Abstract
Minocycline, a tetracycline antibiotic, has been reported to exert beneficial effects in models of Alzheimer’s disease (AD). To characterize the mechanisms underlying the putative minocycline-related neuroprotection, we studied its effect in an in vitro model of AD. Primary hippocampal cultures were treated with β-amyloid peptide (Aβ) and cell viability was assessed by standard MTT-assay. Incubation with 10 μM Aβ for 24 h significantly inhibits cellular MTT-reduction without inducing morphological signs of enhanced cell death or increase in release of lactate dehydrogenase. This indicates that cell viability was not affected. The inhibition of MTT-reduction by Aβ was due to an acceleration of MTT-formazan exocytosis. Intriguingly, the Aβ-triggered increase in MTT-formazan exocytosis was abolished by co-treatment with minocycline. In vehicle-treated cells minocycline had no effect on formazan exocytosis. This hitherto unrecognized property of minocycline has to be noticed in the elucidation of the underlying mechanism of this promising neuroprotectant.
Keywords: β-Amyloid, Minocycline, Neuroprotection, MTT-assay, Alzheimer’s disease, Exocytosis
Introduction
Alzheimer’s disease (AD), the most common form of progressive dementia, is characterized by a progressive loss of neurons from particular regions of the brain, especially the hippocampal formation and the cerebral cortex. Prominent hallmark of affected brains is the abundance of extracellular protein aggregates (senile plaques), which are mainly composed of precipitated β-amyloid (Aβ).
A potential drug for the therapy of AD is the semisynthetic tetracycline derivative minocycline, which has been proven to exert beneficial effects in animal models of AD (Fan et al. 2007; Ryu et al. 2004; Seabrook et al. 2006) and various other neurodegenerative diseases (Nikodemova et al. 2010; Wang et al. 2003; Zhang et al. 2009; Zhu et al. 2002). Minocycline readily passes the blood–brain-barrier and has a proven and safe clinical track record as an antibiotic. It is, therefore, a promising candidate for the treatment of neurodegeneration (Blum et al. 2004).
The neuroprotection conferred by minocycline has been mainly attributed to its inhibitory effect on microglial activation (Fan et al. 2007; Ryu et al. 2004; Seabrook et al. 2006). However, minocycline also protects neuronally differentiated PC12 cells from Aβ-related toxicity, indicating that minocycline can rescue cells independently of its anti-inflammatory action. It inhibits Aβ-induced caspase-12 activation and release of lactate dehydrogenase (LDH) (Choi et al. 2007). These findings suggest that minocycline might also protect against Aβ-related neurotoxicity by interfering with cell death mechanisms.
Our objectives were to investigate early effects of minocycline in an in vitro model of AD. Besides morphological observation and monitoring of LDH-release, cell viability was assessed by the standard MTT-assay, as an inhibition of cellular MTT-reduction is assumed to be an early indicator of Aβ-related toxicity (Isobe et al. 1999; Shearman et al. 1995). Since early in our experiments we found that minocycline may affect the exocytosis of MTT-formazan, we additionally assessed patterns of cellular formazan localization.
Methods
Chemicals, Animals, and Cell Culture
Cell culture media were purchased from PAA (Cölbe, Germany) and B27 was obtained from Invitrogen (Karlsruhe, Germany). Amyloid beta peptide (Aβ25–35) from rPeptide (Bogart, USA) was dissolved in sterile distilled water at a concentration of 1 mM and aliquots were stored at −20°C. The final concentration of Aβ was determined in preliminary experiments at different treatment periods and by monitoring MTT-reduction and LDH-release. In the used in vitro model primary hippocampal cells were incubated with 10 μM Aβ for 24 h, unless otherwise stated.
Procedures for animal use were in strict accordance with the Animal Health and Care Committee of the State Saxony-Anhalt, Germany. Wistar rats (Harlan-Winkelmann, Borchen, Germany) were mated overnight; the following day was considered embryonic day 1 (E1). Primary cultures were prepared from E18-embryos. The hippocampi were removed, placed in DMEM, mechanically triturated, and centrifuged (5 min at 300×g). The cells were seeded in high glucose DMEM plus 2% B27 onto poly-d-lysine-coated 96-well microtiter plates (1 * 105 cells/well) and maintained in a humidified 5% CO2 atmosphere at 37°C. The optimal incubation period was determined by immunohistochemistry using mouse anti-MAP-2 (1:3,000; Sternberger Monoclonals Incorporated, Lutherville, USA) and rabbit anti-GFAP (1:500; Progen, Heidelberg, Germany) to detect neurons and astrocytes, respectively. After 3–6 days in vitro the cultures mostly consisted of neurons (>75%) and were used for experiments. Longer cultivation resulted in an increased proportion of astroglial cells. Before the use of cultures in experiments the cell morphology was routinely checked by phase contrast microscopy.
MTT-Reduction and Formazan Exocytosis Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is a yellow tetrazolium salt which is reduced to purple formazan by active cells. MTT (Sigma, Steinheim, Germany) was added to the cultures yielding 1 mg/ml. After 2 h at 37°C and 5% CO2 medium was replaced by 200 μl DMSO. Absorbance of the dissolved formazan was measured at 570 nm (reference: 620 nm) using a microtiter plate reader (Titertek Plus MS212, ICN, Frankfurt, Germany). To determine the rate of formazan exocytosis, cultures were incubated with MTT for 1 h. Five pictures per well (~75 cells/picture) were taken using an Axiovert 100 M confocal laser scanning microscope (Zeiss, Germany) and the number of cells showing formazan crystals at their surface was divided by the total cell number. To avoid possible problems in discrimination of cell bodies the cell density was controlled by phase contrast microscopy before use of the cultures.
Determination of LDH
LDH-release was measured using the Cytotoxicity Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s protocol.
Statistical Analysis
All experiments were replicated in at least three independent hippocampal preparations. Values obtained were compared by repeated measures ANOVA followed by Dunnett post test using GraphPadPrism (version 5.00, GraphPad Software, San Diego, USA).
Results
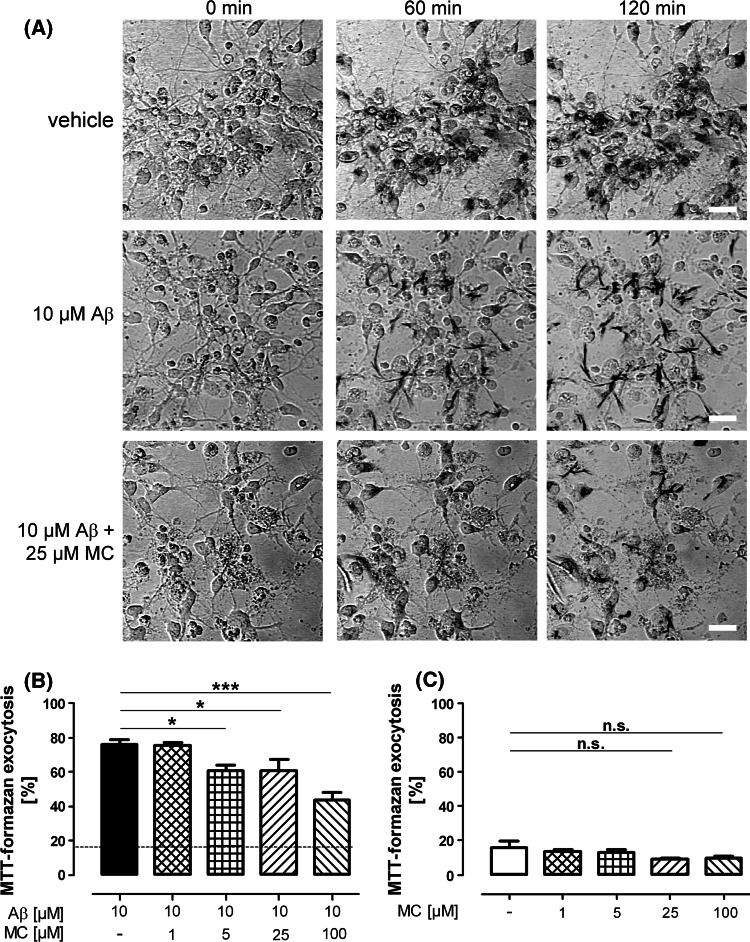
Treatment of hippocampal cultures with ≥1 μM Aβ for 24 h impaired the ability of cells to reduce MTT. While lower concentrations of the peptide were without any significant effect, MTT-reduction decreased to 88.2 ± 4.1% (n = 4, P < 0.05) and 83.5 ± 6.0% (n = 4, P < 0.01; Fig. 1a) of control with 1 or 10 μM Aβ, respectively. MTT-reduction was fully restored by co-treatment with ≥25 μM minocycline (Fig. 1a). Minocycline in the absence of Aβ did not affect MTT-reduction (Fig. 1b). Interestingly, the observed decrease in MTT-reduction triggered by Aβ neither correlated with any morphological signs of cell death (Fig. 2a) nor any statistically significant influence on the LDH-release (Fig. 1c), indicating that cell viability was not impaired.
Fig. 1.
Effects of Aβ on the MTT-reduction and LDH-release of primary hippocampal cultures after 24 h-incubation. a Treatment with 10 μM Aβ significantly impaired the capacity to reduce MTT as compared to control cultures. Co-incubation with 25 or 100 μM minocycline (MC) recovered the MTT-reducing activity of Aβ-treated cells. b In contrast, minocycline did not affect the MTT-reduction of vehicle-treated cells. c Aβ did not alter the release of LDH, indicating that cell viability was not affected. Shown are the mean ± SEM of four preparations (* P < 0.05, ** P < 0.01, *** P < 0.001)
Fig. 2.
Time course and quantification of formazan exocytosis in control and Aβ-treated cultures. a After addition of MTT only few vehicle-treated cells showed extracellular crystals at their surface (upper panel), indicating exocytosis of formazan. In contrast, in Aβ-treated cultures (middle panel), practically all cells had externalized formazan 1 h after starting the incubation with MTT. Co-incubation with 25 μM minocycline (MC) prominently delayed MTT-formazan exocytosis, as most formazan remained localized in intracellular granules. Scale bar: 20 μm. b Quantification revealed that in cultures incubated with 10 μM Aβ 76.2 ± 2.5% of the cells showed external formazan precipitations, while in vehicle-treated control cultures (dotted line) only 15.8 ± 3.8% of cells had undergone formazan exocytosis (P < 0.001, n = 3). Minocycline diminished the amount of formazan-externalizing cells to 60.8 ± 3.3%, 60.6 ± 6.6%, and 43.8 ± 4.2% with 5, 25, and 100 μM minocycline, respectively. c Minocycline did not significantly affect the rate of MTT-formazan exocytosis in the absence of Aβ. Shown are the mean ± SEM of three preparations (* P < 0.05, ** P < 0.01, *** P < 0.001, n.s. P > 0.05)
Aβ has been shown to enhance the externalization of MTT-formazan, thereby inhibiting cellular MTT-reduction in the absence of cell death (Abe and Saito 1998; Isobe et al. 1999). We therefore investigated whether an acceleration of formazan externalization occurred in our model. In control cells (Fig. 2a, upper panel), the vast majority of formazan remained localized in intracellular granules after 2 h of incubation with MTT. In contrast, virtually all cells of Aβ-treated cultures showed a marked accumulation of extracellular formazan crystals at their surface at the same time (Fig. 2a, middle panel). Minocycline in concentration of ≥5 μM significantly abolished the Aβ-related acceleration of formazan exocytosis (Fig. 2a, lower panel; Fig. 2b). Importantly, minocycline alone had no significant effect on MTT-formazan exocytosis (Fig. 2c).
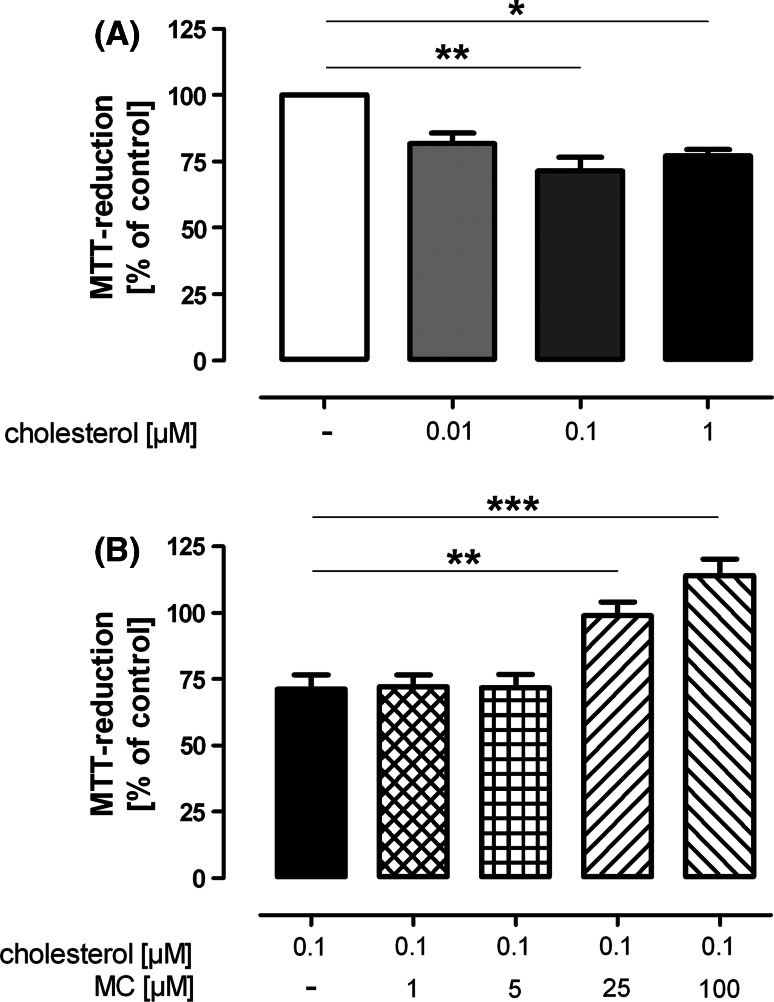
To exclude that minocycline was able to normalize formazan externalization in Aβ-treated cultures by merely inhibiting the uptake of Aβ or promoting the degradation of extracellular Aβ, we investigated whether minocycline also affected formazan exocytosis in cells treated with cholesterol, a sterol known to influence externalization in a way similar to Aβ (Liu et al. 1998). We found that a 3-h-incubation with 0.1 μM cholesterol significantly decreased the MTT-reduction as compared to vehicle-treated cells (71.3 ± 5.2%, P < 0.01, n = 4; Fig. 3a). In cells co-incubated with 25 or 100 μM minocycline, MTT-reduction was restored to 99.0 ± 5.2% (P < 0.01, n = 4) or 114.1 ± 6.2% (P < 0.001, n = 4), respectively (Fig. 3b). Comparable to Aβ, we found an accelerated formazan exocytosis triggered by cholesterol, whereas minocycline (25 μM) clearly inhibited the cholesterol-induced formazan externalization (not shown).
Fig. 3.
Effect of minocycline (MC) on the MTT-reduction of cholesterol-treated cells. a A 3-h-treatment with ≥0.1 μM cholesterol significantly inhibited the ability of hippocampal cultures to reduce MTT. b Minocycline in concentration of 25 or 100 μM was able to recover the MTT-reduction of cholesterol-treated cells. Shown are the mean ± SEM of four preparations (* P < 0.05, ** P < 0.01, *** P < 0.001)
Discussion
Here we show that in hippocampal cell cultures a 24-h-treatment with 10 μM Aβ significantly decreases the ability of cells to reduce MTT. Co-treatment with minocycline recovered the MTT-reduction that initially appeared to be due to a protective effect of minocycline against Aβ-induced cell death. However, we did not observe any morphological signs of cell death such as a retraction of dendritic processes or changes in LDH-release. These findings show that the decrease in MTT-reduction induced by Aβ was not caused by a reduction of cell viability.
The MTT-assay is commonly regarded as an indicator for mitochondrial activity and, hence, cell proliferation and viability. In contrast, Liu et al. (1997) demonstrated that mitochondria do not significantly contribute to cellular MTT-reduction. MTT is internalized into endosomes and reduced to formazan. Formazan-containing vesicles are thought to be subsequently externalized, although the exact mechanism remains unknown. Since chloroquinine (a drug known to facilitate several types of exocytosis) also accelerates the externalization of formazan (Isobe et al. 1999), this process has been denominated as “formazan exocytosis”. At the cell surface, the water-insoluble formazan forms crystals which inhibit further MTT-reduction (Liu and Schubert 1997). It has also been reported that Aβ can affect MTT-reduction in the absence of other signs of toxicity after short treatment period in PC12 cells (Shearman et al. 1994) as well as in astrocytes (Kerokoski et al. 2001). This phenomenon has been attributed to the ability of Aβ to accelerate the exocytosis of MTT-formazan (Abe and Saito 1998; Liu and Schubert 1997).
Our own data confirm such observations, as the treatment with 10 μM Aβ prominently increased the number of cells with externalized formazan. Most interestingly, we found that minocycline abolished the Aβ-induced facilitation of formazan exocytosis, thereby increasing MTT-reduction of Aβ-treated cells. Minocycline did not affect formazan externalization in vehicle-treated cells, but it did block the acceleration of formazan exocytosis when triggered by cholesterol. These data rule out the possibility that minocycline inhibits Aβ-stimulated formazan exocytosis by merely blocking the internalization of Aβ or triggering its degradation. It can, therefore, be concluded that minocycline does not interfere with formazan exocytosis perse, but specifically interrupts signaling pathways which cause an acceleration of formazan exocytosis upon stimulation with Aβ or cholesterol. To our knowledge, this intriguing effect of minocycline has not been described before.
At this point, the question arose whether the Aβ-induced stimulation of formazan exocytosis is causally related to its neurotoxicity. It has been reported that only neurotoxic Aβ-peptides such as Aβ1–40 or Aβ25–35 accelerate formazan exocytosis, while non-toxic peptides (Aβ1–28, Aβ40–1, scrambled Aβ25-35) have no such effect (Liu and Schubert 1997). Furthermore, acceleration of formazan exocytosis and neurotoxicity are common features of fibrillar proteins with β-pleated sheet structure (e.g. insulin and glucagon fibrils), while the proteins perse do not affect formazan exocytosis or cell viability when present in the native, non-fibrillar form (Liu and Schubert 1998). It is, therefore, intriguing to speculate that enhancement of formazan exocytosis is an early step in Aβ-triggered neurotoxicity. Several compounds (e.g. estrogen, genistein) have been, on the other hand, identified which block formazan exocytosis, but do not protect against the toxic effects of Aβ (Liu and Schubert 1997, 1998). Yet, these compounds have in common that they also affect formazan externalization in vehicle-treated cells. It has, thus, been proposed that drugs which specifically block Aβ-stimulated, but not physiological, formazan exocytosis might be neuroprotective (Liu and Schubert 1998). Hong et al. (2007) have, in accordance to that, identified two novel compounds that protect against Aβ-induced neurotoxicity by screening for specific inhibitors of Aβ-stimulated formazan externalization. We therefore suppose that treatment with minocycline might also be beneficial against toxic effects of the peptide.
In conclusion, with the present study we demonstrate for the first time that minocycline prevents Aβ-triggered changes in exocytosis of MTT-formazan, thereby recovering MTT-reduction of Aβ-treated cells. This hitherto unrecognized action of the multifunctional antibiotic, which also possess antioxidative potential (Haroon et al. 2007) and the ability to impair mitochondrial function (Kupsch et al. 2009), however, is an additional characteristic of minocycline that has to be considered in the controversial discussed cytoprotection by this drug (Jordan et al. 2007; Mansson et al. 2010). Whether the effect of minocycline on exocytosis of Aβ-treated cells contributes to its beneficial properties observed in vivo, possibly by interfering with vesicular transport or lipid metabolism, which is impaired in AD (Lukiw et al. 2005; Suzuki et al. 2006), remains to be clarified.
Acknowledgment
This work was supported by funding from Magdeburger Forschungsverbund NBL3 (project number BMBF 01ZZ0407).
References
- Abe K, Saito H (1998) Amyloid beta protein inhibits cellular MTT reduction not by suppression of mitochondrial succinate dehydrogenase but by acceleration of MTT formazan exocytosis in cultured rat cortical astrocytes. Neurosci Res 31:295–305 [DOI] [PubMed] [Google Scholar]
- Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M (2004) Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis 17:359–366 [DOI] [PubMed] [Google Scholar]
- Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH (2007) Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology 32:2393–2404 [DOI] [PubMed] [Google Scholar]
- Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE (2007) Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci 27:3057–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon MF, Fatima A, Scholer S, Gieseler A, Horn TF, Kirches E, Wolf G, Kreutzmann P (2007) Minocycline, a possible neuroprotective agent in Leber’s hereditary optic neuropathy (LHON): studies of cybrid cells bearing 11778 mutation. Neurobiol Dis 28:237–250 [DOI] [PubMed] [Google Scholar]
- Hong HS, Maezawa I, Yao N, Xu B, Diaz-Avalos R, Rana S, Hua DH, Cheng RH, Lam KS, Jin LW (2007) Combining the rapid MTT formazan exocytosis assay and the MC65 protection assay led to the discovery of carbazole analogs as small molecule inhibitors of Abeta oligomer-induced cytotoxicity. Brain Res 1130:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe I, Michikawa M, Yanagisawa K (1999) Enhancement of MTT, a tetrazolium salt, exocytosis by amyloid beta-protein and chloroquine in cultured rat astrocytes. Neurosci Lett 266:129–132 [DOI] [PubMed] [Google Scholar]
- Jordan J, Fernandez-Gomez FJ, Ramos M, Ikuta I, Aguirre N, Galindo MF (2007) Minocycline and cytoprotection: shedding new light on a shadowy controversy. Curr Drug Deliv 4:225–231 [DOI] [PubMed] [Google Scholar]
- Kerokoski P, Soininen H, Pirttila T (2001) Beta-amyloid (1–42) affects MTT reduction in astrocytes: implications for vesicular trafficking and cell functionality. Neurochem Int 38:127–134 [DOI] [PubMed] [Google Scholar]
- Kupsch K, Hertel S, Kreutzmann P, Wolf G, Wallesch CW, Siemen D, Schonfeld P (2009) Impairment of mitochondrial function by minocycline. FEBS J 276:1729–1738 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schubert D (1997) Cytotoxic amyloid peptides inhibit cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction by enhancing MTT formazan exocytosis. J Neurochem 69:2285–2293 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schubert D (1998) Steroid hormones block amyloid fibril-induced 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) formazan exocytosis: relationship to neurotoxicity. J Neurochem 71:2322–2329 [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Kimura H, Schubert D (1997) Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69:581–593 [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Schubert D (1998) Amyloid beta peptide alters intracellular vesicle trafficking and cholesterol homeostasis. Proc Natl Acad Sci USA 95:13266–13271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Pappolla M, Pelaez RP, Bazan NG (2005) Alzheimer’s disease—a dysfunction in cholesterol and lipid metabolism. Cell Mol Neurobiol 25:475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson R, Morota S, Hansson MJ, Sonoda I, Yasuda Y, Shimazu M, Sugiura A, Yanagi S, Miura H, Uchino H, Elmer E (2010) Minocycline sensitizes rodent and human liver mitochondria to the permeability transition: implications for toxicity in liver transplantation. Hepatology 51:347–348 (author reply 349–350) [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Lee J, Fabry Z, Duncan ID (2010) Minocycline attenuates experimental autoimmune encephalomyelitis in rats by reducing T cell infiltration into the spinal cord. J Neuroimmunol 219:33–37 [DOI] [PubMed] [Google Scholar]
- Ryu JK, Franciosi S, Sattayaprasert P, Kim SU, McLarnon JG (2004) Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia 48:85–90 [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Jiang L, Maier M, Lemere CA (2006) Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 53:776–782 [DOI] [PubMed] [Google Scholar]
- Shearman MS, Ragan CI, Iversen LL (1994) Inhibition of PC12 cell redox activity is a specific, early indicator of the mechanism of beta-amyloid-mediated cell death. Proc Natl Acad Sci USA 91:1470–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman MS, Hawtin SR, Tailor VJ (1995) The intracellular component of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction is specifically inhibited by beta-amyloid peptides. J Neurochem 65:218–227 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Araki Y, Yamamoto T, Nakaya T (2006) Trafficking of Alzheimer’s disease-related membrane proteins and its participation in disease pathogenesis. J Biochem 139:949–955 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM (2003) Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci USA 100:10483–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Zhang Z, Fauser U, Schluesener HJ (2009) Improved outcome of EAN, an animal model of GBS, through amelioration of peripheral and central inflammation by minocycline. J Cell Mol Med 13:341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM (2002) Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417:74–78 [DOI] [PubMed] [Google Scholar]