Abstract
Tumor necrosis factor alpha (TNF-α)-mediated death signaling causes the recruitment of monomeric pro- apoptotic Bax into a 500-kDa protein complex. The adenovirus Bcl-2 homologue, E1B 19K, inhibits TNF-α-mediated apoptosis, interacts with Bax, and blocked the formation of the 500-kDa Bax complex. TNF-α and truncated Bid induced Bax-Bax cross-linking, indicative of oligomerization, and E1B 19K expression during infection inhibited this TNF-α-mediated Bax oligomerization. TNF-α signaled conformation changes at the Bax amino and carboxy termini. Exposure of the Bax amino terminus facilitates E1B 19K-Bax binding, which prevented exposure of the carboxy-terminal Bax Bcl-2 homology region 2 epitope. Inhibition of Bax oligomerization by E1B 19K is an activity that bears striking similarity to the means by which bacterial immunity proteins block pore formation by bacterial toxins which have structural homology to Bax.
Infection with mammalian DNA viruses, including adenovirus, results in a host immune response and production of multiple antiviral factors, including tumor necrosis factor alpha (TNF-α), which promote death of virus-infected cells (25). Viruses, in turn, have evolved multiple mechanisms that protect infected cells from the host's antiviral defenses to ensure efficient viral replication, propagation, and persistent infection (44). Adenoviruses inhibit apoptosis induced by TNF-α during productive viral infection through the function of the E1B 19K protein (10, 43) and multiple proteins (10.4K, 14.5K, and 14.7K) encoded by the E3 gene (44). While the antiapoptotic function of the E3 proteins is restricted to that triggered by death receptors, the E1B 19K protein functions in multiple and distinct apoptotic pathways, suggesting that it acts at a point central to the regulation of many pathways (41).
TNF-α is a ligand for the death receptors TNF receptors 1 and -2 (29). TNF-α–receptor interaction causes the recruitment of adaptor proteins which promote the activation of caspase-8 (29), which cleaves 23-kDa Bid, into the 15-kDa fragment tBid (21, 24). tBid binds the proapoptotic proteins Bax and Bak and causes them to undergo a conformation change; in the case of Bax, it occurs at the amino terminus (32, 40). This correlates with the release of cytochrome c from mitochondria, caspase-9 and -3 activation (22, 45), and cleavage of a constellation of cellular caspase substrates which leads to apoptosis.
In adenovirus-infected cells, E1B 19K blocks TNF-α-mediated death signaling at the level of mitochondria. E1B 19K does not block caspase-8 processing or activation, Bid cleavage into tBid, tBid-Bax association, or alteration of Bax conformation at the amino terminus (32). However, E1B 19K interacts preferentially with the TNF-α-induced and conformationally altered Bax and prevents cytochrome c release from mitochondria. This blocks caspase-9 activation, which interrupts the activation of caspase-3, preventing cleavage of cellular substrates and apoptosis in virus-infected cells (32).
These findings indicated a role for Bax in TNF-α-mediated apoptosis, in particular, in facilitating the release of cytochrome c from mitochondria to permit caspase-9 activation. However, how a conformation change in Bax may lead to release of cytochrome c is unknown. To address this question, we examined conformation changes in Bax in cells undergoing TNF-α-mediated apoptosis and found specific and independent alterations in the amino and carboxy termini. E1B 19K interacts with Bax conformationally altered at the amino terminus. We also characterized the Bax protein complex in TNF-α-treated cells and found that monomeric Bax oligomerized into a large 500-kDa complex. E1B 19K expression during adenovirus infection did not block the conformation change in the Bax amino terminus but did prevent exposure of the carboxy-terminal Bcl-2 homology region (BH2) Bax epitope and the oligomerization of Bax into a 500-kDa protein complex. Thus, exposure of the Bax amino terminus promotes an E1B 19K-Bax interaction that may prevent a conformational change at the Bax carboxy terminus necessary for Bax oligomerization and propagation of TNF-α death signaling through mitochondria.
MATERIALS AND METHODS
Antibodies.
The following antibodies were used: rabbit polyclonal E1B 19K antibody generated against baculovirus-expressed full-length His-tagged E1B 19K recombinant protein; rabbit polyclonal carboxy-terminal E1B 19K antibody raised against six carboxy-terminal amino acids of E1B 19K (from Phillip E. Branton, McGill University, Montreal, Quebec, Canada); rat anti-Bid polyclonal antibody that recognizes both Bid and tBid (from Junying Yuan, Harvard Medical School, Boston, Mass.); rabbit polyclonal Bax antibody Bax(11–30), directed against amino acids 11 to 30 of human Bax (Bax N-20; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.); rabbit polyclonal Bax antibody Bax(43–61), directed against amino acids 43 to 61 of human Bax (PharMingen, San Diego, Calif.); mouse monoclonal antibody Bax(55–178), directed against amino acids 55 to 178 of human Bax (BioVision, Inc., Palo Alto, Calif.); and rabbit polyclonal Bax antibody Bax(150–165), directed against amino acids 150 to 165 of human Bax (Bax Ab-1; Oncogene Research Products, Boston, Mass.).
Plasmids and transfection.
Plasmids with Myc-tagged rat Bax in pcDNA3 (pcDNA3-Myc-rBax) and Myc-tagged human tBid in pcDNA3.1/HisB (pcDNA3.1-htBid-Myc) were previously described (15, 32). pCDNA3.1/His B (Invitrogen, San Diego, Calif.) was used as a control vector. HeLa cells were electroporated with 5 μg of pcDNA3.1/His B vector or pcDNA3-Myc-rBax for Bax transient transfection and with 6 μg of pcDNA3.1/His B or pcDNA3.1-htBid-Myc for tBid transient transfection.
Adenovirus infection.
Adenoviruses Ad5dl309 and Ad5dl337 were obtained from T. Shenk (Princeton University, Princeton, N.J.). Ad5dl309 has a deletion in the E3 gene and was used as the wild-type virus (19). Ad5dl337 was derived from Ad5dl309 and has a deletion in the E1B 19K gene (33). HeLa cells were infected as previously described (42).
Gel filtration chromatography.
For gel filtration chromatography, 2.5 × 107 HeLa cells were mock, Ad5dl309, or Ad5dl337 infected for 24 h and were then untreated or treated with TNF-α (2,000 U/ml; Boehringer Mannheim, Indianapolis, Ind.) plus cycloheximide (CHX; 30 μg/ml; Sigma, St. Louis, Mo.) (TNF/CHX) for 4 h. Cell lysates prepared in 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS; Calbiochem, La Jolla, Calif.) lysis buffer (20 mM Tris [pH 7.4], 137 mM NaCl, 2 mM EDTA, 10% glycerol, 2% CHAPS) at a density of 107 cells/ml were centrifuged at 14,000 rpm for 20 min. and the supernatant was loaded onto the column. The Sephacryl S-300 and Sephacryl S-100 gel filtration media (Pharmacia, Piscataway, N.J.) were packed by gravity in 1.5-cm (inside diameter) by 50-cm (length) Econo columns (Bio-Rad, Hercules, Calif.) and run by gravity. The bed and void volumes of the columns were 62 and 20 ml, respectively. The high-molecular-mass (aldolase [158 kDa], catalase [232 kDa], ferritin [440 kDa], and thyroglobulin [669 kDa]) and low-molecular-mass (RNase [13.7 kDa], chymotrypsinogen A [25 kDa], ovalbumin [43 kDa], and albumin [67 kDa]) calibration kits (Pharmacia) were used to calibrate the columns. Columns were equilibriated and run in CHAPS buffer. Fractions (0.5 ml) were collected using a Gilson FC 203B fraction collector (Gilson, Inc., Middleton, Wis.); 30 μl of each fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as previously described (31) and probed with the Bax(11–30) antibody, E1B 19K polyclonal antibody, or anti-Bid antibody.
In vitro cross-linking.
Hela cells (107) were mock or Ad5dl309 infected for 24 h and were then untreated or treated with TNF/CHX for the indicated time periods. Cell lysates prepared in HEPES-CHAPS lysis buffer (10 mM HEPES [pH 7.4], 137 mM NaCl, 2 mM EDTA, 10% glycerol, 2% CHAPS) at a density of 107 cells/ml were incubated with 1 mM disuccinimidyl suberate (DSS; Pierce, Rockford, Ill.) or dimethyl sulfoxide alone for 2 h at 4°C, and quenching was carried out as previously described (39). Microprecipitates formed were spun down at 14,000 rpm for 20 min. A 14-μl aliquot out of the 1-ml cross-linked lysate was resolved by SDS-PAGE and then analyzed by Western blotting with the Bax(11–30) antibody, carboxy-terminal E1B 19K antibody, or an anti-Bid antibody.
Immunoprecipitation.
HeLa cells were prepared for immunoprecipitation, which was carried out as previously described (32) except that all cells were harvested and resuspended in CHAPS lysis buffer or in CHAPS lysis buffer plus Triton X-100 (1%), both with protease inhibitors, as previously described (32). The Sepharose was washed three times in a 0.5% CHAPS buffer or in a 0.5% CHAPS buffer containing 0.2% Triton X-100. Whole-cell extracts and immunoprecipitates were resolved by SDS-PAGE and then analyzed by Western blotting with the Bax(11–30) antibody and the E1B 19K polyclonal antibody.
RESULTS
TNF-α induces formation of a 500-kDa Bax complex.
TNF-α treatment of cells induces a conformational change at the amino terminus of Bax; however, how this conformation change in Bax contributes to cell death is unclear. Nonionic detergents mimic this alteration of Bax in vitro and induce homo- and heterodimerization (18). The zwitterionic detergent CHAPS has been shown to retain Bax in its native monomer conformation in extracts from normal healthy cells (1, 17). To characterize the TNF-α-induced conformation change in Bax, mock-infected HeLa cells were untreated or treated for 4 h with TNF/CHX to block the NFκB-activated survival pathway. Treatment of cells with CHX alone has no effect on caspase activation, Bax conformation, or cell viability (32). At 4 h of TNF/CHX treatment, cell viability is not yet appreciably affected, but caspase-8 is processed, Bid is cleaved, cytochrome c is released, and caspase-9 activation is initiated. At this time in TNF/CHX-treated adenovirus-infected cells, E1B 19K interacts with Bax and blocks cytochrome c release and caspase-9 activation (32). Cell lysates prepared in CHAPS lysis buffer were fractionated on a Sephacryl S-300 gel filtration column to characterize the molecular weight of the Bax protein complex in cells in the presence or absence of TNF-α death signaling.
Western blotting of the column fractions for Bax showed that in untreated mock-infected cells, Bax fractionated with a molecular mass of around 25 kDa, consistent with it being a monomer (Fig. 1). However, in the mock-infected cells treated with TNF/CHX, there was a dramatic shift in the fractionation profile of Bax from 25 kDa to a protein complex migrating at approximately 500 kDa (Fig. 1). Approximately one-third of the total Bax protein shifted from the monomer peak (fractions 35 to 40) to the high-molecular-weight fractions (fractions 22 to 29).
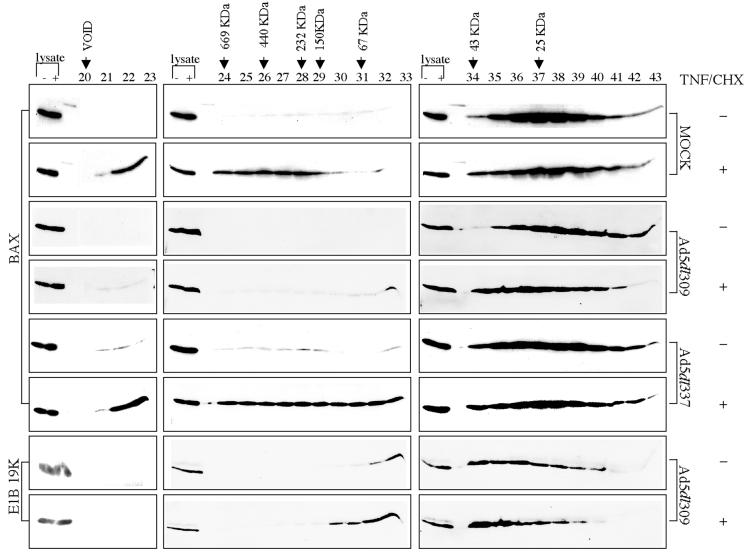
FIG. 1.
E1B 19K inhibits formation of the TNF-α-induced 500-kDa Bax protein complex during adenovirus infection. HeLa cells were mock, Ad5dl309, or Ad5dl337 infected for 24 h and then untreated or treated with TNF/CHX for 4 h. Cell lysates prepared in CHAPS lysis buffer were fractionated on a Sephacryl S-300 gel filtration column. Column fractions 20 to 43 were analyzed by SDS-PAGE followed by Western blotting for Bax or E1B 19K, as indicated. Whole-cell lysates from both the minus- and plus-TNF/CHX samples loaded on the column were included on every Western blot as an internal reference for Bax or E1B 19K levels. The peaks at which the molecular weight markers fractionated are indicated.
E1B 19K inhibits formation of the TNF-α-induced 500-kDa Bax complex.
To determine if the E1B 19K protein affected the formation of the large Bax complex by TNF/CHX during adenovirus infection, HeLa cells were infected with the wild-type virus Ad5dl309 or the E1B 19K deletion mutant virus Ad5dl337 and were then untreated or treated with TNF/CHX. Lysates prepared in CHAPS lysis buffer were fractionated on a Sephacryl S-300 gel filtration column. Western blotting of the column fractions with a Bax antibody showed that Bax fractionated as a monomer in untreated Ad5dl309-infected cells as it did in mock-infected cells (Fig. 1). In contrast to mock-infected cells treated with TNF/CHX, in Ad5dl309-infected cells treated with TNF/CHX, Bax now fractionated in the 25 to 50-kDa molecular mass range, and the 500-kDa Bax complex was strikingly absent (Fig. 1). To directly compare the difference in Bax levels in fractions 22 to 29 from mock- and Ad5dl309-infected TNF/CHX-treated cells, fractions from each were run side-by-side on the same gel and subjected to Western blotting for Bax, which revealed approximately eightfold more Bax in mock-infected cells than in Ad5dl309-infected cells (data not shown). The inhibition of formation of the 500-kDa Bax complex in Ad5dl309-infected cells is probably due to the interaction of E1B 19K with Bax, since 19K coimmunoprecipitates with Bax preferentially in TNF/CHX-treated cells (see below) (32).
To establish that the inhibition of the 500-kDa Bax complex in TNF/CHX-treated cells was solely a function of the E1B 19K protein, HeLa cells were infected with the E1B 19K deletion mutant virus Ad5dl337 and left untreated or treated with TNF/CHX. Western blotting of the Sephacryl S-300 gel filtration column fractions for Bax showed that most of the Bax fractionated as a monomer in untreated Ad5dl337-infected cells, although trace amounts of Bax fractionated in the higher-molecular-weight fractions (Fig. 1), which may be due to the induction of apoptosis by Ad5dl337 in the absence of E1B 19K (32). In Ad5dl337-infected cells treated with TNF/CHX, however, one-third of the Bax monomer population fractionated in the higher-molecular-weight fractions (22 to 29), similar to mock-infected cells treated with TNF/CHX (Fig. 1). Thus, the inhibition of the formation of the Bax 500-kDa complex in Ad5dl309-infected cells treated with TNF/CHX was dependent on expression of the E1B 19K protein during adenovirus infection.
Since E1B 19K binds to Bax in TNF-α-treated cells, the fractionation profile of E1B 19K was examined in Ad5dl309-infected cells with and without TNF/CHX. The E1B 19K peak in the TNF/CHX-treated Ad5dl309-infected cells shifted to a slightly higher-molecular-mass range (45 to 60 kDa) compared to untreated cells. The corresponding Bax Western blots also showed a similar shift in the Bax profile in Ad5dl309-infected cells (Fig. 1). This is consistent with a 19K-Bax association and recruitment of E1B 19K into a low-molecular-weight complex with Bax (Fig. 1).
tBid is not present in the TNF-α-induced 500-kDa Bax complex.
TNF-α induces an interaction between tBid and Bax, and E1B 19K expression has no effect on tBid-Bax association (32). tBid expression is also sufficient to induce an alteration in Bax conformation, as seen by the exposure of an epitope on the amino terminus of Bax by both immunofluorescence and immunoprecipitation (32). Lysates from mock-, Ad5dl309-, and Ad5dl337-infected HeLa cells untreated or treated with TNF/CHX were fractionated on a Sephacryl S-100 gel filtration column to resolve Bax complexes as in Fig. 1. The Sephacryl S-100 gel filtration matrix was used because it has higher resolution in the range from 10 to 100 kDa, where monomeric Bax, Bid, and tBid were expected to fractionate, while still capable of resolving the 500-kDa Bax complex. Column fractions were probed with Bax and E1B 19K antibodies (Fig. 2A) and with an antibody that recognizes Bid and tBid (Fig. 2B) to determine whether tBid fractionated with a specific form of Bax.
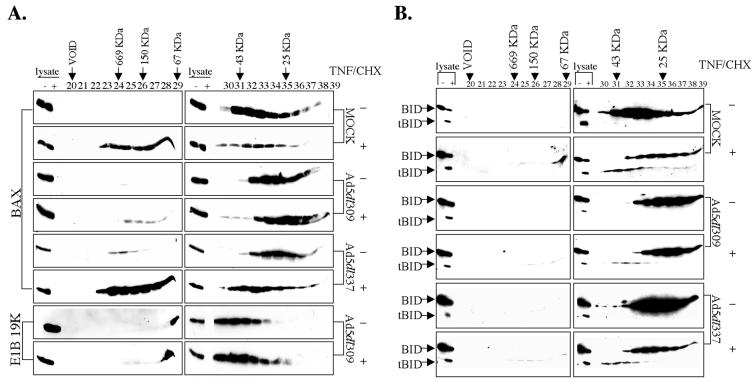
FIG. 2.
tBid is not present in the TNF-α-induced 500-kDa Bax protein complex. Cell lysates prepared in CHAPS lysis buffer were fractionated on a Sephacryl S-100 gel filtration column from cells treated as in Fig. 1. Column fractions 20 to 39 were analyzed by SDS-PAGE followed by Western blotting to Bax or E1B 19K, as indicated (A), or Bid (B). Whole-cell lysates from both the minus- and plus-TNF/CHX samples loaded on the column were included on every Western blot as an internal reference for Bax, E1B 19K, and Bid levels. The peaks at which the molecular weight markers fractionated are indicated.
As with the Sephacryl S-300 column, TNF/CHX induced the formation of the 500-kDa Bax protein complex (fractions 23 to 29) in the mock- and Ad5dl337-infected cells, while the formation of this 500-kDa Bax protein complex was almost completely inhibited in the TNF/CHX-treated Ad5dl309-infected cells in the Sephacryl S-100 column fractionation (Fig. 2A). In the untreated mock-, Ad5dl309-, and Ad5dl337-infected cell extracts, 23-kDa Bid fractionated with a peak with a molecular mass of 30 kDa, consistent with Bid being monomeric (Fig. 2B). Following 4 h of TNF/CHX treatment, a significant proportion of Bid was cleaved into 15-kDa tBid which fractionated with a peak corresponding to a molecular mass of 43 kDa, whereas the remaining uncleaved Bid still fractionated at 30 kDa in all cases (Fig. 2B). These findings are consistent with tBid-Bax coimmunoprecipitation from TNF/CHX-treated cells (32). Strikingly, tBid did not cofractionate with the 500-kDa Bax complex in TNF/CHX-treated cells (Fig. 2B). Although tBid expression is sufficient to induce a conformational change in Bax (32), likely by binding to monomeric Bax, tBid apparently did not enter into the 500-kDa Bax complex. These findings are consistent with a hit-and-run model for tBid-dependent Bax activation. Interestingly, infection of Ad5dl309 and E1B 19K expression did not affect the generation of tBid or its fractionation at 43 kDa in cells treated with TNF/CHX (Fig. 2B). E1B 19K expression during viral infection does not prevent tBid-Bax coimmunoprecipitation in TNF/CHX-treated cells, nor does it prevent the TNF/CHX- and tBid-dependent conformational change in Bax (32). Furthermore, the presence of a tBid-Bax-E1B 19K ternary complex was not detected (32). Thus, it is likely that tBid binds Bax and alters its conformation and that after this transient tBid-Bax interaction, E1B 19K replaces tBid to form an E1B 19K-Bax complex with a molecular mass of approximately 45 to 60 kDa. This E1B 19K-Bax interaction may block the generation of the 500-kDa Bax complex and apoptosis.
TNF-α induces Bax-Bax cross-linking which is inhibited by E1B 19K.
Bax has been shown to homodimerize in vivo and in vitro by coimmunoprecipitation, yeast two-hybrid assays, and chemical cross-linking (1, 5, 12, 20, 36, 39, 46). Bax homodimerization is triggered by different death stimuli, and enforced dimerization of Bax has been shown to result in its translocation to mitochondria and induction of apoptosis (5, 12). As one means to investigate whether TNF-α induced homodimerization of Bax, chemical cross-linking studies were performed. Mock-infected HeLa cells untreated or treated with TNF/CHX for 4 h were lysed in a CHAPS lysis buffer and then incubated with the membrane-permeable, irreversible chemical cross-linking reagent DSS or vehicle alone. Bax cross-linking was then assayed by SDS-PAGE and Western blotting for Bax. To determine what effect, if any, E1B 19K would have on Bax cross-linking, Ad5dl309-infected cells untreated or treated with TNF/CHX were examined in parallel.
In untreated mock-infected cells, Bax migrated at 20 kDa on SDS-polyacrylamide gels, and no cross-linked Bax products were detected. In TNF/CHX-treated mock-infected cells, however, the presence of DSS induced the appearance of a novel Bax-immunoreactive band migrating at 38 kDa (Fig. 3A) that we have designated Bax*. DSS also induced lesser amounts of higher-molecular-weight Bax-immunoreactive bands in TNF/CHX-treated mock-infected cells (data not shown). The formation of a Bax cross-linked product similar to Bax* was also seen when cells were treated with other death stimuli (5, 12). In Ad5dl309-infected cells, no Bax* was detected as in mock-infected cells (Fig. 3A). In contrast to mock-infected cells treated with TNF/CHX, however, there was no Bax* formation by DSS in TNF/CHX-treated Ad5dl309-infected cells (Fig. 3A). Similar results were also obtained with in vivo cross-linking with DSS and in vitro with other chemical cross-linking reagents (data not shown). In vivo cross-linking with DSS induced the formation of Bax* in TNF/CHX-treated AD5dl337-infected cells, similar to that induced in TNF/CHX-treated mock-infected cells (data not shown). Thus, E1B 19K expression during viral infection prevented the formation of the TNF/CHX-induced Bax cross-linked product Bax*.
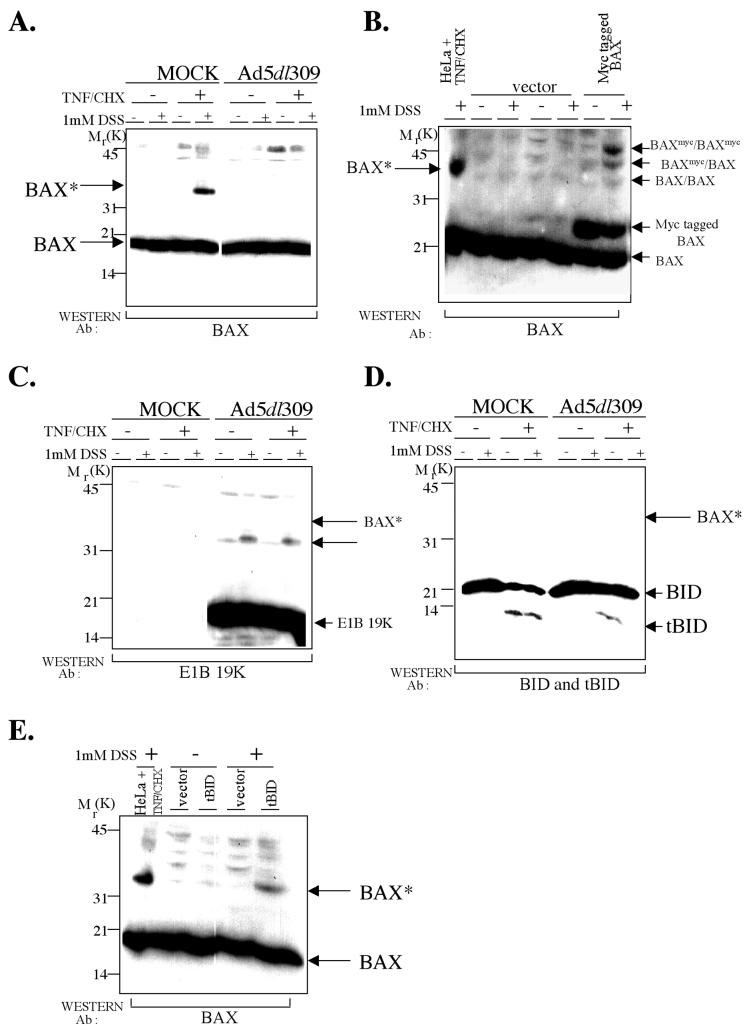
FIG. 3.
TNF-α induces Bax-Bax cross-linking, which is inhibited by E1B 19K during adenovirus infection. (A) E1B 19K expression during viral infection prevents the formation of the TNF/CHX-induced Bax cross-linked product Bax*. Cell lysates prepared in CHAPS lysis buffer were incubated with 1 mM DSS or vehicle alone from cells treated as in Fig. 1. Cross-linked samples were analyzed by SDS-PAGE followed by Western blotting for Bax. The positions of migration of the Bax cross-linked product Bax* and the molecular weight markers on the Western blot are indicated. Ab, antibody. (B) The TNF/CHX-induced Bax cross-linked product Bax* may be a Bax cross-linked dimer. HeLa cells were transfected with either the control vector or pcDNA3-Myc-rBax, as indicated; 24 h posttransfection, cells lysed in CHAPS lysis buffer were incubated with 1 mM DSS or vehicle alone. Cross-linked samples were analyzed by SDS-PAGE followed by Western blotting for Bax. A 4-h TNF/CHX-treated, cross-linked HeLa cell lysate is also included in the first lane, which shows the position of migration of Bax*. The positions of migration of Baxmyc cross-linked to Baxmyc (BAXmyc/BAXmyc), Baxmyc cross-linked to endogenous Bax (BAXmyc/BAX), and endogenous Bax cross-linked to endogenous Bax (BAX/BAX) on the Western blot are indicated. (C and D) E1B 19K, Bid, and tBid are not components of Bax*. The blot in panel A was reprobed with a E1B 19K antibody (C) and with a Bid antibody (D). The position of migration of Bax* is indicated. An arrow in panel C may represent an E1B 19K dimer. (E) tBid is sufficient to induce Bax-Bax cross-linking. HeLa cells were transfected with either the control vector or pcDNA3.1-htBid-Myc, as indicated; 24 h posttransfection, cells lysed in CHAPS lysis buffer were incubated with 1 mM DSS or vehicle alone. Cross-linked samples were analyzed by SDS-PAGE followed by Western blotting for Bax. A 4-h TNF/CHX-treated, cross-linked HeLa cell lysate is also included in the first lane, which shows the position of migration of Bax*.
To investigate if Bax* represented cross-linking of Bax to itself, Myc-tagged Bax (Baxmyc), which has a slightly higher molecular weight than endogenous Bax, was transiently expressed in HeLa cells. Transient expression of Bax has been shown to induce Bax dimerization and apoptosis (12). Bax homodimerization would be expected to produce three different Bax cross-linked products: Baxmyc cross-linked to Baxmyc, Baxmyc cross-linked to endogenous Bax, and endogenous Bax cross-linked to endogenous Bax. Transiently expressed Baxmyc in cells migrated at a molecular mass of 23 kDa, whereas endogenous Bax migrated at 20 kDa on SDS-PAGE (Fig. 3B). Treatment of the Baxmyc-expressing cell lysates with DSS resulted in bands corresponding to the three expected cross-linked Bax products, Baxmyc-Baxmyc migrating at 46 kDa, Baxmyc-Bax migrating at 43 kDa, and Bax-Bax migrating at the same molecular mass as Bax* (38 kDa) (Fig. 3B). In the control vector lysates treated with and without DSS, there were no Bax cross-linked products, as expected (Fig. 3B). Since Bax is known to homodimerize, this suggested that Bax* may represent Bax cross-linked to itself.
As both E1B 19K and tBid coimmunoprecipitate with Bax from TNF/CHX-treated Ad5dl309-infected cells, we investigated whether E1B 19K and/or tBid were present in a Bax cross-linked product. When the blot in Fig. 3A was reprobed for E1B 19K, no Bax-E1B 19K cross-linked product was detected, although E1B 19K was present in the samples (Fig. 3C). A small proportion of the E1B 19K protein was cross-linked into a higher form (Fig. 3C arrow), which may be an E1B 19K dimer, but its appearance was not a TNF/CHX-mediated event, and it was not Bax*, which has a higher molecular weight (Fig. 3C). Western blotting for Bid and tBid revealed that neither was a component of Bax*, as no Bid-reactive band was observed migrating at the position of Bax* (Fig. 3D). Thus, TNF/CHX likely induced the formation of Bax dimers (or oligomers) represented by the induction of Bax* and the 500-kDa Bax complex, and E1B 19K expression inhibited formation of both.
tBid is sufficient to induce Bax-Bax cross-linking.
TNF-α-mediated death signaling induces an interaction between tBid and Bax, and tBid expression is sufficient to induce an alteration in Bax conformation (32). Therefore, we investigated if tBid was sufficient to induce formation of Bax* in cross-linking assays. HeLa cells were transiently transfected either with vector alone or with a tBid expression vector and lysed in a CHAPS lysis buffer with or without DSS. DSS induced the formation of a Bax cross-linked product only in tBid-expressing cells, not in vector control transfections which migrated at the same molecular weight as Bax* formed in TNF/CHX-treated mock-infected cell extracts (Fig. 3E). Thus, tBid expression was sufficient to induce the formation of Bax*, which is likely representative of Bax dimerization or oligomerization.
E1B 19K expression prevents Bax* formation at prolonged periods of TNF-α death signaling.
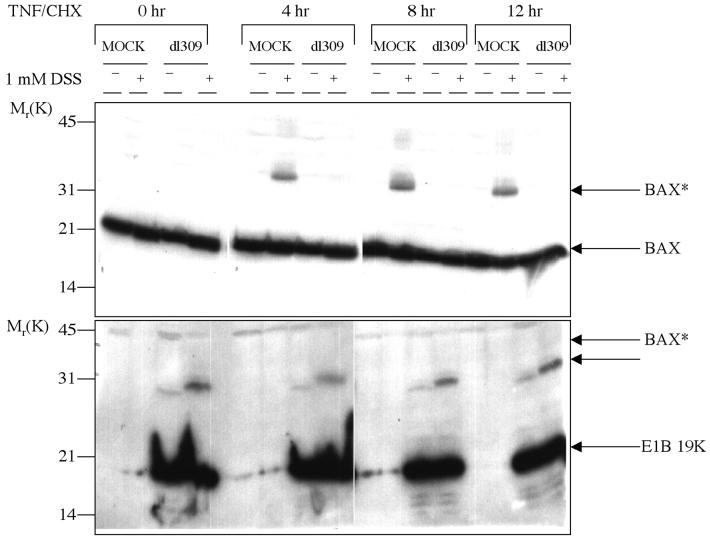
E1B 19K has been shown to block TNF-α-induced cell death for more than 24 h, at which point survival of E1B 19K-negative control cells is negligible (10, 32, 43). To examine if the inhibition of Bax* formation by E1B 19K was transient or was sustained, a time course of TNF/CHX treatment was performed on mock- and Ad5dl309-infected HeLa cells. After 4, 8, and 12 h of TNF/CHX treatment, cells were lysed in a CHAPS lysis buffer and then treated or not treated with DSS. A Bax Western blot revealed that Bax* was present throughout the 12 h of TNF/CHX treatment in mock-infected cells treated with DSS (Fig. 4). In contrast, even after 12 h of TNF/CHX treatment in Ad5dl309-infected cells treated with DSS, no Bax* formed (Fig. 4). The E1B 19K blot of the time course of TNF/CHX treatment showed E1B 19K expression throughout the time course of infection and TNF/CHX treatment (Fig. 4). Therefore, the inhibition of TNF/CHX-dependent generation of Bax* by E1B 19K expression during viral infection was sustained and not transient.
FIG. 4.
E1B 19K expression prevents Bax* formation at prolonged periods of TNF-α death signaling. Hela cells were mock or Ad5dl309 infected for 24 h and then untreated or treated with TNF/CHX for 4, 8, and 12 h. Cell lysates prepared in CHAPS lysis buffer were incubated with 1 mM DSS or vehicle alone. Cross-linked samples were analyzed by SDS-PAGE followed by Western blotting for Bax (top) or E1B 19K (bottom). The positions of migration of the Bax cross-linked product Bax* and the E1B 19K cross-linked product are indicated.
TNF-α induces conformation changes in specific regions of the Bax protein.
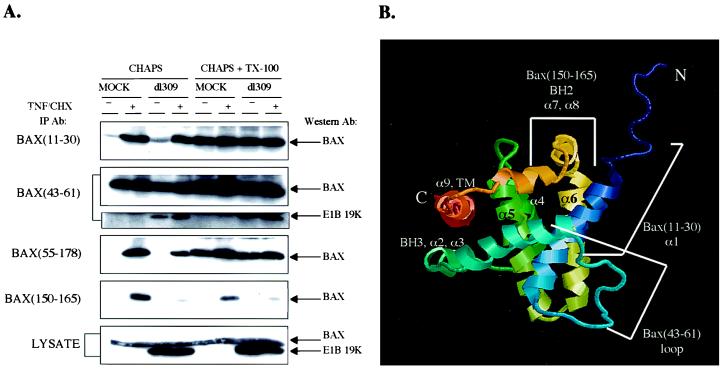
In cells undergoing apoptosis, including that mediated by TNF-α, Bax has been shown to undergo a conformation change resulting in the exposure of the amino terminus by immunoprecipitations in nondetergent conditions or in CHAPS buffer and by immunofluorescence (4, 17, 32). To explore the extent of the conformational changes in Bax, a panel of antibodies with specificities to different epitopes on Bax was used to determine the extent of conformation change of Bax upon TNF/CHX treatment and in adenovirus-infected cells. The Bax(11–30) antibody, which recognizes an epitope contained within amino acids 11 to 30 near the amino terminus of Bax, detects Bax only in TNF/CHX-treated cells (32). The Bax(43–61) antibody recognizes an epitope contained within amino acids 43 to 61, in what was predicted to be an unstructured loop region based on structural homology with Bcl-XL (27). The Bax(55–178) monoclonal antibody recognizes an undefined epitope contained within amino acids 55 to 178 of Bax. The Bax(150–165) antibody recognizes an epitope within amino acids 150 to 165 encompassing BH2, near the carboxy terminus of Bax.
HeLa cells were either mock or Ad5dl309 infected, and cell extracts were prepared in a CHAPS lysis buffer which has been shown not to reveal the Bax amino terminus for immunoprecipitation unless a death stimulus is present. As a control, cell lysates were also prepared in CHAPS lysis buffer with 1% Triton X-100, which results in exposure of the Bax amino terminus in a TNF/CHX-independent fashion. Bax and E1B 19K levels were identical regardless of the detergent lysis conditions and were unaffected by TNF/CHX (Fig. 5).
FIG. 5.
TNF-α induces conformation changes in specific regions of Bax. (A) HeLa cells were either mock or Ad5dl309 infected for 24 h and then untreated or treated with TNF/CHX for 4 h. Cell lysates were prepared either in CHAPS lysis buffer or in CHAPS lysis buffer plus Triton X-100. Equivalent levels of Bax and E1B 19K were present in all lysates (bottom panel). Cell lysates were immunoprecipitated with the four Bax antibodies indicated on the left. Immune complexes were resolved by SDS-PAGE and probed with the Bax(11–30) antibody. The Bax (43–61) immunoprecipitates were examined for 19K coimmunoprecipitation by Western blotting for 19K. (B) Structure of Bax (35), indicating the locations of epitopes for the different antibodies.
The Bax(11–30) antibody immunoprecipitated Bax from mock-infected cells only in the presence of TNF/CHX in CHAPS buffer, suggesting that death signaling by TNF-α reveals the Bax amino terminus (Fig. 5A). Infection with Ad5dl309 and expression of E1B 19K did not affect the ability of Bax(11–30) to immunoprecipitate Bax in CHAPS buffer in a TNF/CHX-dependent fashion (Fig. 5A). In CHAPS-plus-Triton X-100 buffer, Bax was immunoprecipitated equally well from mock- and Ad5dl309-infected cells treated or not treated with TNF/CHX (Fig. 5A), indicating that Triton X-100 can liberate the amino terminus of Bax in vitro to reveal the amino-terminal epitope for the Bax(11–30) antibody.
Interestingly, the Bax(43–61) antibody immunoprecipitated Bax equally well in mock or Ad5dl309-infected cells in the presence or absence of TNF/CHX in CHAPS buffer, which suggested that this predicted loop region of Bax was always exposed (Fig. 5A). In CHAPS-plus-Triton X-100 buffer, Bax was immunoprecipitated equally well with Bax(43–61) from mock or Ad5dl309-infected cells, treated or not treated with TNF/CHX (Fig. 5A). When the Bax(43–61) immunoprecipitations were examined for E1B 19K coimmunoprecipitation with the E1B 19K antibody, more E1B 19K coimmunoprecipitated from Ad5dl309-infected TNF/CHX-treated cells than from the untreated infected cells in CHAPS buffer, as expected (Fig. 5A). Moreover, purified E1B 19K protein on beads pulls down Bax from TNF/CHX-treated cells preferentially (data not shown). In CHAPS-plus-Triton X-100 buffer, slightly more E1B 19K coimmunoprecipitated with Bax from the untreated Ad5dl309-infected cells (Fig. 5A), perhaps due to the conformational change in the Bax amino terminus induced by Triton X-100.
The Bax(55–178) antibody immunoprecipitated Bax from mock-infected cells only in the presence of TNF/CHX in CHAPS buffer (Fig. 5A), which suggested that an epitope between amino acids 55 to 178 of Bax also became exposed in the presence of TNF/CHX. E1B 19K expression slightly reduced the ability of this antibody to immunoprecipitate Bax in CHAPS buffer, indicating that the presence of E1B 19K can partially influence the availablity of this epitope. Unfortunately, since the location of the epitope for this monoclonal antibody within the 55-to-178 region of Bax is not known, it is difficult to relate this observation to Bax conformation. In CHAPS-plus-Triton X-100 buffer, Bax was immunoprecipitated equally well by the Bax(55–178) antibody from mock-infected or Ad5dl309-infected cells treated or not treated with TNF/CHX (Fig. 5A).
E1B 19K expression blocks exposure of an epitope at the Bax carboxy terminus by TNF/CHX.
The Bax(150–165) antibody immunoprecipitated Bax from mock-infected HeLa cells only in the presence of TNF/CHX in CHAPS buffer, suggesting that an epitope encompassing BH2, near the carboxy terminus of Bax, also becomes exposed upon TNF/CHX treatment (Fig. 5A). Strikingly, the expression of E1B 19K in Ad5dl309-infected cells treated with TNF/CHX dramatically inhibited the ability of the Bax(150–165) antibody to immunoprecipitate Bax (Fig. 5A). This suggested that either access of the antibody to the BH2 epitope is blocked or the conformational change in this region of Bax is prevented by E1B 19K expression during viral infection. In CHAPS-plus-Triton X-100 buffer, Bax was again immunoprecipitated only in mock-infected cells treated with TNF/CHX (Fig. 5A), indicating that 1% Triton X-100 did not induce the same conformation change in Bax BH2 as did TNF-α. The differential ability of 1% Triton X-100 to affect the amino but not the carboxy terminus of Bax indicates that a second and distinct conformational change in Bax is necessary to replicate the form of proapoptotic Bax in vivo. E1B 19K-Bax interaction is facilitated by Triton X-100 addition to extracts even in the absence of TNF/CHX (Fig. 5A). Thus, E1B 19K likely binds Bax that has undergone the amino-terminal conformation change prior to the carboxy-terminal change.
DISCUSSION
Bax oligomerization and apoptosis.
Bax undergoes a conformation change at the amino terminus in vivo in cells treated with staurosporine (4) or TNF-α (32) and upon interleukin-3 withdrawal (12). Furthermore, addition of purified Bid to isolated mitochondria in vitro has been shown to cause Bax oligomerization (5). In vitro-purified, oligomerized Bax has channel-forming activity and can release cytochrome c from isolated mitochondria (1). Other Bcl-2 family members have also been shown to form channels in membrane bilayers in vitro (13). Taken together, these data suggest that conformationally altered Bax may oligomerize to form a pore and may have a direct role in release of proteins from mitochondrial intermembrane space. Here, we show that TNF-α induces conformational changes near both the amino and carboxy termini of Bax and induces Bax oligomerization, leading to formation of a 500-kDa Bax complex.
tBid is not a component of the Bax oligomeric complex but rather promotes conformation changes in Bax which lead to the formation of oligomeric Bax. Bak, another Bax-related and E1B 19K binding protein (6) known to undergo a conformation change and oligomerize in response to death stimuli such as Fas ligand (40), is potentially a minor component of the 500-kDa Bax complex (data not shown). Furthermore, Bid-induced Bax cross-linked complexes do not contain Bcl-2, Bcl-xL, Bag-1, Bid, or voltage-dependent anion channel (5). Whether the TNF-α-induced Bax oligomeric complex is composed entirely of Bax or contains other mitochondrial proteins remains to be determined.
Structure of Bax.
Bax consists of nine α helices (35), and the overall fold of helices α1 through α8 resembles that of antiapoptotic Bcl-xL (27) and proapoptotic Bid (3), with amphipathic α helices clustered around two central hydrophobic α helices (α5 and α6) (Fig. 5B). This arrangement is also reminiscent of the membrane translocation domains of bacterial toxins, in particular diphtheria toxin and the colicins (27). It is believed that the trigger for insertion of the toxin pore-forming domain into membranes is a conformational change in the toxin, encompassing the pore-forming domain, which occurs when another domain of the toxin binds to a receptor on the membrane (7). According to the umbrella model, the conformation change is followed by a hydrophobic hairpin insertion into the phospholipid bilayer while the amphipathic helices are spread on the membrane (7, 8). This is followed by oligomerization and channel or pore formation (9, 38).
A conformation change in Bax may be required, as in the bacterial toxin pore-forming domain, to expose the central hydrophobic helices for membrane insertion and oligomerization to form a pore. Bcl-xL has also been found by nuclear magnetic resonance spectroscopy to undergo a dramatic conformational change when in the micelle-bound form (23). Amino acids 1 to 19 of Bax are required for retention of Bax in a membrane insertion incompetent state in vitro (11). BH3 (α2), appears to be essential for function and interaction between the proapoptotic Bcl-2 family members, and a conformation change at the amino terminus may expose Bax BH3, to facilitate its binding to other Bcl-2 family members including Bax itself.
Carboxy-terminal α9 of Bax occupies the hydrophobic pocket proposed previously to mediate heterodimer formation (35). It is the same hydrophobic cleft formed by BH1, BH2, and BH3 which contains the Bak BH3 peptide in the Bcl-xL–Bak structure (34). The orientation of α9 in Bax provides simultaneous control over its mitochondrial targeting and dimer formation. The conformational change that allows the carboxy terminus to enter mitochondrial membranes must dislodge α9 from the protein core, thereby exposing the hydrophobic BH3 binding pocket to participate in dimer formation. A mutation in Bax α9 that promotes dissociation of α9 causes Bax to constitutively localize to mitochondria (30). BH3 alone would not be enough to compete with α9 of Bax for binding to its BH3 pocket; therefore, dimerization via this pocket cannot occur without an energy-driven process, probably tBid-Bax interaction, to disengage α9 (35). It is likely that the TNF-α-induced conformation change in Bax results in insertion of the α9 transmembrane domain and α5 and α6 into the mitochondrial membrane, followed by Bax oligomerization. Although Bax can oligomerize in vitro without its transmembrane domain, it does so to a lesser extent (1). Complete oligomerization might require membrane insertion of both the α9 transmembrane domain and α5 plus α6.
Specific conformation changes in Bax on TNF-α treatment.
We found that in cells treated with TNF-α, epitopes near the Bax amino and carboxy termini become exposed, but an epitope in the less structured loop remains constitutively available (Fig. 5). Conformation changes at the Bax amino and carboxy termini may be important to expose α5, α6, and α9 for membrane insertion and oligomerization. Nonionic detergents such as Triton X-100 cause only exposure of an epitope on the amino terminus without exposure of the BH2 epitope near the carboxy terminus; however, TNF-α causes exposure of both of these Bax epitopes (Fig. 5). Thus, detergents may induce only dimerization of Bax, whereas TNF-α may induce all the conformation changes needed for Bax to form the authentic 500-kDa Bax complex. Indeed, addition of Triton X-100 to CHAPS lysates induced Bax dimerization but not oligomerization (data not shown).
E1B 19K interacts with and prevents Bax oligomerization.
E1B 19K interacts with Bax, as shown by in vivo coimmunoprecipitation, yeast two-hybrid, and in vitro binding assays (14–16, 32). The highly conserved central region of E1B 19K (positions 30 to 146) acts as receptor for the Bax BH3, and a 28-amino-acid Bax peptide containing BH3 is sufficient for E1B 19K interaction (14, 15). Furthermore, binding of E1B 19K to Bax is required for inhibition of the proapoptotic function of Bax (14, 15). Thus, conformation changes in Bax to expose BH3 may be required for E1B 19K interaction. Indeed, E1B 19K binds poorly to Bax in the absence of a death stimulus like TNF-α (Fig. 5) (32). Point mutations in the vicinity of the BH1 and BH3 of E1B 19K that abolish Bax binding also abolish protection from TNF-α-mediated apoptosis, while missense mutations elsewhere in the protein do not (2, 16, 43). Taken together, these data suggest that the large central region of the E1B 19K protein, which has homology to other Bcl-2 family members, may act as a binding pocket for an exposed Bax BH3.
E1B 19K expression blocked access of the BH2 epitope of Bax in vivo during TNF-α-mediated death signaling. E1B 19K may interact with Bax in that region, thereby blocking access to the antibody, or may prevent a change in conformation in BH2. Regardless, the E1B 19K-Bax interaction prevents the oligomerization of Bax during TNF-α-mediated death signaling, which may prevent Bax from forming a pore in the mitochondrial membrane and releasing cytochrome c from mitochondria.
Whether other antiapoptotic Bcl-2 family members function similarly to E1B 19K in the TNF-α-mediated death signaling pathway is not known. Bcl-2 blocks Bax-Bax cross-linking upon interleukin-3 withdrawal (12) and inhibits a Fas-induced conformational change in the Bax amino terminus and mitochondrial translocation (28). On one hand, recombinant Bcl-xL has been shown to prevent purified Bid-induced Bax oligomerization in isolated mitochondria, and a recombinant Bcl-xL mutant that cannot bind Bax does not inhibit Bax cross-linking (5). On the other hand, Bcl-xL has been shown to bind bacterially expressed, oligomeric Bax, without requiring Bax to dissociate to monomers in vitro (36). These discrepancies may be due to technical differences, and it remains to be seen if other antiapoptotic Bcl-2 family members are functionally analogous to E1B 19K, and whether inhibition of signaling by other death receptors by E1B 19K occurs by the same mechanism. Similarly, we do not know if the inhibition of other apoptotic pathways by E1B 19K such as p53-dependent apoptosis in E1B 19K-expressing stable cell lines also relies on binding to and inhibition of Bax oligomerization.
Homology of Bax and E1B 19K to bacterial pore-forming toxins and their corresponding immunity proteins.
The ability of E1B 19K to interact with conformationally altered Bax and prevent Bax oligomerization is very reminiscent to how bacterial immunity proteins inhibit pore formation by bacterial toxins. Conformational changes occur in the pore-forming domains of the bacterial toxins, which result in the exposure of the two central hydrophobic α helices and insertion of these helices into the phospholipid bilayer. This is followed by oligomerization to form the transmembrane channel, which leads to damage of cell membranes by pore formation and colloid osmotic lysis (9, 26). The corresponding bacterial immunity proteins function by specific and direct interaction between their helices and the helices of the toxin channel-forming domain within the membrane bilayer to inhibit oligomerization and productive channel formation (37, 47). Analogously, E1B 19K binding to Bax may prevent Bax oligomerization and pore formation in the mitochondrial membrane, thereby blocking the release of cytochrome c and perhaps other proteins from the mitochondria. Adenoviruses may have evolved a function in E1B 19K analogous to that of the bacterial immunity proteins. Thus, there may be an evolutionary conservation in the structure and function of bacterial pore-forming toxins and their corresponding immunity proteins to the proapoptotic mitochondrial proteins and their corresponding antagonists.
ACKNOWLEDGMENTS
We thank Junying Yuan and Phillip E. Branton for generous gifts of antibodies. We also thank Denise Perez, Andrea Cuconati, Kurt Degenhardt, Holly Henry, and Deirdre Nelson for critical reading and Thomasina Sharkey for assistance with preparation of the manuscript.
This work was supported by a grant from the National Institutes of Health (CA53370) to E.W. and the Howard Hughes Medical Institute.
REFERENCES
- 1.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou J-C. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochemistry. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 2.Chiou S-K, Tseng C C, Rao L, White E. Functional complementation of the adenovirus E1B 19K protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou J J, Li H, Salvesen g S, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 4.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release and apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eskes R, Desagher S, Antonsson A, Martinou J. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrow S N, White J H M, Martinou I, Raven T, Pun K-T, Grinham C J, Martinou J-C, Brown R. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature (London) 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 7.Gazit E, LaRocca P, Sansom M S P, Shai Y. The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis δ-endotoxin are consistent with an “umbrella-like” structure of the pore. Proc Natl Acad Sci USA. 1998;95:12289–12294. doi: 10.1073/pnas.95.21.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber D, Shai Y. Insertion and organization within membranes of the δ-endotoxin pore-forming domain, helix 4-loop-helix 5, and inhibition of its activity by a mutant helix 4 peptide. J Biol Chem. 2000;275:23602–23607. doi: 10.1074/jbc.M002596200. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert R J, Jimenez J L, Chen S, Tickle I J, Rossjohn J, Parker M, Andrew P, Saibil H. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell. 1999;97:647–655. doi: 10.1016/s0092-8674(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 10.Gooding L R, Aquino L, Duerksen-Hughes P J, Day D, Horton T M, Yei S, Wold W S M. The E1B–19K protein of group C adenoviruses prevents cytolysis by tumor necrosis factor of human cells but not mouse cells. J Virol. 1991;65:3083–3094. doi: 10.1128/jvi.65.6.3083-3094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goping E S, Gross A, Lavoie J N, Nguyen M, Jemmerson R, Roth K, Korsmeyer S J, Shore G C. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross A, Jockel J, Wei M C, Korsmeyer S J. Enforced dimerization of Bax results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross A, McDonnell J M, Korsmeyer S J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 14.Han J, Modha D, White E. Interaction of E1B 19K with Bax is required to block Bax-induced loss of mitochondrial membrane potential and apoptosis. Oncogene. 1998;17:2993–3005. doi: 10.1038/sj.onc.1202215. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996a;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Wallen H D, Nuñez G, White E. E1B 19,000 molecular-weight protein interacts with and inhibits CED-4-dependent, FLICE-mediated apoptosis. Mol Cell Biol. 1998;18:6052–6062. doi: 10.1128/mcb.18.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu Y-T, Youle R J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 18.Hsu Y-T, Youle R J. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 19.Jones N, Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci USA. 1979;76:3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis S, Bethell S S, Patel S, Martinou J-C, Antonsson B. Purification and biochemical properties of soluble recombinant human Bax. Protein Expr Purif. 1998;13:120–126. doi: 10.1006/prep.1997.0871. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zhu H, Xu C-J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Nijihawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 23.Losonczi J A, Olejniczak E T, Betz S F, Harlan J E, Mack J, Fesik S W. NMR studies of the anti-apoptotic protein Bcl-xL in micelles. Biochemistry. 2000;39:11024–11033. doi: 10.1021/bi000919v. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 25.Mahr J A, Gooding L R. Immune evasion by adenoviruses. Immunol Rev. 1999;168:121–130. doi: 10.1111/j.1600-065x.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 26.Manoj K A S, Aronson A I. Analysis of mutations in the pore-forming region essential for insecticidal activity of a Bacillus thuringiensis δ-endotoxin. J Bacteriol. 1999;181:6103–6107. doi: 10.1128/jb.181.19.6103-6107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S-L, Ng S-C, Fesik S W. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 28.Murphy K M, Streips U N, Lock R B. Bcl-2 Inhibits a Fas-induced conformational change in the Bax N terminus and Bax mitochondrial translocation. J Biol Chem. 2000;275:17225–17228. doi: 10.1074/jbc.C900590199. [DOI] [PubMed] [Google Scholar]
- 29.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 30.Nechushtan A, Smith C L, Hsu Y-T, Youle R J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez D, White E. E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J Cell Biol. 1998;141:1255–1266. doi: 10.1083/jcb.141.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez D, White E. TNF-α signals apoptosis through a Bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol Cell. 2000;6:53–63. [PubMed] [Google Scholar]
- 33.Pilder S, Logan J, Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1B 21,000-molecular weight polypeptide leads to degradation of viral and cellular DNA. J Virol. 1984;52:664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, Eberstadt M, Yoon H S, Shuker S B, Chang B S, Minn A J, Thompson C B, Fesik S W. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Youle R J, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 36.Tan Y-J, Beerheide W, Ting A E. Biophysical characterization of the oligomeric state of Bax and its complex formation with Bcl-XL. Biochemistry. 1999;255:334–339. doi: 10.1006/bbrc.1999.0222. [DOI] [PubMed] [Google Scholar]
- 37.Taylor R M, Zakharov S D, Heymann J B, Girvin M E, Cramer W A. Folded state of the integral membrane colicin E1 immunity protein in solvents of mixed polarity. Biochemistry. 2000;39:12131–12139. doi: 10.1021/bi000206c. [DOI] [PubMed] [Google Scholar]
- 38.Wallace A, Stillman T, Atkins A, Jamieson S J, Bullough P A, Green J, Artymiuk P J. E. coli hemolysis E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell. 2000;100:265–276. doi: 10.1016/s0092-8674(00)81564-0. [DOI] [PubMed] [Google Scholar]
- 39.Wang K, Gross A, Waksman G, Korsmeyer S J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei M C, Lindsten T, Mootha V K, Weiler S, Gross A, Ashiya M, Thompson C B, Korsmeyer S J. tBid, a membrane-targeted death ligand, oligomerizes Bak to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 41.White E. Regulation of apoptosis by adenovirus E1A and E1B oncogenes. Semin Virol. 1998;8:505–513. [Google Scholar]
- 42.White E, Blose S H, Stillman B. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol Cell Biol. 1984;4:2865–2875. doi: 10.1128/mcb.4.12.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White E, Sabbatini P, Debbas M, Wold W S M, Kusher D I, Gooding L. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992;12:2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wold W S M, Doronin K, Toth K, Kuppuswamy M, Lichtenstein D L, Tollefson A E. Immune responses to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr Opin Immunol. 1999;11:380–386. doi: 10.1016/S0952-7915(99)80064-8. [DOI] [PubMed] [Google Scholar]
- 45.Wolf B B, Green D R. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 46.Zha H, Aimé-Sempé C, Sato T, Reed J C. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;21:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y L, Cramer W A. Intramembrane helix-helix interactions as the basis of inhibition of the colicin E1 ion channel by its immunity protein. J Biol Chem. 1993;268:10176–10184. [PubMed] [Google Scholar]