB analysis, we have identified the presence of the first redox complex and cytochrome c in C6 membrane fraction and of COX IV by immunofluorescence. Spectrophometric assays showed that the ETC complexes were functional. Moreover, these activities were sensitive to the specific inhibitors (Fig. 3) which indicates specificity. In fact, ATP production by ATP synthase is always linked to the presence of a proton gradient, produced by the respiration chain complexes (Mitchell 1961). Aerobic ATP production is always coupled to a proton gradient built by the mitochondrial I to IV complexes, composed of NADH dehydrogenase (I), succinate dehydrogenase (II), ubiquinol-cytochrome c oxidoreductase (III), cytochrome c, cytochrome c oxidase (IV), which transfers electrons from NADH or FADH2 to O2, forming a proton gradient across the mitochondrial inner membrane with the negative charge on the F1 subunit side of the membrane. The ATP synthase couples the proton flow to phosphorylation of ADP. Interestingly, the plasma membrane-associated ATP synthase and ETC proteins were found to be detergent-resistant lipid raft proteins (Bae 2004).
Lipid rafts are plasma membrane microdomains composed of cholesterol and glycosphingolipids, that segregate proteins among which many receptors with poorly understood consequences for membrane organization (Carver and Schnitzer 2003). Lipid raft proteome analyses from various mammalian cells and tissues have revealed that these contain many unexpected proteins including parts of ETC and endoplasmic reticulum, indicating that plasma membrane rafts might communicate with these intracellular organelles (Bae et al. 2004; Foster et al. 2003; Bini et al. 2003; Kim et al. 2004, 2006). This localization was inferred by the observation of a patchy and punctate staining pattern (Bae et al. 2004), very similar to hat we find for COX IV on surface of non-permeabilized C6 glioma cells (Figure 5b, d). There seems to be the intriguing possibility that the putative mitochondrial proteins expressed on C6 glioma cell membranes are localized in lipid rafts. Within a raft, a proton gradient for ATP synthase may be locally produced. Figure 5 shows that the putative ectopic C6 glioma cell COX IV is expressed on the surface of the cells and colocalizes with WGA, a typical plasma membrane protein. The COX IV pattern was patchy and punctuate (Fig. 5), as if COX IV was localized on discrete sites. Interestingly, by immunocytochemical detection, the OXPHOS chain was found also onto osteosarcoma cell line, observing a punctuate distribution of three of the four respiratory chain complexes on the surface of these cells (Yonally and Capaldi 2006). All the components of the F1 ATP synthase catalytic core were visualized also on the endothelial cell surface, as discrete punctuate structures (Moser et al. 2001).
It is tempting to speculate that plasma membrane lipid rafts have an ability to synthesize extracellular ATP by oxidative phosphorylation through the expression of mitochondrial proteins. C6 glioma cell plasma membranes consume oxygen using NADH and succinate as respiring substrates. Respiration was sensitive to the common inhibitors, like rotenone, antimycin A, and cyanide (Fig. 4). The ability for C6 cells to use NADH as respiring substrate is in favour of an extramitochondrial respiration.
In our experimental conditions, mitochondrial contamination seems to play a negligible role. In fact, the staining for TIM and ANT, two typical inner mitochondrial membrane proteins, on C6 glioma cell membrane fractions and activity of AK3, a typical mitochondrial matrix enzyme, were absent (Fig. 1). Moreover, the membrane fraction was prepared in the absence of cyclosporine A and 2-Aminoethoxydiphenyl borate, inhibitors of the opening of the mitochondrial permeability transition pore (MTP; Beutner et al. 1996). So, mitochondria, if any would not be functional, as they need a coupled membrane (Boyer 1997).
Mitochondrial proteins may be exported, likely by fusion with other cellular membranes. Further studies are necessary to understand how this happens. Examples of mitochondrial matrix proteins found at “extramitochondrial” locations exist (Soltys and Gupta 1999; Soltys and Gupta 2000). Particularly interesting seem the possibility of a fusion among mitochondria and other membranes (Soltys and Gupta 1999) including the nucleus, endoplasmic reticulum and chloroplasts, and with the plasma membrane (Crotty and Ledbetter 1973). We may hypothesize that the ETC present in C6 membrane fraction is the same as expressed in mitochondria. In fact, the Abs against the redox chains I to IV recognised the proteins in both C6 membrane fraction and mitochondrial membranes, indicating a sequence homology. A pivotal role of mitochondria in the pathobiology of cancer and other diseases where the ATP depletion plays as a central role has been suggested.
ATP is an important signaling molecule in the nervous system in physiological and pathological conditions (Morrone et al. 2005). In particular, in pathological conditions, large amounts of intracellular ATP can be released from damaged cells, and this could induce cell death in different cell types, including microglial and myeloid cells (Morrone et al. 2005). On the other hand, in glioma cell lines nucleotides and nucleosides induce proliferation (Morrone et al. 2003). In fact, glioma cells presented a clear resistance to death induced by cytotoxic concentrations of ATP respect to the normal brain tissue (Morrone et al. 2005), suggesting that ATP may be involved in the mechanism associated with malignance of these tumors (Morrone et al. 2003). Interestingly, the authors hypothesized that the inhibition of ATP release by glioma or the blockade of ATP receptors could be an alternative strategy in the management of patients with malignant glioma (Morrone et al. 2003). These data are coherent with our results that show a cytostatic effect on glioma growth when resveratrol, the ATP synthase inhibitor, was added to medium culture. In fact it is known that resveratrol inhibits cellular events associated with tumor initiation, promotion, and progression (Jang et al. 1997).
Our report is not the first example of blocking of tumor growth through the ATP synthase inhibition. In fact, several authors have demonstrated that the angiostatin which binds and inhibits Fo F1-ATP synthase on the endothelial cell surface (Chi and Pizzo 2006a), blocks tumor angiogenesis in vivo (Jung et al. 2007; Moser et al. 2001), inhibiting the endothelial cell migration and proliferation (Moser et al. 2001).
Such results may contribute to the understanding of the generation of ectopic ATP by ATP-synthase and ETC that plays a role in the abnormal growth (Grobben et al. 2002), and on the correlation between ATP signalling and tumour growth, providing a potential direction to follow for the design of new targets for future therapies
Acknowledgment
The work was supported by grants from Compagnia di San Paolo, for the Neuroscience Program, for the research project entitled: “Energetic metabolism in myelinated axon: a new trophic role of myelin sheath”.
Abbreviations
- ANT
Adenylate nucleotide translocase
- COX IV
Subunit IV of cytochrome c oxidase
- ETC
Electron transfer chain
- TIM
Transport inner membrane protein
- WGA
Wheat germ agglutinin
References
- Arakaki N, Nagao T, Niki R, Toyofuku A, Tanaka H, Kuramoto Y, Emoto Y, Shibata H, Magota K, Higuti T (2003) Possible role of cell surface H+-ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res 1:931–939 [PubMed] [Google Scholar]
- Avgeropoulos NG, Batchelor TT (1999) New treatment strategies for malignant gliomas. Oncologist 4:209–224 [PubMed] [Google Scholar]
- Bae TJ, Kim MS, Kim JW, Kim BW, Choo HJ, Lee JW, Kim KB, Lee CS, Kim JH, Chang SY, Kang CY, Lee SW, Ko YG (2004) Lipid raft proteome reveals ATP synthase complex in the cell surface. Proteomics 4:3536–3548 [DOI] [PubMed] [Google Scholar]
- Benda P, Lightbody J, Sato G, Levine L, Sweet W (1968) Differentiated rat glial cell strain in tissue culture. Science 161:370–371 [DOI] [PubMed] [Google Scholar]
- Benda P, Someda K, Messer J, Sweet WH (1971) Morphological and immunochemical studies of rat glial tumors and clonal strains propagated in culture. J Neurosurg 34:310–323 [DOI] [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Welte W, Brdiczka D (1996) Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett 396:189–195 [DOI] [PubMed] [Google Scholar]
- Bini L, Pacini S, Liberatori S, Valensin S, Pellegrini M, Raggiaschi R, Pallini V, Baldari CT (2003) Extensive temporally regulated reorganization of the lipid raft proteome following T-cell antigen receptor triggering. Biochem J 369:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer PD (1997) The ATP synthase—a splendid molecular machine. Annu Rev Biochem 66:717–749 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25:1327–1333 [DOI] [PubMed] [Google Scholar]
- Carver LA, Schnitzer JE (2003) Caveolae: mining little caves for new cancer targets. Nat Rev Cancer 3:571–581 [DOI] [PubMed] [Google Scholar]
- Chi SL, Pizzo SV (2006a) Angiostatin is directly cytotoxic to tumor cells at low extracellular pH: a mechanism dependent on cell surface-associated ATP synthase. Cancer Res 66:875–882 [DOI] [PubMed] [Google Scholar]
- Chi SL, Pizzo SV (2006b) Cell surface F1Fo ATP synthase: a new paradigm? Ann Med 38:429–438 [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Starkov AA, Fiskum G (2003) Cyclosporin A-insensitive permeability transition in brain mitochondria: inhibition by 2-aminoethoxydiphenyl borate. J Biol Chem 278:27382–27389 [DOI] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A (1988) Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255:357–360 [PMC free article] [PubMed] [Google Scholar]
- Crotty WJ, Ledbetter MC (1973) Membrane continuities involving chloroplasts and other organelles in plant cells. Science 182:839–841 [DOI] [PubMed] [Google Scholar]
- Foster LJ, De Hoog CL, Mann M (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA 100:5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobben B, De Deyn PP, Slegers H (2002) Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res 310:257–270 [DOI] [PubMed] [Google Scholar]
- Hong S, Pedersen PL (2008) ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol Mol Biol Rev 72:590–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220 [DOI] [PubMed] [Google Scholar]
- Jung KH, Song SH, Paik JY, Koh BH, Choe YS, Lee EJ, Kim BT, Lee KH (2007) Direct targeting of tumor cell F(1)F(0) ATP-synthase by radioiodine angiostatin in vitro and in vivo. Cancer Biother Radiopharm 22:704–712 [DOI] [PubMed] [Google Scholar]
- Kaye AH, Hill JS (1993) Photodynamic therapy of brain tumours. Ann Acad Med Singap 22:470–481 [PubMed] [Google Scholar]
- Kaye AH, Morstyn G, Gardner I, Pyke K (1986) Development of a xenograft glioma model in mouse brain. Cancer Res 46:1367–1373 [PubMed] [Google Scholar]
- Kim BW, Choo HJ, Lee JW, Kim JH, Ko YG (2004) Extracellular ATP is generated by ATP synthase complex in adipocyte lipid rafts. Exp Mol Med 36:476–485 [DOI] [PubMed] [Google Scholar]
- Kim KB, Lee JW, Lee CS, Kim BW, Choo HJ, Jung SY, Chi SG, Yoon YS, Yoon G, Ko YG (2006) Oxidation-reduction respiratory chains and ATP synthase complex are localized in detergent-resistant lipid rafts. Proteomics 6:2444–2453 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Mangiullo R, Gnoni A, Leone A, Gnoni GV, Papa S, Zanotti F (2008) Structural and functional characterization of F(o)F(1)-ATP synthase on the extracellular surface of rat hepatocytes. Biochim Biophys Acta 1777:1326–1335 [DOI] [PubMed] [Google Scholar]
- McMorris FA, Smith TM, Sprinkle TJ, Auszmann JM (1985) Induction of myelin components: cyclic AMP increases the synthesis rate of 2′,3′-cyclic nucleotide 3′-phosphohydrolase in C6 glioma cells. J Neurochem 44:1242–1251 [DOI] [PubMed] [Google Scholar]
- Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148 [DOI] [PubMed] [Google Scholar]
- Morrone FB, Jacques-Silva MC, Horn AP, Bernardi A, Schwartsmann G, Rodnight R, Lenz G (2003) Extracellular nucleotides and nucleosides induce proliferation and increase nucleoside transport in human glioma cell lines. J Neurooncol 64:211–218 [DOI] [PubMed] [Google Scholar]
- Morrone FB, Horn AP, Stella J, Spiller F, Sarkis JJ, Salbego CG, Lenz G, Battastini AM (2005) Increased resistance of glioma cell lines to extracellular ATP cytotoxicity. J Neurooncol 71:135–140 [DOI] [PubMed] [Google Scholar]
- Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV (2001) Endothelial cell surface F1–F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA 98:6656–6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Baker L, Jorgensen SL, Norenberg MD (1994) Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol 87:8–13 [DOI] [PubMed] [Google Scholar]
- Nobile M, Monaldi I, Alloisio S, Cugnoli C, Ferroni S (2003) ATP-induced, sustained calcium signalling in cultured rat cortical astrocytes: evidence for a non-capacitative, P2X7-like-mediated calcium entry. FEBS Lett 538:71–76 [DOI] [PubMed] [Google Scholar]
- Panfoli I, Musante L, Bachi A, Ravera S, Calzia D, Cattaneo A, Bruschi M, Bianchini P, Diaspro A, Morelli A, Pepe IM, Tacchetti C, Candiano G (2008) Proteomic analysis of the retinal rod outer segment disks. J Proteome Res 7:2654–2669 [DOI] [PubMed] [Google Scholar]
- Panfoli I, Calzia D, Bianchini P, Ravera S, Diaspro A, Candiano G, Bachi A, Monticone M, Aluigi MG, Barabino S, Calabria G, Rolando M, Tacchetti C, Morelli A, Pepe IM (2009) Evidence for aerobic metabolism in retinal rod outer segment disks. Int J Biochem Cell Biol 41:2555–2565 [DOI] [PubMed] [Google Scholar]
- Parker KK, Norenberg MD, Vernadakis A (1980) “Transdifferentiation” of C6 glial cells in culture. Science 208:179–181 [DOI] [PubMed] [Google Scholar]
- Ravera S, Panfoli I, Calzia D, Aluigi MG, Bianchini P, Diaspro A, Mancardi G, Morelli A (2009) Evidence for aerobic ATP synthesis in isolated myelin vesicles. Int J Biochem Cell Biol 41:1581–1591 [DOI] [PubMed] [Google Scholar]
- Ravera S, Panfoli I, Aluigi MG, Calzia D, Morelli A (2010) Characterization of myelin sheath FoF1-ATP synthase and its regulation by IF1. Cell Biochem Biophys. doi:10.1007/s12013-010-9112-1 [DOI] [PubMed]
- Sgobbo P, Pacelli C, Grattagliano I, Villani G, Cocco T (2007) Carvedilol inhibits mitochondrial complex I and induces resistance to H2O2-mediated oxidative insult in H9C2 myocardial cells. Biochim Biophys Acta 1767:222–232 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS (1999) Mitochondrial-matrix proteins at unexpected locations: are they exported? Trends Biochem Sci 24:174–177 [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Gupta RS (2000) Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol 194:133–196 [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA (2008) Signaling at purinergic P2X receptors. Annu Rev Physiol 71:333–359 [DOI] [PubMed] [Google Scholar]
- Volpe JJ, Fujimoto K, Marasa JC, Agrawal HC (1975) Relation of C-6 glial cells in culture to myelin. Biochem J 152:701–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburn KW, Hill JS, Stylli S, Kaye AH, Reiss JA, Phillips DR (1994) Evaluation of a morpholinothiolporphyrin for use in photodynamic therapy. Br J Cancer 70:398–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonally SK, Capaldi RA (2006) The F(1)F(0) ATP synthase and mitochondrial respiratory chain complexes are present on the plasma membrane of an osteosarcoma cell line: an immunocytochemical study. Mitochondrion 6:305–314 [DOI] [PubMed] [Google Scholar]