Abstract
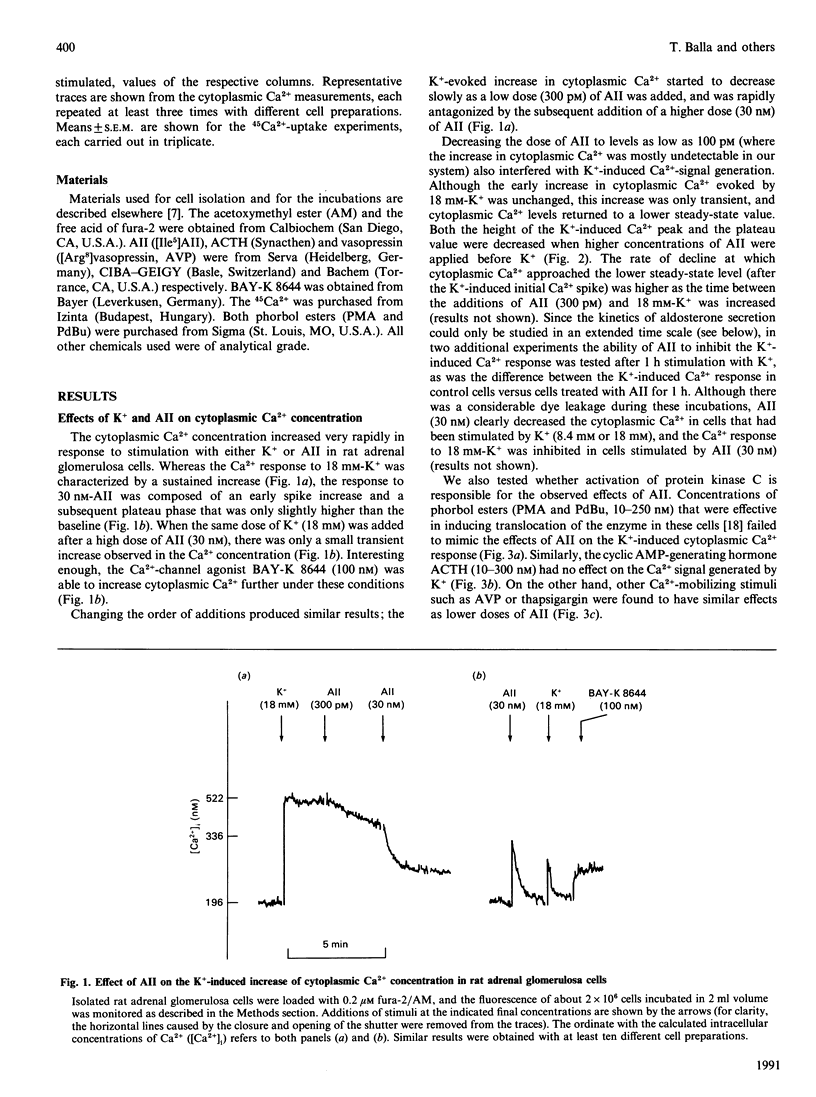
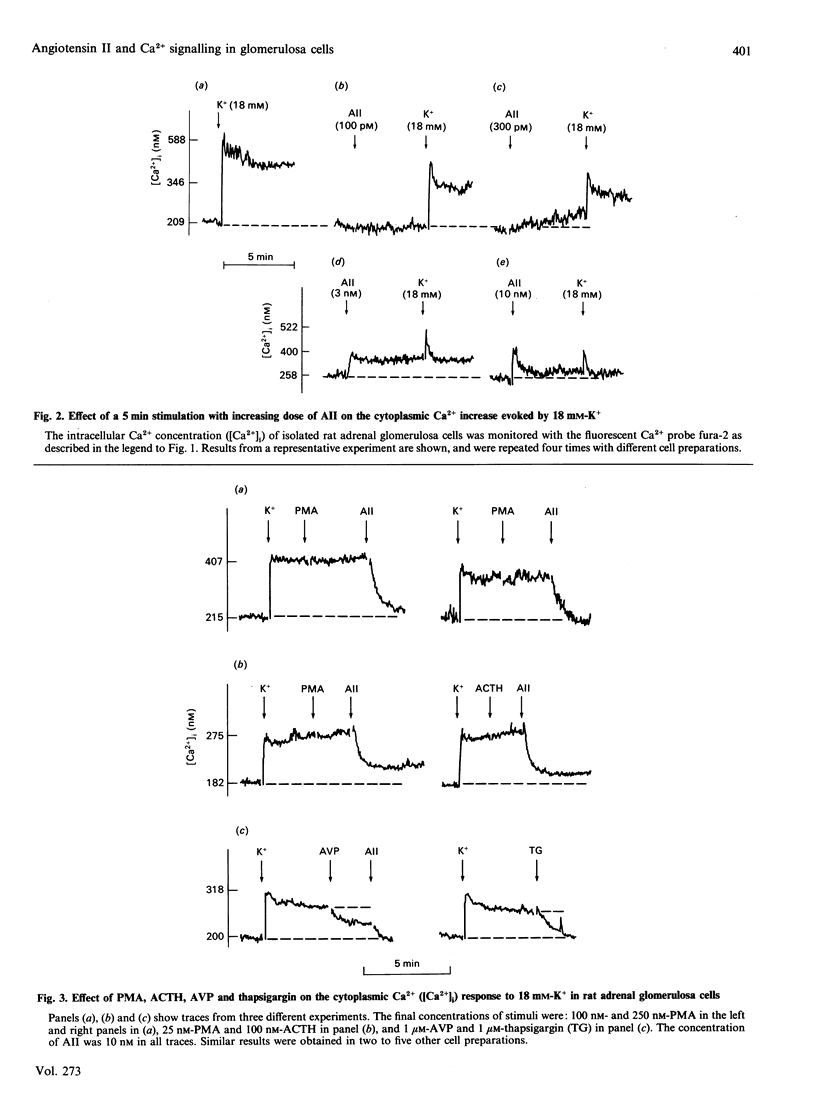
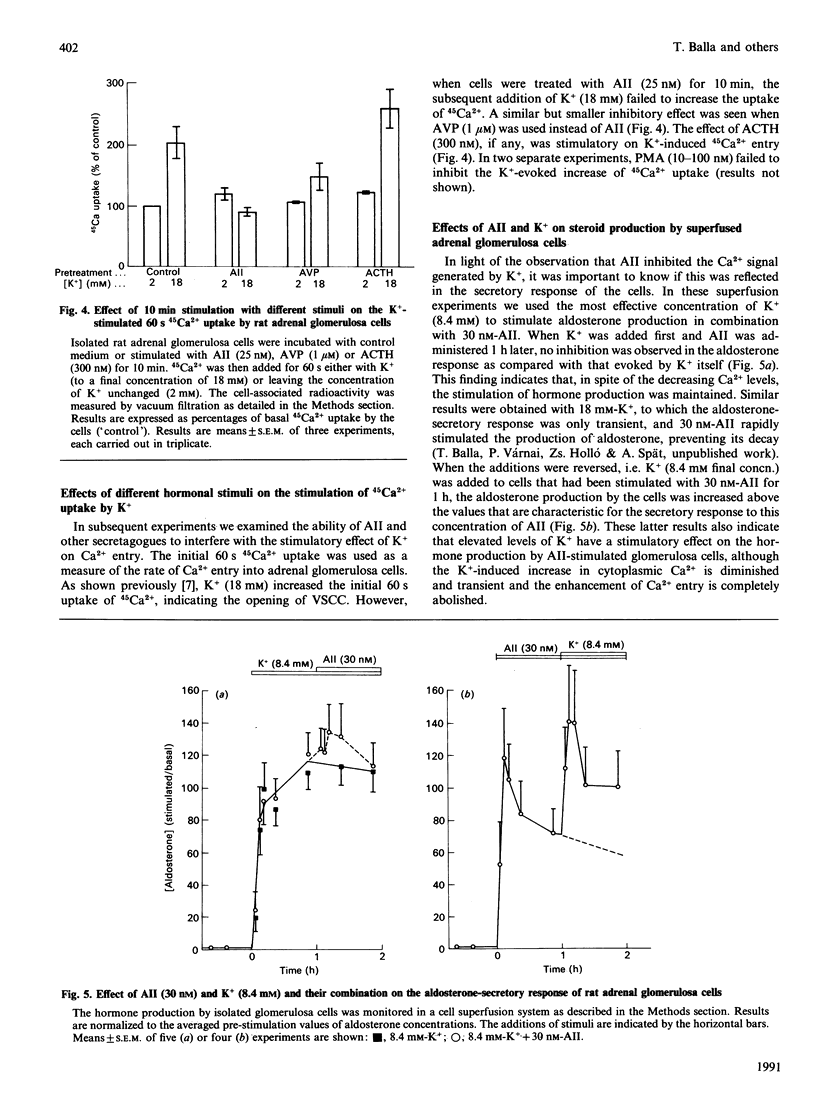
The Ca2(+)-mobilizing hormone angiotensin II (AII) dose-dependently inhibited the K(+)-induced sustained increase of cytoplasmic Ca2+ concentration in adrenal glomerulosa cells and caused a rapid decrease of cytoplasmic Ca2+ when added to cells already stimulated with K+. These effects of AII on the K(+)-induced Ca2+ signal were mimicked, although less effectively, by other Ca2(+)-mobilizing agonists such as [Arg8]vasopressin (AVP) and thapsigargin. Phorbol esters did not show such effects, nor did corticotropin (ACTH), a secretagogue acting via cyclic AMP. The K(+)-stimulated initial 45Ca2+ uptake, a measure of Ca2+ entry into glomerulosa cells, was also prevented by AII pretreatment, and was inhibited by AVP, but not by ACTH. The stimulatory effect of K+ on aldosterone production, however, was not inhibited by AII, and the AII-induced aldosterone production was further increased by increasing K+. These data indicate that AII is able to inhibit static increases in cytoplasmic Ca2+ by inhibiting Ca2+ entry through voltage-sensitive Ca2+ channels and, possibly, by activating Ca2+ extrusion from the cells. It is also concluded that the Ca2+ signal evoked by AII is very efficient in stimulating hormone secretion, and the secretory response of the cells becomes more sensitive to any further increase of Ca2+ entry through voltage-sensitive Ca2+ channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Catt K. J. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology. 1986 Jan;118(1):112–118. doi: 10.1210/endo-118-1-112. [DOI] [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Guillemette G., Morgan R. O., Catt K. J. Angiotensin-stimulated production of inositol trisphosphate isomers and rapid metabolism through inositol 4-monophosphate in adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9323–9327. doi: 10.1073/pnas.83.24.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T., Szebeny M., Kanyar B., Spät A. Angiotensin II and FCCP mobilizes calcium from different intracellular pools in adrenal glomerulosa cells; analysis of calcium fluxes. Cell Calcium. 1985 Aug;6(4):327–342. doi: 10.1016/0143-4160(85)90003-x. [DOI] [PubMed] [Google Scholar]
- Balla T., Várnai P., Holló Z., Spät A. Effects of high potassium concentration and dihydropyridine Ca2(+)-channel agonists on cytoplasmic Ca2+ and aldosterone production in rat adrenal glomerulosa cells. Endocrinology. 1990 Aug;127(2):815–822. doi: 10.1210/endo-127-2-815. [DOI] [PubMed] [Google Scholar]
- Barrett P. Q., Bollag W. B., Isales C. M., McCarthy R. T., Rasmussen H. Role of calcium in angiotensin II-mediated aldosterone secretion. Endocr Rev. 1989 Nov;10(4):496–518. doi: 10.1210/edrv-10-4-496. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Jornot L., Vallotton M. B. Correlation between cytosolic free Ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin II and potassium. J Biol Chem. 1984 Jul 25;259(14):8863–8869. [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Vallotton M. B. Quantitative analysis of the cytosolic-free-Ca2+-dependency of aldosterone production in bovine adrenal glomerulosa cells. Different requirements for angiotensin II and K+. Biochem J. 1987 Oct 15;247(2):335–340. doi: 10.1042/bj2470335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T., Barrett P. Q., Rasmussen H. Ca channels in adrenal glomerulosa cells: K+ and angiotensin II increase T-type Ca channel current. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2412–2416. doi: 10.1073/pnas.85.7.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Pozzan T., Wollheim C. B., Vicentini L. M., Meldolesi J. Tumor promoter phorbol myristate acetate inhibits Ca2+ influx through voltage-gated Ca2+ channels in two secretory cell lines, PC12 and RINm5F. J Biol Chem. 1986 Jan 5;261(1):32–35. [PubMed] [Google Scholar]
- Drummond A. H. Bidirectional control of cytosolic free calcium by thyrotropin-releasing hormone in pituitary cells. 1985 Jun 27-Jul 3Nature. 315(6022):752–755. doi: 10.1038/315752a0. [DOI] [PubMed] [Google Scholar]
- Duddy S. K., Kass G. E., Orrenius S. Ca2(+)-mobilizing hormones stimulate Ca2+ efflux from hepatocytes. J Biol Chem. 1989 Dec 15;264(35):20863–20866. [PubMed] [Google Scholar]
- Elliott M. E., Goodfriend T. L. Angiotensin alters 45Ca2+ fluxes in bovine adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3044–3048. doi: 10.1073/pnas.78.5.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Büki B., Muscsi I., Spät A. Polyphosphoinositide metabolism in adrenal glomerulosa cells. Mol Cell Endocrinol. 1985 Jun;41(1):105–112. doi: 10.1016/0303-7207(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Szabó B., Spät A. Reduced responsiveness of glomerulosa cells after prolonged stimulation with angiotensin II. Am J Physiol. 1985 Feb;248(2 Pt 1):E209–E214. doi: 10.1152/ajpendo.1985.248.2.E209. [DOI] [PubMed] [Google Scholar]
- Faragó A., Seprõdi J., Spät A. Subcellular distribution of protein kinase C in rat adrenal glomerulosa cells. Biochem Biophys Res Commun. 1988 Oct 31;156(2):628–633. doi: 10.1016/s0006-291x(88)80889-1. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Aguilera G., Catt K. J. Selective enhancement of angiotensin II- and potassium-stimulated aldosterone secretion by the calcium channel agonist BAY K 8644. Endocrinology. 1986 Feb;118(2):869–874. doi: 10.1210/endo-118-2-869. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Hinsch K. D., Wulfern M., Trautwein W., Schultz G. Angiotensin II-induced stimulation of voltage-dependent Ca2+ currents in an adrenal cortical cell line. EMBO J. 1988 Mar;7(3):619–624. doi: 10.1002/j.1460-2075.1988.tb02855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985 Aug 5;260(16):9177–9184. [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Pertussis toxin blocks angiotensin II-induced calcium influx but not inositol trisphosphate production in adrenal glomerulosa cell. FEBS Lett. 1986 Aug 18;204(2):347–351. doi: 10.1016/0014-5793(86)80841-9. [DOI] [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Time-dependent restoration of the trigger pool of calcium after termination of angiotensin II action in adrenal glomerulosa cells. J Biol Chem. 1987 Apr 5;262(10):4557–4563. [PubMed] [Google Scholar]
- Kojima K., Kojima I., Rasmussen H. Dihydropyridine calcium agonist and antagonist effects on aldosterone secretion. Am J Physiol. 1984 Nov;247(5 Pt 1):E645–E650. doi: 10.1152/ajpendo.1984.247.5.E645. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Quinn S. J., Cornwall M. C., Williams G. H. Electrophysiological responses to angiotensin II of isolated rat adrenal glomerulosa cells. Endocrinology. 1987 Apr;120(4):1581–1589. doi: 10.1210/endo-120-4-1581. [DOI] [PubMed] [Google Scholar]
- Rane S. G., Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986 Jan;83(1):184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spät A., Balla I., Balla T., Cragoe E. J., Jr, Hajnóczky G., Hunyady L. Angiotensin II and potassium activate different calcium entry mechanisms in rat adrenal glomerulosa cells. J Endocrinol. 1989 Jul;122(1):361–370. doi: 10.1677/joe.0.1220361. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Rojas E., Stutzin A., Izumi S., Catt K. J. Desensitization of pituitary gonadotropin secretion by agonist-induced inactivation of voltage-sensitive calcium channels. J Biol Chem. 1989 Jul 5;264(19):10939–10942. [PubMed] [Google Scholar]
- Williams B. C., McDougall J. G., Tait J. F., Tait S. A. Calcium efflux and steroid output from superfused rat adrenal cells: effects of potassium, adrenocorticotropic hormone, 5-hydroxytryptamine, adenosine 3':5'-cyclic monophosphate and angiotensins II and III. Clin Sci (Lond) 1981 Nov;61(5):541–551. doi: 10.1042/cs0610541. [DOI] [PubMed] [Google Scholar]
- Williams C. L., Lennon V. A. Activation of M3 muscarinic acetylcholine receptors inhibits voltage-dependent calcium influx in small cell lung carcinoma. J Biol Chem. 1990 Jan 25;265(3):1443–1447. [PubMed] [Google Scholar]


