Abstract
Taurine is one of the most abundant free amino acids in the central nervous system, where it displays several functions. However, its molecular targets remain unknown. It is well known that taurine can activate GABA-A and strychnine-sensitive glycine receptors, which increases a chloride conductance. In this study, we describe that acute application of taurine induces a dose-dependent inhibition of voltage-dependent calcium channels in chromaffin cells from bovine adrenal medullae. This taurine effect was not explained by the activation of either GABA-A, GABA-B or strychnine-sensitive glycine receptors. Interestingly, glycine mimicked the modulatory action exerted by taurine on calcium channels, although the acute application of glycine did not elicit any ionic current in these cells. Additionally, the modulation of calcium channels exerted by both taurine and glycine was prevented by the intracellular dialysis of GDP-β-S. Thus, the modulation of voltage-dependent calcium channels by taurine seems to be mediated by a metabotropic-like glycinergic receptor coupled to G-protein activation in a membrane delimited pathway.
Electronic supplementary material
The online version of this article (doi:10.1007/s10571-010-9574-0) contains supplementary material, which is available to authorized users.
Keywords: Taurine, Calcium channels, Glycine receptors, G-proteins
Introduction
Taurine (2-aminoetanosulphonic acid) is one of the most abundant free amino acids in the central nervous system, where it can reach concentrations between 20 and 90 nmol/mg protein depending on analyzed brain area (Palkovits et al. 1986). Although taurine has been implicated in several functional processes in the brain (Huxtable 1992), its molecular targets in this structure are not well identified. It is widely accepted that brain cells (both neurons and glia) use taurine as an organic osmolyte to regulate their volumes under conditions causing cell swelling (Pasantes-Morales and Schousboe 1988; Solís et al. 1988; Kimelberg et al. 1990). To achieve this task, taurine moves from intracellular to extracellular compartment trough anionic channels regulated by volume changes (Mongin et al. 1999). Taurine is a structural analogue of γ-aminobutyric acid (GABA) and glycine which can activate some types of receptors for these amino acids (GABA-A and strychnine-sensitive glycine receptors) (Horikoshi et al. 1988). Despite taurine acts as a weak agonist of GABA-A receptors in several brain areas (del Olmo et al. 2000; Ye et al. 1997), it is not clear whether endogenous taurine can behave as the natural neurotransmitter on this type of receptor. Only in the thalamus, it has been recently reported that taurine activates with high affinity a special combination of GABA-A subunits located extrasynaptically (Jia et al. 2008). On the other hand, taurine may act as the endogenous ligand of glycine receptors (Hussy et al. 1997; Flint et al. 1998; Mori et al. 2002), when glycine release is insufficient to activate strychnine-sensitive receptors. Nevertheless, taurine is not considered as a conventional neurotransmitter because a specific receptor for taurine has not been identified yet (Huxtable 1992), and because taurine release is largely independent of the presence of extracellular calcium (Hanretta and Lombardini 1986; Pasantes-Morales et al. 1988).
Many groups working in different tissues have found taurine actions on calcium movements (Huxtable 1992). For example, in miocites, taurine facilitates the calcium influx at low external calcium concentration and prevents the toxic effects of high calcium by decreasing calcium influx by a process mediated by voltage-dependent calcium channels (Satoh and Sperelakis 1998) and/or the sodium–calcium exchanger (Satoh and Sperelakis 1993). It has also been reported that taurine increases the cytosolic calcium concentration in hippocampal neurons (Zhao et al. 1999). In addition, taurine also seems to promote calcium uptake by the mitochondria (Palmi et al. 1999; El Idrissi 2008).
In this study, we used chromaffin cells isolated from bovine adrenal medulla (an accessible experimental preparation where calcium currents are well identified) to evaluate the possible taurine actions on calcium currents. We found that taurine reduces voltage-dependent calcium currents through the activation of a new metabotropic-like glycine receptor coupled to G-proteins.
Materials and Methods
Preparation of Cells
Adrenal glands were obtained from the city slaughterhouse under the supervision of the local veterinary service. Bovine adrenal medullary chromaffin cells were isolated as described previously (Livett 1984). We used the Percoll gradients for the cell isolation procedure; thus, we had a mixture of adrenergic (60–70%) and noradrenergic cells (30–40%) in our cultures. Cells were suspended in DMEM supplemented with 5% foetal bovine serum, 50 IU/ml penicillin and 50 μg/ml streptomycin. Cells were preplated for 30 min and proliferation inhibitors (10 μM cytosine arabinoside, 10 μM fluorodeoxyuridine and 10 μM leucine methyl ester) were added to the medium to prevent excessive growth of fibroblasts that would mask the chromaffin cell death measurements. For patch-clamp studies, cells were plated on 1-cm-diameter glass coverslips at low density (5 × 104 cells per coverslip). Cultures were maintained in an incubator at 37°C in a water-saturated atmosphere with 5% CO2; they were used within 1–5 days after plating.
Current Measurements and Analysis
Ca2+ (I Ca), Na+ (I Na), glycinergic (I gly) and GABAergic (I GABA) currents were recorded using the whole-cell configuration of the patch-clamp technique. Whole-cell recordings were made with fire-polished electrodes (resistance 2–5 MΩ when filled with the standard intracellular solutions) mounted on the headstage of an EPC-10 patch-clamp amplifier (HEKA Electronic, Lambrecht, Germany), allowing cancellation of capacitative transients and compensation of series resistance. Data were acquired with a sample frequency ranging between 5 and 10 kHz and filtered at 1–2 kHz. Recording traces with leak currents >100 pA or series resistance >20 MΩ were discarded. Data acquisition and analysis were performed using PULSE programs (HEKA Elektronic, Lambrecht, Germany).
Coverslips containing the cells were placed on an experimental chamber mounted on the stage of a Nikon Eclipse T2000 inverted microscope. During the preparation of the seal with the patch pipette, the chamber was replaced with a control Tyrode solution containing (in mM): 137 NaCl, 1 MgCl2, 10 HEPES/NaOH, 0.005 tetrodotoxin (TTX), pH 7.4 (no TTX was added when measuring I Na, I gly and I GABA). Once the patch membrane was ruptured and the whole-cell configuration of the patch-clamp technique was established, the cell was locally, rapidly and constantly superfused with an extracellular solution of similar composition to the chamber solution, but containing nominally 0 mM Ca2+ (no EGTA added; to measure I Na), 2 mM Ca2+ (to measure I gly and I GABA) or 10 mM Ca2+ (to record I Ca) (see “Results” section for specific experimental protocols). For the ionic currents recording, the cells were dialysed with an intracellular solution containing (in mM): 10 NaCl, 100 CsCl, 14 EGTA, 20 TEA.Cl, 5 Mg-ATP, 0.3 Na-GTP and 20 HEPES/CsOH (pH 7.3). The external solutions were rapidly exchanged using electronically driven miniature solenoid valves coupled to a multi-barrel concentration-clamp device, the common outlet of which was placed within 100 μm of the cell to be patched. The flow rate was 1 ml/min and regulated by gravity. Experiments were performed at room temperature (22–24°C). The cells were maintained in culture 2–4 days after recordings.
Immunocytochemical Identification
In order to study the distribution of taurine, the cells were doubly immunostained with antibodies against taurine (rabbit polyclonal antibody) and anti-taurine transporter (rabbit polyclonal antibody). Briefly, slices from adrenal medullae were fixed for 24 h in a formaldehyde phosphate-buffered saline mixture. After blocking in 0.3% normal donkey serum (NDS) with primary antibodies (rabbit anti-taurine 1:100 or rabbit anti-taurine transporter 1:500) over night at room temperature. After three washes in Tris-buffered saline (TBS) a secondary antibody was added (polyclonal swine anti-rabbit immunoglobulins). Immunocytochemical controls were prepared as described, omitting primary antibodies and incubating with 0.3% NDS instead. Sections were then incubated with streptavidin-peroxidase conjugate. The peroxidase activity was revealed using 3′-diaminobenzidine tetrahydrochloride (DAB) as chromogen. Finally, sections were counterstained with hematoxylin. To test the specificity of the observed staining pattern, we prepared immunocytochemical controls as described above but omitting primary antibodies and incubating with 0.3% NDS instead.
Chemicals
Taurine, baclofen, GABA, glycine, GDP-β-S, ATP and the salts to make the saline solutions were obtained from Sigma (Madrid, Spain). Tetrodotoxin citrate (TTX) and CGP 55845 were purchased from Tocris (Biogen Científica, Spain). Dulbecco’s modified Eagle’s medium (DMEM), HRP-streptavidin conjugate, foetal calf serum and penicillin/streptomycin were purchased from Invitrogen (Madrid, Spain). Antibodies AB13 (anti-taurine), AB5414P (anti-taurine transporter) and E0353 (Polyclonal Swine anti-rabbit) were obtained from Chemicon.
Statistical Analysis
Data were expressed as means ± S.E.M. of the number of cells (n) studied, from at least 3 different cell cultures. Student′s t test or one-way ANOVA followed by Newman–Keuls multiple comparison test were used to determine statistical significance between means. The statistical significance was established at P values smaller than 0.05 (*) and 0.01 (**).
Results
Expression of Taurine among Chromaffin Cells and Distribution of Taurine Transporter
By using a specific antibody against taurine, we detected taurine immunoreactivity in nearly 50% of chromaffin cells and in the whole population of cortical cells (see supplementary Fig. 1). Panel 1A1 depicts a strong taurine immunoreactivity in the cortical layer. However, half of adrenal medullae cells are prominently labelled in the nucleus, whereas other cells were not labelled or display a very faint reactivity to taurine antibody.
Once immunoidentified the localisation of taurine in adrenal cells we wanted to know if the presence of taurine was due to the activity of its specific transporter (TAUT). To evaluate this point, we used a specific antibody anti-TAUT. Panel 1B1 shows a prominent immunoreactivity to TAUT in the cells of cortical region, whereas, no immunostaining was observed in adrenal medullae (Panel 1B2). This result indicates that TAUT is not expressed in the plasma membrane of chromaffin cells. Thus, the origin of taurine in this cellular type may come from its endogenous synthesis or alternatively, by the action of an unidentified taurine transporter different from TAUT.
Voltage Clamped Analysis of GABA Acid Response in Chromaffin Cells
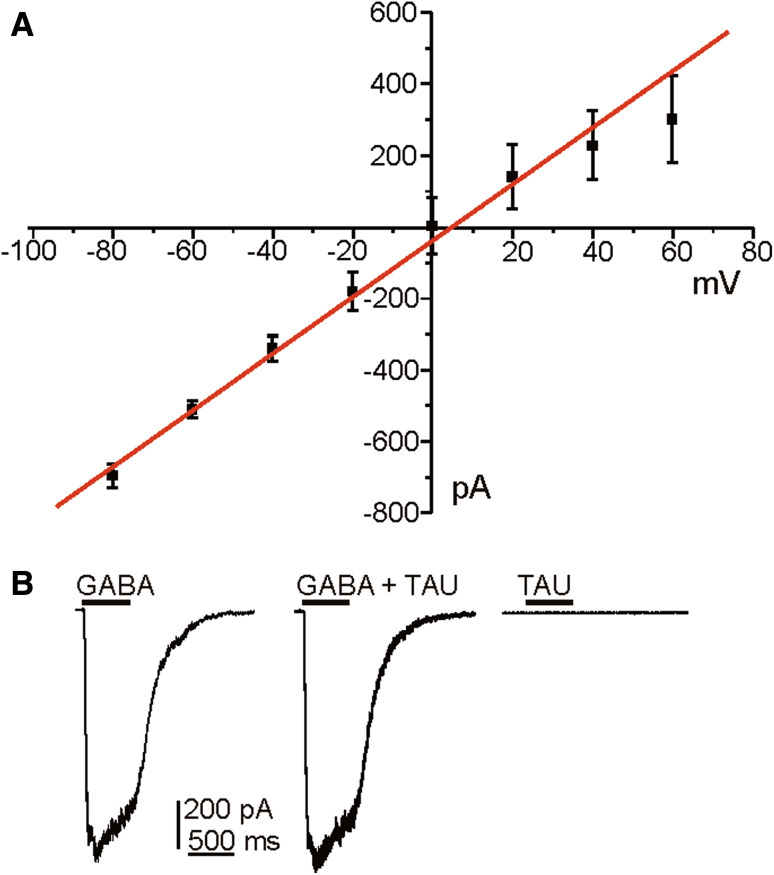
Considering that taurine can activate GABA-A receptors (del Olmo et al. 2000) we first wanted to know whether chromaffin cells express this type of receptors and whether they are activated by taurine. We evaluated the current–voltage relationship obtained with a chloride-based solutions, in response to the application of 100 μM GABA (500-ms pulse at 10-s intervals, HP −80 mV and 10 mV increment steps) in 13 cells. The amplitude of the I GABA varied in a linear manner with holding potential, no rectification of the response was observed. Applications of GABA produced outward or inward currents depending on the cell holding potential and the E Cl for this internal solutions. Thus, the response reversed at a membrane potential of 0.3 mV. The apparent reversal potential of the GABA-evoked current from the Nerst equation for a chloride-based solution is −3.1 mV (for 135 mM Cl− inside). For the same driving force, outward GABA currents, obtained at membrane potentials positive to the reversal potential, were similar to inward currents observed at potential negative (Fig. 1a). As shown in Fig. 1b, GABA-induced currents recorded at −80 mV holding potential (left panel) was not modified by the application of taurine (middle panel). Furthermore, 100 μM taurine, tested at all holding potentials did not evoke any chloride current in chromaffin cells (right panel). This group of results indicates that taurine does not activate GABA-A receptors in chromaffin cells.
Fig. 1.
GΑBA-induced response in chromaffin cell. a Graph plots current–response relationship obtained by application of 100 μM of GABA at various membrane potentials using an intracellular solution of 130 mM Cl−. Each point represents the mean of individual measurements at each potential in 13 cells. Note the linearity of the I GABA–voltage relationship (values for R 2 equal to 0.975). Data are means ± S.E.M. b Original traces recorded at −80 mV holding potential when applying GABA (left panel), GABA plus taurine evoked current (middle) or taurine alone (right). Note that taurine was not able to induce chloride current
Effects of Taurine on Voltage-Dependent Calcium Currents
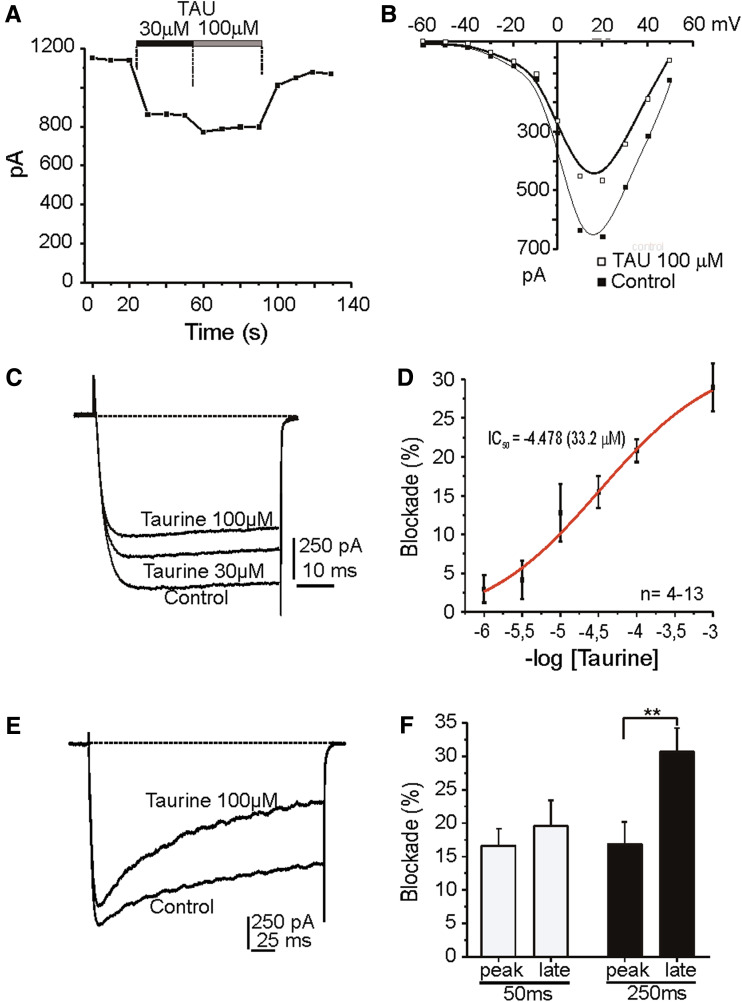
Calcium channels are responsible for the neurosecretory process, so, we performed experiments in voltage clamped cells to ascertain whether taurine was affecting calcium channel functionality. I Ca were elicited in −80 mV clamped cells by depolarising pulses to 20 mV at 10 s intervals, and 10 mM Ca2+ as charge carrier. In 46% of cell tested (64 out of 139 cells), taurine (30 and 100 μM) caused an inhibition of I Ca (15 and 20%, respectively; Fig. 2a, d); in the rest of the cells no effect was detected. This effect of taurine not seems to be related to the proportion of noradrenergic and adrenergic cells observed in the primary culture of chromaffin cell (10 and 90%, respectively). However, no co-immuroreactivity with PNMT or DBH to support this consideration has been done. Initial I Ca was recovered promptly after taurine washout (80% recovery in approximately 20 s). Figure 2b illustrates representative current–voltage curve; the activation threshold was approximately −40 mV, I Ca peaked at +15 mV, and the apparent reversal potential was approximately at +60 mV. At 100 μM taurine, the peak current was reduced by 30%; without shifting the I/V curve. Figure 2c shows original traces obtained in the cell of panel A under the indicated experimental conditions. No modification in the kinetic parameters was observed. Figure 2d shows a sigmoid plotting to obtain a concentration–response curve for the taurine blockade of calcium channels yielding an IC50 of 33.2 μM.
Fig. 2.
Blockade of whole-cell I Ca by taurine. a Time course of I Ca generated by test pulses to 20 mV. Taurine (TAU, 30 and 100 μM) was applied when indicated by the top horizontal bars. b I–V curve plots the current peak obtained by stimulating with test pulses in 10 mV increment steps, in the absence (control) and in the presence of taurine (100 μM). c Original I Ca traces recorded at the condition indicated. d Concentration–response curve for the inhibition of I Ca by taurine (4–13 cells). e Original records of I Ca elicited by 250 ms depolarising pulses (HP −80 mV) in 10 mM Ca2+. Taurine (100 μM) was superfused during 2 min. Note the enhancement of current inactivation exerted by taurine. f Averaged results on the fraction of peak current and late current blocked by taurine at 50 and 250 ms stimulation pulses (n = 19). Data are means ± S.E. from at least three different cultures. * P < 0.05, Student’s t test
Figure 3e shows a cell depolarised with 250 ms voltage-steps to 10 mV applied at 20 s intervals. The application of taurine (100 μM) caused a decline of peak I Ca (18% blockade after 10 s). Initially, the amplitude of the current decreased, showing no signs of inactivation, however, after 40 s the current tended to inactivate. Thus, the blockade of the late current by taurine was more pronounced than of the initial peak current. A second interesting feature was observed during current recovery after taurine washout. Recovery of the late current seems to be more rapid than that of the peak current but at the end both reached similar amplitude, 90% of the initial current. This incomplete recovery might be due to current run-down after such a long stimulation period. Since inactivation of the current was time-dependent and extended along the 250 ms depolarising pulse, it was thought that the magnitude of current inactivation in the presence of taurine depends on the duration of test depolarising pulse. Figure 2f shows averaged results from several cells. Blockade of peak current rose from 16.6 ± 2.5% at 50 ms and 17.9 ± 4% at 250 ms pulses. Inhibition of the late current was significantly higher, 19.6 ± 4% at 50 ms, and 33 ± 4% at 250 ms.
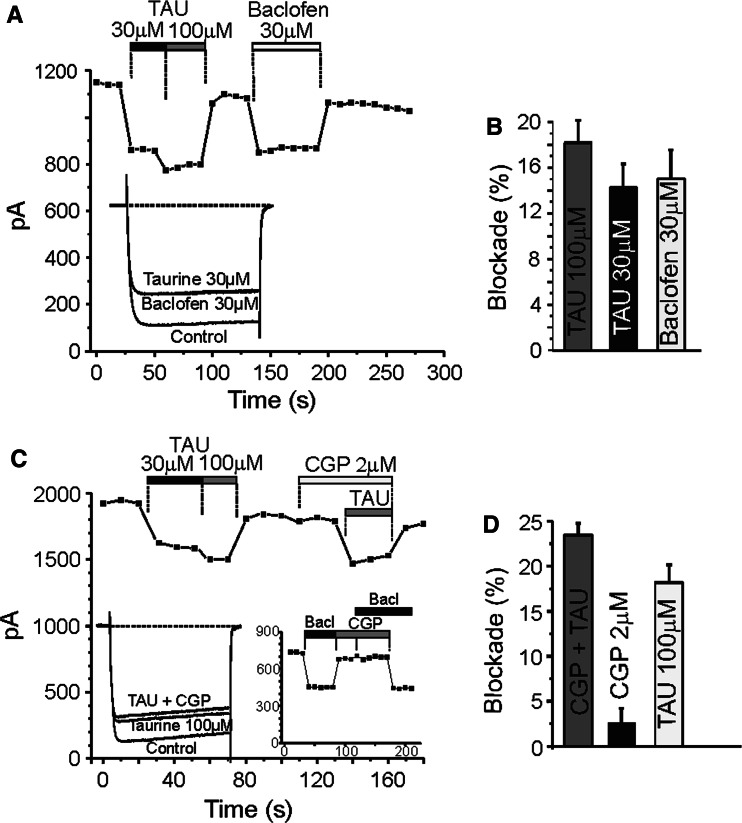
Fig. 3.
GABA-B receptors are not involved in the blockade of calcium current exerted by taurine. Time courses of I Ca recorded in 10 mM Ca2+. a Taurine (30–100 μM) and baclofen (30 μM) were superfused during the time period indicated by the top horizontal bars. Inset: original traces recorded at 20 mV test potential at the indicated experimental conditions. b Averaged results on the fraction of current blocked by taurine and baclofen (n = 13). c Taurine (TAU, 30–100 μM), CGP 55845 (2 μM), or both (TAU 100 μM plus CGP) were superfused during the time period indicated by the top horizontal bars. Note that the inhibition of GABA-B receptors did not modify I Ca indicating that there is not a tonic activation of these receptors. Insets: original traces at the indicated experimental conditions (left). Effects exerted by baclofen (30 μM) applied alone or in combination with the CGP 55845 (2 μM) were evaluated (right). d Averaged results on the fraction of current blocked by taurine, CGP55845 or both (n = 8). Data are means ± S.E
The Blockade of GABA-B Receptor Did Not Modify the Effect of Taurine on Calcium Currents
These experiments were performed in an attempt to find out whether the interaction of taurine with calcium channels is dependent on the activation of metabotropic GABA-B receptors (Nicoll 2004). Figure 3 shows the time course of blockade by 30 and 100 μM taurine in bovine chromaffin. At a −80 mV HP, 50 ms depolarising pulses to 10 mV applied at 10-s intervals evoked an initial I Ca attaining 1351.3 ± 110 pA (n = 22 cells). This initial current declined abruptly in the presence of 30 and 100 μM taurine (14.2 ± 2% and 18.2 ± 2%, respectively) and fully recovered after taurine wash-out. The application of a selective agonist of GABA-B receptors, baclofen (30 μM), decreased the I Ca (15 ± 2%) at about the same rate that 30 μM taurine, also in a reversible manner after washout. In a group of experiments carried out to determine the current–voltage relationship, taurine and baclofen depressed calcium current similarly at all test depolarising potentials without shifting I–V curves (data not shown).
Baclofen-induced decrease of I Ca seems to be due to the activation of GABA-B receptors, because it was fully prevented by CGP55845, a GABA-B antagonist (Fig. 3c, right inset). However, taurine effect on I Ca was not modified in the presence of CGP55845 (Fig. 4c, d). Examining the current–voltage relationship, it becomes clear that taurine reduces I Ca in the presence and absence of CGP55845 similarly at all applied test depolarising potentials, without shifting I–V curves (data not shown). Thus, taurine effect on I Ca does not appear to involve GABA-B receptor activation.
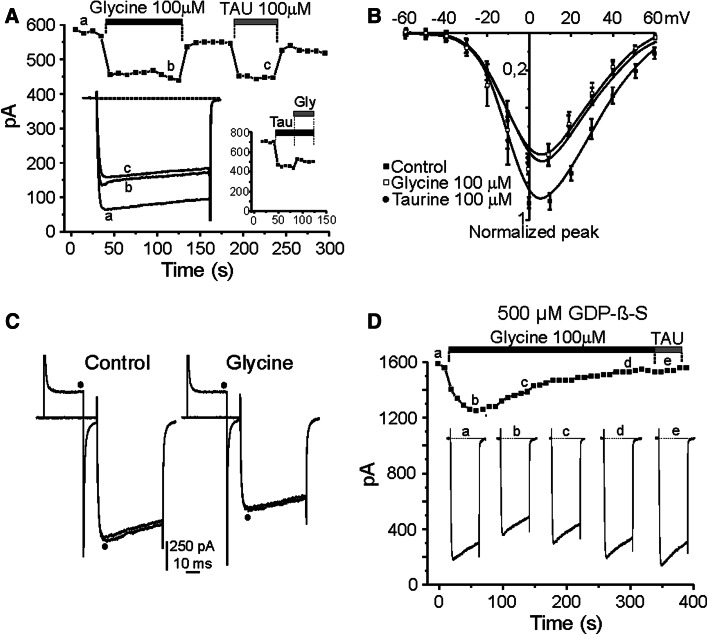
Fig. 4.
Glycine elicited a G-protein-mediated blockade of whole-cell calcium currents. a Time course for I Ca. Glycine (100 μM) and taurine (100 μM) were applied when indicated by the top horizontal bars. Insets represent original traces corresponding at the indicated experimental conditions (left panel) and time course of simultaneous application of taurine and glycine (right panel). b I–V curve plots current peaks evoked by test pulses in 10-mV steps, applied in the absence (control, filled squares) and in the presence of taurine (100 μM, filled circles) or glycine (100 μM, open squares). c Traces recorded after a regular test pulse or preceded by a pre-pulse (filled circle) in control conditions (left) and during 100 μM glycine application (right). d Time course for I Ca (10 mM Ca2+) during the intracellular application of GDP-β-S (500 μM). 50-ms pulses (20 mV) applied at 10-s intervals (HP −80 mV). Glycine (100 μM) and taurine (100 μM) were applied during the time indicated by the top horizontal bars. Insets represent current traces obtained at the beginning of recording and after the progressive G-protein inactivation by GDP-β-S as indicated. The first I Ca was obtained soon after having established the whole-cell recording conditions. Note that the strong inhibition observed at the beginning of recording was almost fully removed after 200 s of cell dialysis
G-Protein-Mediated Modulation of Voltage-Dependent Calcium Channels by Glycine
It is known that taurine can activate strychnine-sensitive glycine receptors (Hussy et al. 1997). In another group of experiments we determined the possible expression of this inhibitory receptor in the plasma membrane of chromaffin cell by recording the chloride current evoked by the application of 500-ms pulse of glycine (100 μM) at different holding potential. In four cells tested no chloride current was observed (data not shown). Thus, chromaffin cell do not express ionotropic glycine receptors.
The existence of a metabotropic glycine receptor has been recently described in salamander retina (Hou et al. 2008). We wonder whether taurine action on I Ca may be mediated through the activation of this type of receptor complex. To test this hypothesis we measured I Ca at a holding potential of −80 mV, elicited by 50 ms depolarising test pulses to 10 mV in 10 mM Ca2+. The I Ca amounted to 1,178 ± 156 pA, and then declined abruptly in the presence of 100 μM glycine, in a reversible manner after washout. Figure 4a shows the blocking effects exerted by glycine (19.2 ± 2%), similar than that elicited by 100 μM taurine (15.2 ± 2%), both amino acids were evaluated in the same 9 cells. Co-application of both drugs did not exert an additive blockade (see inset right). Figure 4b depicts how current–voltage relationship is modified by glycine and taurine. Current peaks were obtained during the application of 50-ms pulses in 10-mV steps from −80 mV HP, in control or in the presence of taurine (100 μM) or glycine (100 μM). The activation threshold was approximately −40 mV, I Ca peaked at +10 mV, and the apparent reversal potential was near +60 mV. At 100 μM, taurine and glycine reduced I Ca by about 30%; there was no shift in the I/V curve (n = 4). To evaluate whether this inhibition of I Ca was due to the activation by glycine of a metabotropic receptor linked to a voltage-sensitive G-protein mechanism, the chromaffin cells were depolarised to 10 mV; and 10 s later, the regular test pulse of 50-ms was preceded by a 30-ms prepulse to +80 mV (Fig. 4c; black circle). With this protocol, neither in control conditions nor in the presence of 100 μM glycine (n = 5) a facilitation of calcium current was observed. These results indicate that the glycine-dependent modulation of calcium channels is voltage-independent (Hernández-Guijo et al. 1999).
In bovine chromaffin cells, it has been demonstrated that the inhibition of Ca2+-channels by endogenous down-regulation processes or by the exogenous application of either opioids or ATP (Hernández-Guijo et al. 1998, 1999) are mediated through G-proteins sensitive to pertussis toxin. Here, we tested whether G-proteins were also involved in the inhibition of calcium channels exerted by glycine by examining whether intracellular dialysis with GDP-β-S prevents calcium channel inhibition.
Intracellular dialysis of GDP-β-S was able to remove most of the inhibition of I Ca. Figure 4d exemplifies the gradual effect of the cellular dialysis with GDP-β-S (500 μM added to the pipette solution) on I Ca amplitude as a function of time. In this example, the application of glycine (100 μM) after breaking into the whole-cell configuration inhibited I Ca by 17 ± 9%, but the progressive replacement of endogenous GTP by the GDP-β-S from the pipette caused a continuous a complete reduction of this inhibition. The time course of the reduction of glycine effect on I Ca probably reflects the time required for GDP-β-S to replace all endogenous GTP. Voltage-dependent Ca2+ channel inhibition was maximal during the first seconds of recordings in the presence of glycine and then, progressively diminished to nearly zero within the next 3–4 min. When G-proteins are fully inactivated by the intracellular dialysis of GDP-β-S, the perfusion of taurine (100 μM) did not elicit any effect on the I Ca (trace e). Thus, both glycine and taurine require endogenous GTP to modulate I Ca.
Discussion
Here, we report in primary cultures of bovine adrenal chromaffin cells, that taurine, one of the most abundant free amino acids in the central nervous system after glutamate, reduces voltage-dependent calcium currents in a concentration-dependent manner. Our results seem to indicate that this taurine effect may be mediated through the activation of a metabotropic-like glycine receptor coupled to G-protein activation.
We have found, by using a specific antibody against taurine, that this amino acid is highly concentrated in all the cells of cortical area of adrenal gland; whereas, only a half of chromaffin cells in adrenal medullae showed taurine immunoreactivity mainly localised in the nucleus. Taurine presence in cell nuclei has been observed in different cell types from several tissues (Ottersen 1988; Terauchi et al. 1998; Lobo et al. 2000), although, at present, its functional role in this cell compartment is unknown. The existence of the taurine transporter (TAUT) in the plasmatic membrane of cortical cells, as was revealed by using an antibody against TAUT, is consistent with the accumulation of taurine in these cells. Nevertheless, chromaffin cells do not appear to express TAUT as judged by the lack TAUT immunoreactivity. Thus, the presence of taurine in this cellular type might come from the activity of the biosynthetic pathway for taurine (Stipanuk et al. 2002) or from the existence of an unidentified taurine transport system different to TAUT.
Taurine modulates synaptic transmission in hippocampal and striatal synapses (del Olmo et al. 2004; Sergeeva et al. 2003). Therefore, taurine presence in bovine adrenal medulla might have a functional meaning. For example, taurine, once released to the extracellular space, might act on chromaffin cells in an autocrine/paracrine manner. In our experimental preparation, taurine did not affect sodium current (data not shown); however, we show that taurine, at a range of concentrations similar to that found in extracellular fluid (10–100 μM; Lerma et al. 1986), modulates voltage-dependent calcium channels in a concentration-dependent manner. The τon and τoff were very fast; in a 10-s pulse the full blockade was archived, indicating a membrane delimited process that was maintained during the time of taurine application. The washout of the drug quickly removed the inhibition and recovered the initial I Ca values. However, this effect was observed approximately in half of cells studied; this fact may be related to a different expression on the receptor for taurine associated to a different population of cells, similar that account for β-adrenergic receptors (Cesetti et al. 2003). One additional effect of taurine was the increment in the inactivation kinetic that leads to a blockade of late current (at the end of the 250-ms pulse) 2–3 folds higher than observed on peak current.
We have tried to determine the possible receptor mediating the effect of taurine on I Ca. Taurine is known to activate GABA-A receptors (del Olmo et al. 2000, 2004; Jia et al. 2008) and chromaffin cell are endowed with functional GABA-A receptors (Castro et al. 2003). However, we demonstrated that taurine was unable to induce chloride current mediated by activation of this receptor, and it did not modified the chloride current evoked by acute application of GABA. Furthermore, we have also considered that the effect exerted by taurine could be mediated by activation of GABA-B receptors because baclofen, a selective agonist, mimicked the inhibition exerted by taurine. However, the fact that CGP55845, a GABA-B antagonist, did not prevent the blockade exerted by taurine is difficult to reconcile with the idea that taurine action were mediated by GABA-B receptor activation.
Another receptor target for taurine is the strychnine-sensitive glycine receptor (Hussy et al. 1997; Flint et al. 1998; Mori et al. 2002). We evaluated whether bovine chromaffin cells express functional glycinergic receptors. The result was negative because the acute perfusion of glycine did not induce any chloride current. Nevertheless, glycine application induced a modulation of voltage-dependent calcium channels similar in magnitude and time course to that observed with taurine. Given that it has been recently found in salamander retina (Hou et al. 2008), that I Ca can be inhibited by the activation of a new metabotropic glycine receptor coupled to G-protein, we hypothesised that this type of receptor could be involved in the modulation of voltage-dependent calcium channels displayed by both glycine and taurine. Our experiments, in which the inhibitory effect of both amino acids was prevented by dialysing the cell under recording with GDP-β-S, support this proposal. Additionally, the application of strong depolarising prepulse did not recover the modulatory current indicating that this modulation is voltage-independent (Hernández-Guijo et al. 1999).
In conclusion, our study made attention that in chromaffin cells a G-protein-dependent modulation of voltage-activated calcium channels by taurine may be exerted through the activation of a metabotropic glycine receptor. This result provides new molecular target to assess the physiological function of taurine in the nervous system.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Inmunofluorescence images showing the localisation of taurine in chromaffin cells. A 1 Inmunostaining with antibodies against taurine in an adrenal gland slice. Note the strong signal detected in the cortical tissue. A 2 Magnification of medullae region. Note the labelling of the nucleus in approximately half of adrenal chromaffin cells. B 1 Immunostaining with antibodies anti-taurine transporter in an adrenal gland slice. Note the strong signal detected in the cortical tissue. B 2 Magnification of medullae region. Note the lack of signal in the totality of adrenal chromaffin cells. Controls without primary antibodies for TAU (C 1) and TAUT (C 2) show a very faint immunostaining, these photographs were obtained with the same exposure times used in A and B. Scale bars: A 1, B 1, C 1, C 2: 50 μm; A 2, B 2: 25 μm. l (TIFF 1,613 kb)
Acknowledgments
This study was supported by Instituto de Salud Carlos III (grant PI080227 to J.M.H-G; and grant PI081067 to J.M.S.). E.A. is a fellow of Fundación Teófilo Hernando. We also acknowledge financial support from the CEAL-UAM-Banco de Santander.
Footnotes
A commentary to this article can be found at doi: 10.1007/s10571-010-9611-z.
References
- Castro E, Gonzalez MA, Oset-Gasque MJ (2003) Distribution of γ-aminobutyric acid receptors in cultured adrenergic and noradrenergic bovine chromaffin cells. J Neurosci Res 71:375–382 [DOI] [PubMed] [Google Scholar]
- Cesetti T, Hernández-Guijo JM, Baldelli P, Carabelli V, Carbone E (2003) Opposite action of β1- and β2-adrenergic receptors on Cav1 L-Channel current in rat chromaffin cells. J Neurosci 23:3–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Olmo N, Bustamante J, Martín del Rio R, Solís JM (2000) Taurine activates GABAA but not GABAB receptors in rat hippocampal CA1 area. Brain Res 864:298–307 [DOI] [PubMed] [Google Scholar]
- del Olmo N, Suárez LM, Orensanz LM, Suárez F, Bustamante J, Duarte JM, Martín del Río R, Solís JM (2004) Role of taurine uptake on the induction of long-term synaptic potentiation. Eur J Neurosci 19:1875–1886 [DOI] [PubMed] [Google Scholar]
- El Idrissi A (2008) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34:321–328 [DOI] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR (1998) Nonsynaptic glycine receptor activation during early neocortical development. Neuron 20:43–53 [DOI] [PubMed] [Google Scholar]
- Hanretta AT, Lombardini JB (1986) Properties of spontaneous and evoked release of taurine from hypothalamic crude P2 synaptosomal preparations. Brain Res 378:205–215 [DOI] [PubMed] [Google Scholar]
- Hernández-Guijo JM, Gandía L, Lara B, García AG (1998) Autocrine/paracrine modulation of calcium channels in bovine chromaffin cells. Pflügers Arch Eur J Physiol 437:104–113 [DOI] [PubMed] [Google Scholar]
- Hernández-Guijo JM, Carabelli V, Gandía L, García AG, Carbone E (1999) Voltage independent autocrine modulation of L-type channels mediated by ATP, opioids and catecholamines in rat chromaffin cells. Eur J Neurosci 11:3574–3584 [DOI] [PubMed] [Google Scholar]
- Horikoshi T, Asanuma A, Yanagisawa K, Anzai K, Goto S (1988) Taurine and beta-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Res 464:97–105 [DOI] [PubMed] [Google Scholar]
- Hou M, Duan L, Slaughter M (2008) Synaptic inhibition by glycine acting at a metabotropic receptor in tiger salamder retina. J Physiol 586:2913–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F (1997) Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol 502:609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable R (1992) Physiological actions of taurine. Physiol Rev 7:101–163 [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL (2008) Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J Neurosci 28:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA (1990) Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 10:1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384:145–155 [DOI] [PubMed] [Google Scholar]
- Livett BG (1984) Adrenal medullary chromaffin cells in vitro. Physiol Rev 64:1103–1161 [DOI] [PubMed] [Google Scholar]
- Lobo MV, Alonso FJ, Martín del Rio R (2000) Immunocytochemical localization of taurine in different muscle cell types of the dog and rat. Histochem J 32:53–61 [DOI] [PubMed] [Google Scholar]
- Mongin AA, Cai Z, Kimelberg HK (1999) Volume-dependent taurine release from cultured astrocytes requires permissive [Ca2+]I and calmodulin. Am J Physiol 277:823–832 [DOI] [PubMed] [Google Scholar]
- Mori M, Gahwiler BH, Gerber U (2002) Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol 539:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA (2004) My close encounter with GABA-B receptors. Biochem Pharmacol 68:1667–1674 [DOI] [PubMed] [Google Scholar]
- Ottersen OP (1988) Quantitative assessment of taurine-like immunoreactivity in different cell types and processes in rat cerebellum: an electronmicroscopic study based on a postembedding immunogold labelling procedure. Anat Embryol 178:407–421 [DOI] [PubMed] [Google Scholar]
- Palkovits M, Elekes I, Lang T, Patthy A (1986) Taurine levels in discrete brain nuclei of rats. J Neurochem 47:1333–1335 [DOI] [PubMed] [Google Scholar]
- Palmi M, Youmbi GT, Fusi F, Sgaragli GP, Dixon HB, Frosini M, Tipton KF (1999) Potentiation of mitochondrial Ca2+ sequestration by taurine. Biochem Pharmacol 58:1123–1131 [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Schousboe A (1988) Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res 20:503–509 [DOI] [PubMed] [Google Scholar]
- Satoh H, Sperelakis N (1993) Effects of taurine on Ca2+ currents in young embryonic chick cardiomyocytes. Eur J Pharmacol 231:443–449 [DOI] [PubMed] [Google Scholar]
- Satoh H, Sperelakis N (1998) Review of some actions of taurine on ion channels of cardiac muscle cells and others. Gen Pharmacol 30:451–463 [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Chepkova AN, Doreulee N, Eriksson KS, Poelchen W, Monnighoff I, Heller-Stilb B, Warskulat U, Haussinger D, Haas HL (2003) Taurine-induced long-lasting enhancement of synaptic transmission in mice: role of transporters. J Physiol 550:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís JM, Herranz AS, Herreras O, Lerma J, Martín del Rio R (1988) Does taurine act as an osmoregulatory substance in the rat brain? Neurosci Lett 91:53–58 [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF (2002) Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr 132:3369–3378 [DOI] [PubMed] [Google Scholar]
- Terauchi A, Nakazaw A, Johkura K, Yan L, Usuda N (1998) Immunohistochemical localization of taurine in various tissues of the mouse. Amino Acids 15:151–160 [DOI] [PubMed] [Google Scholar]
- Ye G, Tse AC, Yung W (1997) Taurine inhibits rat substantia nigra pars reticulata neurons by activation of GABA- and glycine-linked chloride conductance. Brain Res 749:175–179 [DOI] [PubMed] [Google Scholar]
- Zhao P, Huang YL, Chen JS (1999) Taurine antagonizes calcium overload induced by glutamate or chemical hypoxia in cultured hippocampal neurons. Neurosci Lett 268:25–28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Inmunofluorescence images showing the localisation of taurine in chromaffin cells. A 1 Inmunostaining with antibodies against taurine in an adrenal gland slice. Note the strong signal detected in the cortical tissue. A 2 Magnification of medullae region. Note the labelling of the nucleus in approximately half of adrenal chromaffin cells. B 1 Immunostaining with antibodies anti-taurine transporter in an adrenal gland slice. Note the strong signal detected in the cortical tissue. B 2 Magnification of medullae region. Note the lack of signal in the totality of adrenal chromaffin cells. Controls without primary antibodies for TAU (C 1) and TAUT (C 2) show a very faint immunostaining, these photographs were obtained with the same exposure times used in A and B. Scale bars: A 1, B 1, C 1, C 2: 50 μm; A 2, B 2: 25 μm. l (TIFF 1,613 kb)