Abstract
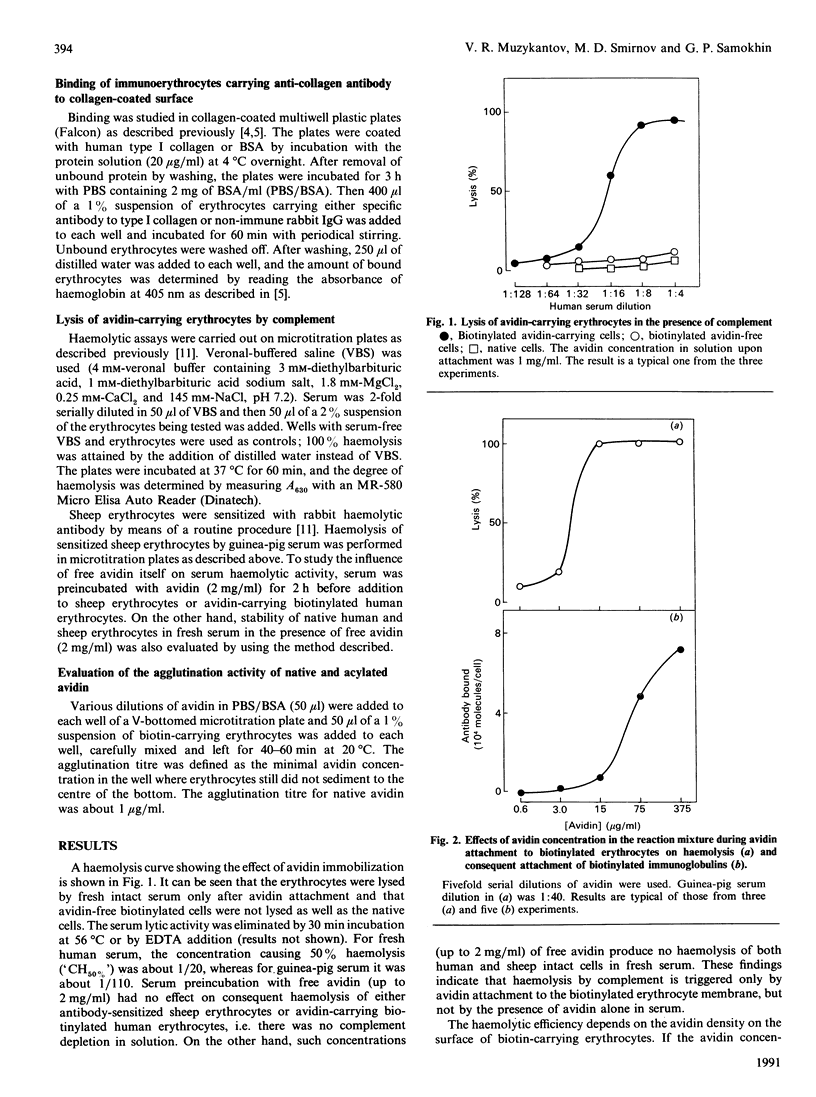
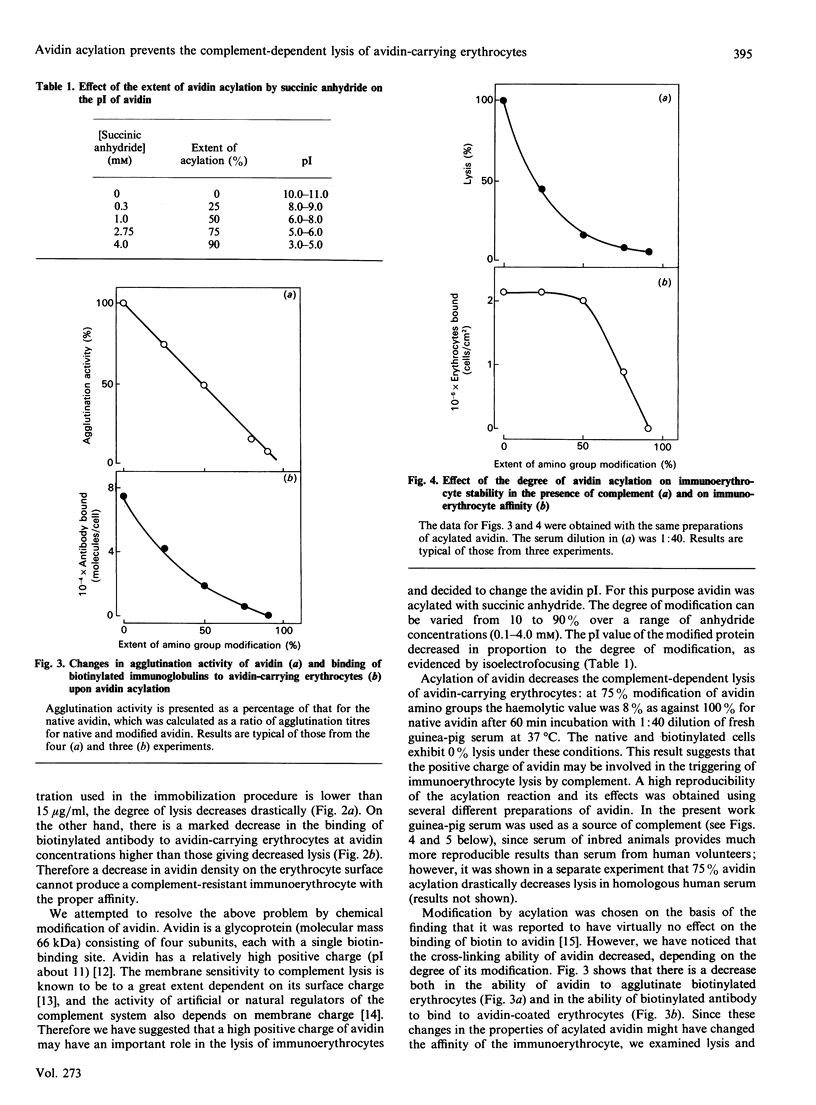
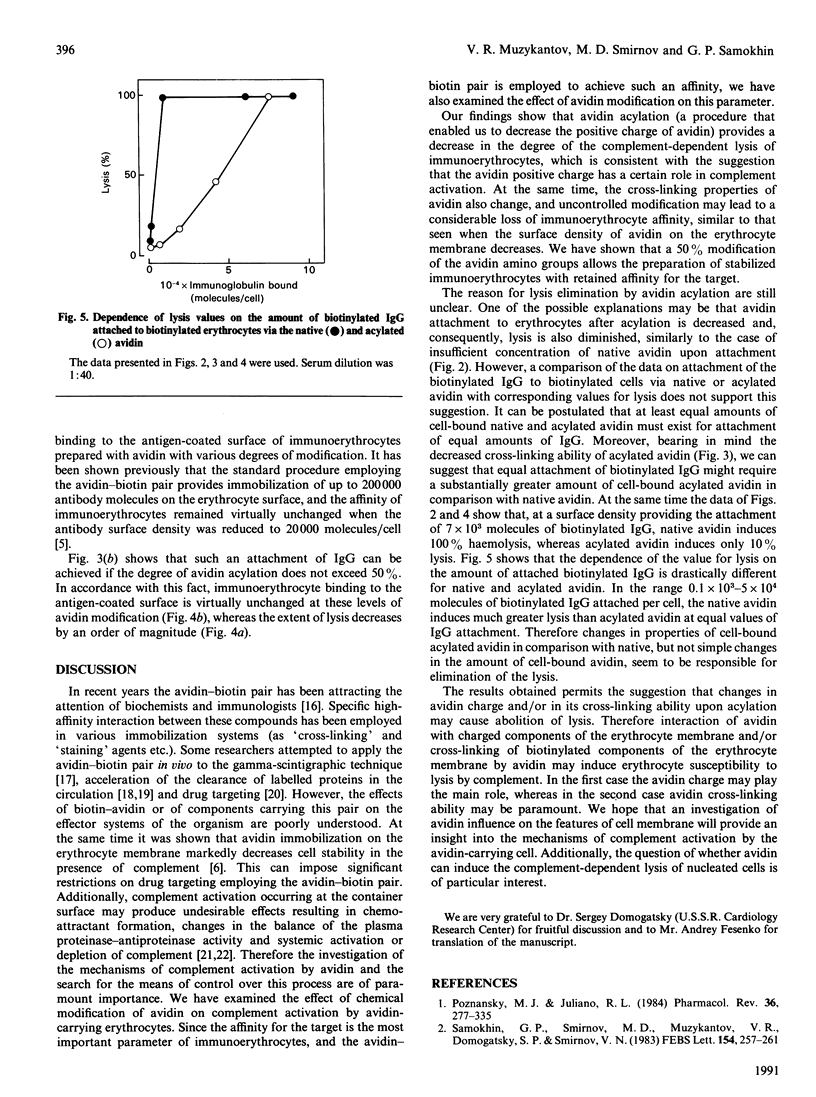
Non-covalent binding of avidin to biotinylated erythrocytes results in complement-dependent haemolysis. Biotinylated erythrocytes, as well as native cells, are not lysed by complement. Complement activation requires a tight contact between avidin and the erythrocyte membrane, since avidin does not in itself activate complement and does not inhibit lysis of sensitized sheep erythrocytes. The efficiency of haemolysis depends on avidin's surface density. When the avidin concentration in the reaction mixture is less than 15 micrograms/ml, erythrocyte lysis is not induced. However, the attachment of biotinylated antibodies to avidin-carrying erythrocytes decreases dramatically. Acylation of avidin with succinic anhydride strongly decreases its ability to induce complement-dependent haemolysis. However, the ability of avidin to cross-link the biotin-containing structures decreases after acylation. A 50% modification of avidin by succinic anhydride (pI about 7.0) allows preparation of 'immunoerythrocytes', which retain their affinity to antigen and stability in the presence of complement.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asghar S. S. Pharmacological manipulation of complement system. Pharmacol Rev. 1984 Dec;36(4):223–244. [PubMed] [Google Scholar]
- Colman R. W. Surface-mediated defense reactions. The plasma contact activation system. J Clin Invest. 1984 May;73(5):1249–1253. doi: 10.1172/JCI111326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten H. R. Molecular basis of complement deficiency syndromes. Lab Invest. 1985 May;52(5):468–474. [PubMed] [Google Scholar]
- Cunningham C. M., Kingzette M., Richards R. L., Alving C. R., Lint T. F., Gewurz H. Activation of human complement by liposomes: a model for membrane activation of the alternative pathway. J Immunol. 1979 Apr;122(4):1237–1242. [PubMed] [Google Scholar]
- Fields R. The measurement of amino groups in proteins and peptides. Biochem J. 1971 Sep;124(3):581–590. doi: 10.1042/bj1240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn F. M., Titus G., Montibeller J. A., Hofmann K. Hormone-receptor studies with avidin and biotinylinsulin-avidin complexes. J Biol Chem. 1980 Jun 25;255(12):5742–5746. [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of streptavidin. Tryptophan residues involved in the active site. Biochem J. 1988 Nov 15;256(1):279–282. doi: 10.1042/bj2560279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J. S., Salter D. N., Shepherd B. P. A study of some factors that influence the iodination of ox insulin. Biochem J. 1967 Apr;103(1):120–128. doi: 10.1042/bj1030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnatowich D. J., Virzi F., Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987 Aug;28(8):1294–1302. [PubMed] [Google Scholar]
- Klibanov A. L., Martynov A. V., Slinkin M. A., Sakharov IYu, Smirnov M. D., Muzykantov V. R., Danilov S. M., Torchilin V. P. Blood clearance of radiolabeled antibody: enhancement by lactosamination and treatment with biotin-avidin or anti-mouse IgG antibodies. J Nucl Med. 1988 Dec;29(12):1951–1956. [PubMed] [Google Scholar]
- Muzykantov V. R., Sakharov D. V., Smirnov M. D., Domogatsky S. P., Samokhin G. P. Targeting of enzyme immobilized on erythrocyte membrane to collagen-coated surface. FEBS Lett. 1985 Mar 11;182(1):62–66. doi: 10.1016/0014-5793(85)81154-6. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R., Sakharov D. V., Smirnov M. D., Samokhin G. P., Smirnov V. N. Immunotargeting of erythrocyte-bound streptokinase provides local lysis of a fibrin clot. Biochim Biophys Acta. 1986 Nov 19;884(2):355–362. doi: 10.1016/0304-4165(86)90184-4. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R., Samokhin G. P., Smirnov M. D., Domogatsky S. P. Hemolytic complement activity assay in microtitration plates. J Appl Biochem. 1985 Jun;7(3):223–227. [PubMed] [Google Scholar]
- Poznansky M. J., Juliano R. L. Biological approaches to the controlled delivery of drugs: a critical review. Pharmacol Rev. 1984 Dec;36(4):277–336. [PubMed] [Google Scholar]
- Samokhin G. P., Smirnov M. D., Muzykantov V. R., Domogatsky S. P., Smirnov V. N. Red blood cell targeting to collagen-coated surfaces. FEBS Lett. 1983 Apr 18;154(2):257–261. doi: 10.1016/0014-5793(83)80160-4. [DOI] [PubMed] [Google Scholar]
- Sinitsyn V. V., Mamontova A. G., Checkneva Y. Y., Shnyra A. A., Domogatsky S. P. Rapid blood clearance of biotinylated IgG after infusion of avidin. J Nucl Med. 1989 Jan;30(1):66–69. [PubMed] [Google Scholar]
- Smirnov M. D., Samokhin G. P., Muzykantov V. R., Idelson G. L., Domogatsky S. P., Smirnov V. N. Type I and III collagens as a possible target for drug delivery to the injured sites of vascular bed. Biochem Biophys Res Commun. 1983 Oct 14;116(1):99–105. doi: 10.1016/0006-291x(83)90386-8. [DOI] [PubMed] [Google Scholar]
- Smirnov V. N., Domogatsky S. P., Dolgov V. V., Hvatov V. B., Klibanov A. L., Koteliansky V. E., Muzykantov V. R., Repin V. S., Samokhin G. P., Shekhonin B. V. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6603–6607. doi: 10.1073/pnas.83.17.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]


