Abstract
GTPases of the Rho family are molecular switches that play an important role in a wide range of membrane-trafficking processes including neurotransmission and hormone release. We have previously demonstrated that RhoA and Cdc42 regulate calcium-dependent exocytosis in chromaffin cells by controlling actin dynamics, whereas Rac1 regulates lipid organisation. These findings raised the question of the upstream mechanism activating these GTPases during exocytosis. The guanine nucleotide exchange factors (GEFs) that catalyse the exchange of GDP for GTP are crucial elements regulating Rho signalling. Using an RNA interference approach, we have recently demonstrated that the GEFs Intersectin-1L and β-Pix, play essential roles in neuroendocrine exocytosis by controlling the activity of Cdc42 and Rac1, respectively. This review summarizes these results and discusses the functional importance of Rho GEFs in the exocytotic machinery in neuroendocrine cells.
Keywords: PC12 cells, Calcium-regulated exocytosis, Rho, Guanine nucleotide exchange factor, β-Pix, Intersectin, Cancer
Rho GTPases Regulate Exocytosis but what Regulates the Rhos?
Rho GTPases, a subfamily of the small GTPase Ras superfamily are key regulators of numerous membrane-trafficking events including exocytosis (Ridley 2001). Exocytosis is a fundamental cellular process involved in various physiological functions like cell migration, axonal membrane growth, neurotransmission, and hormone release. In neurons and endocrine cells, exocytosis occurs in response to extrinsic stimuli leading to an elevation of intracellular calcium concentration that triggers trafficking of secretory vesicles towards the plasma membrane. Subsequently, secretory vesicles dock and fuse with the plasma membrane, which results in the release of secretory products into the extracellular space. Among the cell models that have provided insight into the molecular machinery underlying exocytosis, adrenal chromaffin cells and their derivate PC12 cell line have a prominent place. Large dense core vesicles of these cells contain catecholamines (adrenalin, noradrenalin and dopamine), various proteins and neuropeptides (like granins, neuropeptide Y, enkephalin, and different peptide processing enzymes) as well as nucleotide and calcium (Crivellato et al. 2008; Winkler 1993).
In neuroendocrine chromaffin and PC12 cells, we previously demonstrated that calcium-regulated exocytosis is under the combined control of three members of the Rho GTPases family: RhoA bound to secretory granules, Rac1 and Cdc42 both associated with the plasma membrane. Interestingly, we observed that Cdc42 and Rac1 are strongly activated during secretion (Gasman et al. 2004; Momboisse et al. 2009a), whereas RhoA is more substantially activated in resting cells (Gasman et al. 1998; our unpublished data). Moreover, functional experiments using RNA interference or the expression of constitutively active GTP-loaded proteins indicate that Cdc42 and RhoA influence the exocytotic machinery by spatially and temporally coupling actin dynamics to the traffic of secretory granules at the plasma membrane (Bader et al. 2004; Gasman et al. 1998, 2004, 1999; Malacombe et al. 2006; Momboisse et al. 2009a), whereas Rac1 controls the activity of the lipid-modifying enzyme phospholipase D, which produces fusogenic lipids at exocytotic sites (see model in Fig. 1) (Vitale et al. 2001, 2002; Zeniou-Meyer et al. 2007). In addition, Rho GTPases have been implicated in other exocytotic processes. Rac1 and Cdc42 regulate multiple steps of mast cell degranulation, (Hong-Geller and Cerione 2000), Rac2 controls primary granule release in neutrophils (Abdel-Latif et al. 2004), and the active participation of Rac1 and Cdc42 has been demonstrated during insulin secretion in pancreatic-β-cells (Amin et al. 2003; Nevins and Thurmond 2003). Rac1 has also been shown to regulate calcium-dependent exocytosis in neurons (Doussau et al. 2000; Humeau et al. 2002), and pancreatic acini (Bi and Williams 2005). Finally, secretion from AtT-20 corticotropes is controlled by Rac1 and RhoG (Ferraro et al. 2007; Xin et al. 2004). Altogether, these data clearly demonstrate the importance of Rho GTPases in many secretory pathways. However, despite these major efforts to decipher the function of Rho GTPases, the upstream events leading to Rho activation during exocytosis remain elusive.
Fig. 1.
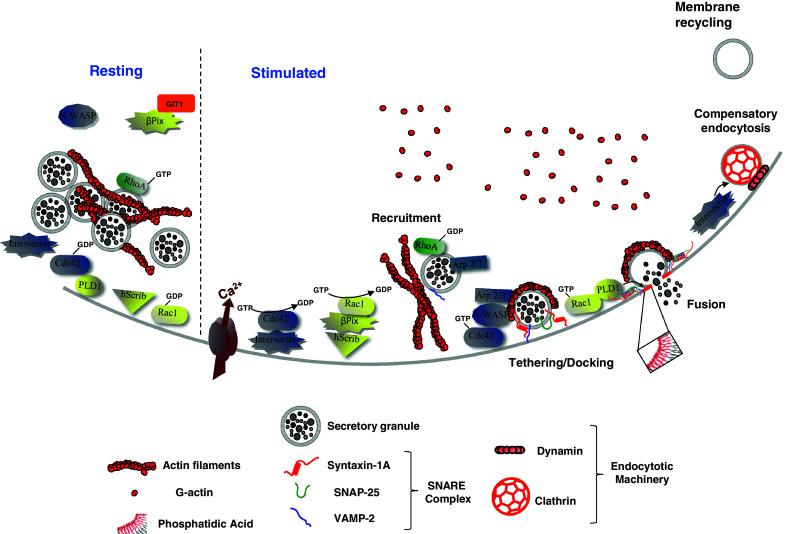
Model of the hypothetical roles of Rho GTPases, Intersectin-1L and β-Pix in calcium-regulated exocytosis from neuroendocrine chromaffin cells. In resting chromaffin cells, secretory granule-associated RhoA is activated and contributes to the stabilization of the cortical actin network. Secretagogue stimulation triggers the recruitment and docking of secretory granules to the plasma membrane. In parallel, while RhoA is presumably inactivated to facilitate actin reorganisation, intersectin-1L activates Cdc42 at exocytotic sites, whereas hScrib recruits β-Pix at the plasma membrane, which in turn activates Rac1. At a post-docking stage, activated-Cdc42 triggers the formation of actin filaments through N-WASP and the granule-bound Arp 2/3 complex. These de novo actin structures, localized at exocytotic sites, are required for some late stages of exocytosis. GTP-bound Rac1 stimulates phospholipase D1 to produce membrane-localized phosphatidic acid at the granule docking site to facilitate formation of the fusion pore. With a well documented function in endocytosis, Intersectin-1L may couple the Cdc42-dependent actin remodelling necessary for exocytosis and the recruitment of the endocytotic machinery required for the subsequent granule membrane retrieval and recycling. When cells return to a resting state, Rac1 and Cdc42 are inactivated through currently unidentified GTPase-activating proteins
Rho GTPases are molecular switches that cycle between an inactive GDP-bound state and an active GTP-bound state. The cycling between these two states is tightly controlled by three different regulators: the guanine nucleotide dissociation inhibitors (GDIs) that maintain the Rhos in an inactive GDP-bound state and regulate their subcellular distribution, the guanine nucleotide exchange factors (GEFs) that promote activation of the GTP-binding protein through the exchange of bound-GDP to GTP and the GTPase-activating proteins (GAPs) that stimulate the hydrolysis of GTP to GDP by the GTPase and thus its inactivation (DerMardirossian and Bokoch 2005; Moon and Zheng 2003; Rossman et al. 2005). By finely and precisely tuning Rho activity, GDIs, GEFs and GAPs are crucial players in all cellular functions regulated by the Rho GTPases including secretion. Indeed, Rho-GDI has been shown to interact with Rac1 in chromaffin cells (Li et al. 2003) and with Cdc42 in pancreatic β-cells (Wang and Thurmond 2010). Overexpression of Rho-GDI has been shown to inhibit exogenously expressed growth hormone release in chromaffin cells (Li et al. 2003). Accordingly, depletion of Rho-GDI in pancreatic mouse islet facilitated glucose-stimulated insulin release, whereas expression of Rho-GDI inhibited it in MIN-6 cells (Wang and Thurmond 2010). So far, the role of Rho-GAP in regulated secretion has been poorly documented. The GAP activity of Nadrin, a neuronal GTPase-activating protein that inhibits RhoA, Rac1 and Cdc42, has been proposed to be required for calcium-mediated release in PC12 cells (Harada et al. 2000). However, the precise mechanisms by which Nadrin could exert this potential function remain to be elucidated, especially given the fact that Nadrin does not seem to be endogenously expressed in adrenal medulla tissue (Harada et al. 2000).
To date, only a few studies have investigated the potential importance of Rho GEFs in regulated secretory pathways. For instance, the Rac1 specific GEF Tiam has been proposed to regulate glucose-stimulated insulin secretion in pancreatic beta-cells (Veluthakal et al. 2009) and β-Pix, a GEF for Cdc42 and Rac1, seems to control hexoasaminidase secretion in mast cells (Chahdi et al. 2004). The group of Richard E. Mains has largely contributed to the field by proposing that Kalirin and/or Trio, two GEFs for RhoA, RhoG and Rac1 regulate pituitary hormone secretion (Ferraro et al. 2007; Mains et al. 1999; Xin et al. 2004). Interestingly, they also demonstrated that the GEF activity of Kalirin and/or Trio tightly controls the balance between constitutive-like secretion and regulated secretion from mature secretory granules in ACTH-secreting AtT-20 corticotropes (Ferraro et al. 2007).
Over the recent years, we have focused our efforts on elucidating the signalling pathways that spatially and temporally coordinate Cdc42 and Rac1 guanine nucleotide exchange and their relationship to membrane-trafficking events underlying exocytosis in neuroendocrine chromaffin cells from the adrenal medulla and PC12 cells, their rat pheochromocytoma derivatives.
Intersectin-1L: The Intersection Between Cdc42 Signalling, Actin Dynamics, Exocytosis and Endocytosis
Intersectin-1L, the long isoform of the Intersectin-1, is a Rho GEF belonging to the Dbl family that is mainly expressed in neurons. It contains, in addition to the DH-PH domains, two N-terminal Eps15 homology domains (EH1 and EH2), a central coiled-coil region, five Src homology 3 (SH3) domains and a carboxy-terminal C2 domain (Guipponi et al. 1998). Intersectin-1L interacts with SNAP-25 (Okamoto et al. 1999), a protein of the SNARE complex that is critical for the docking and fusion of secretory granules to the plasma membrane. We observed intersectin-1L at the exocytotic sites in stimulated PC12 and chromaffin cells (Malacombe et al. 2006). Furthermore, using an RNA interference strategy coupled with expression of constructs encoding the guanine nucleotide exchange domain, we obtained evidence that intersectin-1L is an essential component of the exocytotic machinery (Malacombe et al. 2006). Indeed, by measuring exogenously expressed growth hormone release from PC12 cells, we demonstrated that silencing of intersectin-1L strongly inhibited exocytosis and simultaneously prevented secretagogue-induced activation of Cdc42. These results indicate that intersectin-1L acts as a factor that links Cdc42 activation to the exocytotic pathway (Malacombe et al. 2006). In support of this idea, expression of the GEF domain (DH-PH) was shown to trigger actin polymerization at the cell periphery of secretagogue-stimulated PC12 cells (Momboisse et al. 2009b). This effect is similar to that observed in cells transfected with the GTPase deficient Cdc42Q61L mutant (Gasman et al. 2004) and is abolished in cells depleted of endogenous Cdc42 (Momboisse et al. 2009b). Taken together, these results indicate that intersectin-1L is a component of the pathway that activates Cdc42-dependent reactions, thereby linking extracellular signals to the actin rearrangements required for exocytosis.
Intersectin-1L has been proposed to act as a scaffold regulating the assembly of various endocytotic proteins at the sites of endocytosis, such as dynamin (Hussain et al. 1999; Sengar et al. 1999), AP2 (Pechstein et al. 2010), Eps 15 (Sengar et al. 1999), synaptojanin (Yamabhai et al. 1998). By extending the role of intersectin-1L from endocytosis to exocytosis, our findings support the possibility that intersectin-1L is an adaptor that precisely coordinates Cdc42-mediated actin dynamics involved in the late stages of exocytosis to the process of granule membrane retrieval, thereby coupling calcium-regulated exocytosis to the subsequent compensatory endocytosis in secretory cells (see model in Fig. 1).
β-Pix Controls Fusion Through the Rac1-Induced Activation of PLD
The Cool/Pix proteins are members of the Dbl family of Rho GEFs that specifically activate Rac1 and/or Cdc42 (Chahdi and Sorokin 2008; Feng et al. 2006; Filipenko et al. 2005; ten Klooster et al. 2006). Cool/Pix members have been implicated in various biological processes including cell motility, adhesion, neurite outgrowth as well as the maintenance of neuronal cell polarity (Nayal et al. 2006; Osmani et al. 2006; Shin et al. 2002; Za et al. 2006). The members of this family of protein share a similar domain organization (Zeniou-Meyer et al. 2005). They contain a SH3 (src homology domain), DH-PH domains (responsible for the gunanine-nucleotide exchange activity), a GIT binding region, and a leucine-zipper domain (responsible for the dimerization of the PIX proteins). The α isoforms of PIX proteins differ from the β isoforms by an extension in the amino-terminal region preceding the SH3 domain. Interestingly, a mutation in the gene coding for α-Pix causes a X-linked mental retardation and highlights the importance of Pix proteins in neuronal cell activity (Kutsche et al. 2000).
In an attempt to investigate the importance of β-Pix in calcium-regulated exocytosis, we first demonstrated that β-Pix is mainly cytosolic in resting PC12 cells, but is recruited to the plasma membrane upon cell stimulation through its interaction with hScrib, the mammalian homologue of the Drosophila neoplasic tumour suppressor Scribble (Audebert et al. 2004; Momboisse et al. 2009a). We then demonstrated that overexpression of β-Pix stimulates growth hormone secretion from PC12 cells (Audebert et al. 2004), whereas a reduction of β-Pix expression using a siRNA approach resulted in significant inhibition (Momboisse et al. 2009a). These results are in line with the idea that β-Pix is an essential element of the exocytotic machinery in neuroendocrine cells and led us to probe the possible relationship between β-Pix and Rac1 in the exocytotic machinery. In agreement with this model, knockdown of β-Pix in PC12 cells prevented the secretagogue-induced activation of Rac1 (Momboisse et al. 2009a). Moreover, PLD1 activity was greatly reduced in stimulated cells depleted of β-Pix (Momboisse et al. 2009a). These results indicate that β-Pix functions as a Rac activator within the exocytotic pathway. To strengthen this idea, we tried to directly link the GEF activity of β-Pix with Rac1 and PLD1 activities by using the β-PixL238R-L239R mutant that lacks guanine nucleotide exchange activity (Manser et al. 1998). Expression of this ‘GEF-dead’ β-Pix mutant largely abolished the secretagogue-induced activation of both Rac1 and PLD1 (Momboisse et al. 2009a) and concomitantly the exocytotic activity (Audebert et al. 2004). These results clearly demonstrate that β-Pix is the nucleotide exchange factor that catalyses the activation of Rac1 at the plasma membrane, which is required for PLD1-induced phosphatidic acid synthesis, an important stage in the late fusion step of exocytosis (see model in Fig. 1).
Rho GEFs and Secretion in Neuroendocrine Cancers
Tumours from endocrine and neuroendocrine tissues are often associated with a dysfunction of hormone, neurotransmitter or metabolite secretion (Gratzl et al. 2004). For example, patients with a pheochromocytoma, a tumour-derived from adrenal medullary chromaffin cells have an important hypersecretion of catecholamines indicating an alteration of the secretory machinery from the chromaffin cells (Lenders et al. 2005). Interestingly, our data clearly demonstrated that over-activation of Cdc42 and Rac1 triggers hypersecretion in PC12 cells. While Rho proteins have been largely implicated in human cancers (Vega and Ridley 2008), the GTPases themself are generally not mutated. However, numerous studies have shown that the dysregulation of Rho GTPase cycling plays a crucial role in the initiation and the progression of cancer (Ellenbroek and Collard 2007; Vega and Ridley 2008). Thus, uncovering the molecular pathways controlling the exocytotic steps that involve Rho proteins clearly constitute a biological, pharmaceutical and therapeutic challenge. We have identified part of the signalling pathways that link Rac1 and Cdc42 to calcium-regulated hormone release. An appealing feature is that most of the actors identified in these pathways have also been implicated in cancer. Indeed, β-Pix and Intersectin-1L, as well as many members of the Dbl family of guanine nucleotide exchange factors trigger cell transformation (Cerione and Zheng 1996; Feng et al. 2010; Wang et al. 2005). N-WASP expression has been correlated with different types of tumours (Desmarais et al. 2009; Martin et al. 2008; Wang et al. 2010), whereas elevated PLD activity has been demonstrated in a number of tumorigenic processes, including cell proliferation, survival signalling or cell transformation (Foster 2007; Shi et al. 2007). Finally, Scribble has been classified as a tumour suppressor according to its ability to regulate and maintain cell polarity (Humbert et al. 2003). Altogether, these observations make Rho GTPases regulators and effectors attractive candidates for therapeutic intervention.
Conclusion
Secretion is a ubiquitous and fundamental process governing cell communication. In neuroendocrine cells, exocytosis is intricately controlled by the small GTPases from the Rho family. Since dysregulation of Rho activity is implicated in multiple pathological conditions, it is crucial to understand how the activation/inactivation cycle of the different Rho GTPases are regulated in a given cellular process. We clearly demonstrated that secretion of hormones from the adrenal medulla require the activation of both Rac1 and Cdc42, a process that is controlled by β-Pix and Intersectin-1L, respectively (see model in Fig. 1). However, most of these models derived from studies performed in continuously dividing PC12 cells line. It will be essential to determine in the future whether the regulation of the Rho GTPases signalling during exocytosis obeys similar rules in living organisms. GDIs and GAPs that regulate Rac1 and Cdc42 cycling during the exo-endocytosis process are currently unidentified. Moreover, the coordination between Rac1 and Cdc42 activation, as well as the regulation of β-Pix and Intersectin-1L themselves, during exocytosis remain unknown. Interestingly, β-Pix was shown to interact directly with GIT1 (Audebert et al. 2004), an ARF-GAP, which also regulates exocytosis in neuroendocrine cells by promoting the GTPase activity of ARF6 (Meyer et al. 2006). Resolving theses issues in greater detail will certainly help us to understand precisely how the complex and interconnected Rho pathways control the secretory machinery in normal and pathological conditions.
Acknowledgments
We wish to thank Dr. Nancy Grant for critical reading of the manuscript. The work presented in this review was supported by a Human Frontier Science Program (HFSP) grant (RGY40-2003C), an ANR grant (ANR-07-JCJC-088-01), a ‘Association pour la Recherche sur le Cancer’ grant (ARC #1055) to S.G, an ANR grant (ANR-09-BLAN-0264-01) and a ‘Association pour la Recherche sur le Cancer’ grant (ARC #4051) to N.V as well as by the ‘Fondation pour la Recherche Médicale’ (FRM, fellowship to M.C.).
Footnotes
A commentary to this article can be found at doi:10.1007/s10571-010-9610-0.
References
- Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P (2004) Rac2 is critical for neutrophil primary granule exocytosis. Blood 104:832–839 [DOI] [PubMed] [Google Scholar]
- Amin RH, Chen HQ, Veluthakal R, Silver RB, Li J, Li G, Kowluru A (2003) Mastoparan-induced insulin secretion from insulin-secreting betaTC3 and INS-1 cells: evidence for its regulation by Rho subfamily of G proteins. Endocrinology 144:4508–4518 [DOI] [PubMed] [Google Scholar]
- Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM et al (2004) Mammalian scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 14:987–995 [DOI] [PubMed] [Google Scholar]
- Bader MF, Doussau F, Chasserot-Golaz S, Vitale N, Gasman S (2004) Coupling actin and membrane dynamics during calcium-regulated exocytosis: a role for Rho and ARF GTPases. Biochim Biophys Acta 1742:37–49 [DOI] [PubMed] [Google Scholar]
- Bi Y, Williams JA (2005) A role for Rho and Rac in secretagogue-induced amylase release by pancreatic acini. Am J Physiol Cell Physiol 289:C22–C32 [DOI] [PubMed] [Google Scholar]
- Cerione RA, Zheng Y (1996) The Dbl family of oncogenes. Curr Opin Cell Biol 8:216–222 [DOI] [PubMed] [Google Scholar]
- Chahdi A, Sorokin A (2008) Protein kinase A-dependent phosphorylation modulates beta1Pix guanine nucleotide exchange factor activity through 14-3-3beta binding. Mol Cell Biol 28:1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahdi A, Sorokin A, Dunn MJ, Landry Y (2004) The Rac/Cdc42 guanine nucleotide exchange factor beta1Pix enhances mastoparan-activated Gi-dependent pathway in mast cells. Biochem Biophys Res Commun 317:384–389 [DOI] [PubMed] [Google Scholar]
- Crivellato E, Nico B, Ribatti D (2008) The chromaffin vesicle: advances in understanding the composition of a versatile, multifunctional secretory organelle. Anat Rec (Hoboken) 291:1587–1602 [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM (2005) GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 15:356–363 [DOI] [PubMed] [Google Scholar]
- Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J (2009) N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton 66:303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussau F, Gasman S, Humeau Y, Vitiello F, Popoff M, Boquet P, Bader MF, Poulain B (2000) A Rho-related GTPase is involved in Ca(2+)-dependent neurotransmitter exocytosis. J Biol Chem 275:7764–7770 [DOI] [PubMed] [Google Scholar]
- Ellenbroek SI, Collard JG (2007) Rho GTPases: functions and association with cancer. Clin Exp Metastasis 24:657–672 [DOI] [PubMed] [Google Scholar]
- Feng Q, Baird D, Peng X, Wang J, Ly T, Guan JL, Cerione RA (2006) Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nat Cell Biol 8:945–956 [DOI] [PubMed] [Google Scholar]
- Feng Q, Baird D, Yoo S, Antonyak M, Cerione RA (2010) Phosphorylation of the cool-1/beta-Pix protein serves as a regulatory signal for the migration and invasive activity of Src-transformed cells. J Biol Chem 285:18806–18816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro F, Ma XM, Sobota JA, Eipper BA, Mains RE (2007) Kalirin/Trio Rho guanine nucleotide exchange factors regulate a novel step in secretory granule maturation. Mol Biol Cell 18:4813–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko NR, Attwell S, Roskelley C, Dedhar S (2005) Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene 24:5837–5849 [DOI] [PubMed] [Google Scholar]
- Foster DA (2007) Regulation of mTOR by phosphatidic acid? Cancer Res 67:1–4 [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Hubert P, Aunis D, Bader MF (1998) Identification of a potential effector pathway for the trimeric Go protein associated with secretory granules. Go stimulates a granule-bound phosphatidylinositol 4-kinase by activating RhoA in chromaffin cells. J Biol Chem 273:16913–16920 [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Popoff MR, Aunis D, Bader MF (1999) Involvement of Rho GTPases in calcium-regulated exocytosis from adrenal chromaffin cells. J Cell Sci 112(Pt 24):4763–4771 [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF (2004) Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell 15:520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzl M, Breckner M, Prinz C (2004) Mechanisms of storage and exocytosis in neuroendocrine tumors. Endocr Pathol 15:1–16 [DOI] [PubMed] [Google Scholar]
- Guipponi M, Scott HS, Chen H, Schebesta A, Rossier C, Antonarakis SE (1998) Two isoforms of a human intersectin (ITSN) protein are produced by brain-specific alternative splicing in a stop codon. Genomics 53:369–376 [DOI] [PubMed] [Google Scholar]
- Harada A, Furuta B, Takeuchi K, Itakura M, Takahashi M, Umeda M (2000) Nadrin, a novel neuron-specific GTPase-activating protein involved in regulated exocytosis. J Biol Chem 275:36885–36891 [DOI] [PubMed] [Google Scholar]
- Hong-Geller E, Cerione RA (2000) Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. J Cell Biol 148:481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert P, Russell S, Richardson H (2003) Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays 25:542–553 [DOI] [PubMed] [Google Scholar]
- Humeau Y, Popoff MR, Kojima H, Doussau F, Poulain B (2002) Rac GTPase plays an essential role in exocytosis by controlling the fusion competence of release sites. J Neurosci 22:7968–7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Yamabhai M, Ramjaun AR, Guy AM, Baranes D, O’Bryan JP, Der CJ, Kay BK, McPherson PS (1999) Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J Biol Chem 274:15671–15677 [DOI] [PubMed] [Google Scholar]
- Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth U, Boavida MG, David D, Chelly J, Fryns JP et al (2000) Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet 26:247–250 [DOI] [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K (2005) Phaeochromocytoma. Lancet 366:665–675 [DOI] [PubMed] [Google Scholar]
- Li Q, Ho CS, Marinescu V, Bhatti H, Bokoch GM, Ernst SA, Holz RW, Stuenkel EL (2003) Facilitation of Ca(2 +)-dependent exocytosis by Rac1-GTPase in bovine chromaffin cells. J Physiol 550:431–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains RE, Alam MR, Johnson RC, Darlington DN, Back N, Hand TA, Eipper BA (1999) Kalirin, a multifunctional PAM COOH-terminal domain interactor protein, affects cytoskeletal organization and ACTH secretion from AtT-20 cells. J Biol Chem 274:2929–2937 [DOI] [PubMed] [Google Scholar]
- Malacombe M, Ceridono M, Calco V, Chasserot-Golaz S, McPherson PS, Bader MF, Gasman S (2006) Intersectin-1L nucleotide exchange factor regulates secretory granule exocytosis by activating Cdc42. EMBO J 25:3494–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1:183–192 [DOI] [PubMed] [Google Scholar]
- Martin TA, Pereira G, Watkins G, Mansel RE, Jiang WG (2008) N-WASP is a putative tumour suppressor in breast cancer cells, in vitro and in vivo, and is associated with clinical outcome in patients with breast cancer. Clin Exp Metastasis 25:97–108 [DOI] [PubMed] [Google Scholar]
- Meyer MZ, Deliot N, Chasserot-Golaz S, Premont RT, Bader MF, Vitale N (2006) Regulation of neuroendocrine exocytosis by the ARF6 GTPase-activating protein GIT1. J Biol Chem 281:7919–7926 [DOI] [PubMed] [Google Scholar]
- Momboisse F, Lonchamp E, Calco V, Ceridono M, Vitale N, Bader MF, Gasman S (2009a) BetaPIX-activated Rac1 stimulates the activation of phospholipase D, which is associated with exocytosis in neuroendocrine cells. J Cell Sci 122:798–806 [DOI] [PubMed] [Google Scholar]
- Momboisse F, Ory S, Calco V, Malacombe M, Bader MF, Gasman S (2009b) Calcium-regulated exocytosis in neuroendocrine cells: intersectin-1L stimulates actin polymerization and exocytosis by activating Cdc42. Ann N Y Acad Sci 1152:209–214 [DOI] [PubMed] [Google Scholar]
- Moon SY, Zheng Y (2003) Rho GTPase-activating proteins in cell regulation. Trends Cell Biol 13:13–22 [DOI] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR (2006) Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol 173:587–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins AK, Thurmond DC (2003) Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol 285:C698–C710 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Schoch S, Sudhof TC (1999) EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J Biol Chem 274:18446–18454 [DOI] [PubMed] [Google Scholar]
- Osmani N, Vitale N, Borg JP, Etienne-Manneville S (2006) Scrib Controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol 16:2395–2405 [DOI] [PubMed] [Google Scholar]
- Pechstein A, Bacetic J, Vahedi-Faridi A, Gromova K, Sundborger A, Tomlin N, Krainer G, Vorontsova O, Schafer JG, Owe SG et al (2010) Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2. Proc Natl Acad Sci USA 107:4206–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ (2001) Rho proteins: linking signaling with membrane trafficking. Traffic 2:303–310 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6:167–180 [DOI] [PubMed] [Google Scholar]
- Sengar AS, Wang W, Bishay J, Cohen S, Egan SE (1999) The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J 18:1159–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Zheng Y, Garcia A, Xu L, Foster DA (2007) Phospholipase D provides a survival signal in human cancer cells with activated H-Ras or K-Ras. Cancer Lett 258:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, Kim YG, Cha CI, Kim SR, Park D et al (2002) Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J Biol Chem 277:44417–44430 [DOI] [PubMed] [Google Scholar]
- ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL (2006) Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol 172:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega FM, Ridley AJ (2008) Rho GTPases in cancer cell biology. FEBS Lett 582:2093–2101 [DOI] [PubMed] [Google Scholar]
- Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A (2009) Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol 77:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale N, Caumont AS, Chasserot-Golaz S, Du G, Wu S, Sciorra VA, Morris AJ, Frohman MA, Bader MF (2001) Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J 20:2424–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale N, Chasserot-Golaz S, Bader MF (2002) Regulated secretion in chromaffin cells: an essential role for ARF6-regulated phospholipase D in the late stages of exocytosis. Ann NY Acad Sci 971:193–200 [DOI] [PubMed] [Google Scholar]
- Wang Z, Thurmond DC (2010) Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion. J Biol Chem 285:6186–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Wu WJ, Cerione RA (2005) Cdc42 and Ras cooperate to mediate cellular transformation by intersectin-L. J Biol Chem 280:22883–22891 [DOI] [PubMed] [Google Scholar]
- Wang WS, Zhong HJ, Xiao DW, Huang X, Liao LD, Xie ZF, Xu XE, Shen ZY, Xu LY, Li EM (2010) The expression of CFL1 and N-WASP in esophageal squamous cell carcinoma and its correlation with clinicopathological features. Dis Esophagus 23:512–521 [DOI] [PubMed] [Google Scholar]
- Winkler H (1993) The adrenal chromaffin granule: a model for large dense core vesicles of endocrine and nervous tissue. J Anat 183(Pt 2):237–252 [PMC free article] [PubMed] [Google Scholar]
- Xin X, Ferraro F, Back N, Eipper BA, Mains RE (2004) Cdk5 and Trio modulate endocrine cell exocytosis. J Cell Sci 117:4739–4748 [DOI] [PubMed] [Google Scholar]
- Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK (1998) Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem 273:31401–31407 [DOI] [PubMed] [Google Scholar]
- Za L, Albertinazzi C, Paris S, Gagliani M, Tacchetti C, de Curtis I (2006) BetaPIX controls cell motility and neurite extension by regulating the distribution of GIT1. J Cell Sci 119:2654–2666 [DOI] [PubMed] [Google Scholar]
- Zeniou-Meyer M, Borg JP, Vitale N (2005) The GIT-PIX protein complex: a hub to ARF and Rac/Cdc42 GTPases. Med Sci (Paris) 21:849–853 [DOI] [PubMed] [Google Scholar]
- Zeniou-Meyer M, Zabari N, Ashery U, Chasserot-Golaz S, Haeberle AM, Demais V, Bailly Y, Gottfied I, Nakanishi H, Neiman AM et al (2007) PLD1 production of phosphatidic acid at the plasma membrane promotes exocytosis of large dense-core granule at a late stage. J Biol Chem 37(4):467–476 [DOI] [PubMed] [Google Scholar]


