Abstract
Chromaffin granules are similar organelles to the large dense core vesicles (LDCV) present in many secretory cell types including neurons. LDCV accumulate solutes at high concentrations (catecholamines, 0.5–1 M; ATP, 120–300 mM; or Ca2+, 40 mM (Bulenda and Gratzl Biochemistry 24:7760–7765, 1985). Solutes seem to aggregate to a condensed matrix to elude osmotic lysis. The affinity of solutes for LDCV matrix is responsible for the delayed release of catecholamines during exocytosis. The aggregation of solutes occurs due to a specific H+ pump denominated V-ATPase that maintains an inner acidic media (pH ≈5.5). This pH gradient against cytosol is also responsible for the vesicular accumulation of amines and Ca2+. When this gradient is reduced by modulation of the V-ATPase activity, catecholamines and Ca2+ are moved toward the cytosol. In addition, some drugs largely accumulate inside LDCV and not only impair the accumulation of natural solutes, but also act as false neurotransmitters when they are co-released with catecholamines. There is much experimental evidence to conclude that the physiological modulation of vesicle pH and the manipulation of intravesicular media with drugs affect the LDCV cargo and change the kinetics of exocytosis. Here, we will present some experimental data demonstrating the participation of drugs in the kinetics of exocytosis through changes in the composition of vesicular media. We also offer a model to explain the regulation of exocytosis by the intravesicular media that conciliate the experimentally obtained data.
Keywords: Amperometry, Chromogranins, Vesicular catecholamines, Vesicular calcium, Vesicular ATP
The high concentration of solutes that chromaffin granules (or LDCV) can accumulate in their inner media has intrigued scientists for over half a century (Winkler and Westhead 1980). The theoretical osmolarity calculated by summarizing the concentration of vesicular solutes can be estimated to be about 1,500 mOsm. This large osmotic gradient can only be reduced by the aggregation of solutes thus forming a condensed matrix below acidic pH (≈5.5) to elude osmotic lysis. The affinity of solutes for LDCV matrix is responsible for the delayed release of catecholamines observed by amperometry during exocytosis. We have accumulated experimental evidence to conclude that the manipulation of intravesicular pH affects the LDCV cargo and change the kinetics of exocytosis.
The pH gradient is maintained by the V-ATPase activity (Nelson and Harvey 1999). This gradient of pH is also responsible for the accumulation of amines, ATP, and Ca2+, because their transporters use H+ as the counter-ion (Henry et al. 1998; Machado et al. 2009). We showed that the alkalinization of secretory vesicles slows down exocytosis, whereas acidification causes the opposite effect (Camacho et al. 2006). In addition, alkalinization of vesicular pH causes the leak of catecholamines and Ca2+ (Camacho et al. 2008). This Ca2+ increases the motion of LDCV and can directly trigger exocytosis as demonstrated by amperometry in the absence of external Ca2+. The manipulation of the chromogranin content in LDCV by chga/chgb gene ablation also confirms the importance of intravesicular matrix in the regulation of LDCV cargo and exocytosis (Montesinos et al. 2008; Diaz-Vera et al. 2010).
We can divide intravesicular compounds into two major groups depending on their capacity to move across the vesicle’s membrane. Hence, amines, ascorbate, H+, Ca2+, and ATP are “mobile components” as they are moving in and out of the vesicle, whereas Cgs and other proteins like enzymes are “immobile components” as they cannot easily leave the vesicles (Winkler and Westhead 1980). All mobile compounds of the vesicular cocktail are in equilibrium with the cytosol and the matrix, and as such, they are all principle candidates to be involved in the regulation of exocytosis since changes in any one of these species will affect the others.
Many Drugs Act as False Neurotransmitters, Others Modify the Vesicular pH
In addition to the naturally occurring compounds, several drugs have been recognized as false neurotransmitters like α-methyl-norepinephrine or tyramine, and they can accumulate in secretory vesicles displacing the natural species (Philippu and Schumann 1965; Crout et al. 1962). Other weak bases such as amphetamines have received attention since they have been thought to accumulate in a pH-dependent manner inside vesicles (Sulzer et al. 2005), reducing the quantum size (the amount of catecholamines released per single exocytotic event) by displacing catecholamines toward the cytosol (Sulzer et al. 1993; Fon et al. 1997; Mundorf et al. 1999). However, many other drugs share this characteristic of being permeable weak bases. A circumstance that is frequently ignored is that they accumulate strongly in the acidic organelles like secretory vesicles, and that many of them also bind to Cgs. We described how antihypertensive drugs like hydralazine (Machado et al. 2002b), β-adrenergic blockers (Montesinos et al. 2010), or adrenergic-like fluorescent compounds (Gubernator et al. 2009) accumulate in the LDCV and reduce the quantum size of catecholamine exocytosis. The accumulation of such drugs inside the vesicles could also produce marked changes in the composition of the “mobile components,” like amines, Ca2+ and perhaps ATP. In addition, these drugs are co-released with catecholamines as false neurotransmitters.
The acidic nature of the LDCV is a crucial factor in understanding the equilibrium of its inner components. Hence, the pH of vesicles (5.5) coincides with the maximum stability of Cgs and their optimal capacity to bind soluble species (Helle et al. 1985). Their high content of glutamic and aspartic acid residues produces a pI of 4.4–5.4 (Falkensammer et al. 1985). The pH gradient depends on the activity of a vesicular H+-proton pump ATPase (V-ATPase), which is continuously pumping H+ to acidify the vesicle (Nelson and Harvey 1999). Following the effects of rapid vesicle alkalinization, for instance, using the V-ATPase blocker bafilomycin, can test the role of the pH gradient. Bafilomycin reduces the quantum size and slows down the catecholamine release by exocytosis, as readily observed by amperometry (Camacho et al. 2006).
The regulation of the pH gradient across the LDCV membrane is probably the target of several second messengers which modify the kinetics of exocytosis, and our group has explored two of these second messenger signaling pathways (Borges et al. 2002). For example, the activation of the classical cGMP/PKG pathway by nitric oxide (NO) and other agents promotes the slowing down of catecholamine release in single exocytotic events, without changing the quantum size, an effect that can be rapidly reverted using NO scavengers (Machado et al. 2000). Similar results were found after activation of the cAMP/PKA pathway, although strong stimulation of this kinase also causes a notable increase in quantum size (Machado et al. 2001, Borges et al. 2002). Other drugs like estrogens also slow down exocytosis through a non-genomic mechanism that involves cAMP (Machado et al. 2002a). The activation of these two pathways produces a rapid alkalinization of LDCV (Camacho et al. 2006).
Vesicles behave like a bi-compartmental storage site where the free portion accounts for only ≈10% of the total catecholamines (Schroeder et al. 1996), this portion is probably associated with the halo observed in electron microscopy (Colliver et al. 2000). Changes in pH will rapidly affect this free fraction as it modifies the Donnan equilibrium between the matrix and the halo (Helle et al. 1985). This will initially result in changes in the kinetics of exocytosis without altering the quantum size. However, strong or long lasting inhibition of the V-ATPase also causes the leakage of amines and other soluble components like Ca2+ and ATP, which despite the decrease in the quantum content also promotes granule movement and exocytosis (Camacho et al. 2006, 2008).
The Delayed Release of Catecholamines During the Exocytosis of LDCV
The release of adrenaline following single LDCV fusion events is two–three orders of magnitude slower than that predicted by the diffusion coefficient of catecholamines in aqueous media (Gerhardt and Adams 1982; Hafez et al. 2005). Two mechanisms could explain why catecholamines are retained inside the fused vesicle. One might be the diameter of the fusion pore that could limit the free escape of soluble species from the vesicle. The second candidate is the slow diffusion of solutes from the LDCV matrix (Amatore et al. 2000; Schroeder et al. 1996). Measurements obtained with patch-amperometry, a technique that combines amperometry with cell-attached capacitance, revealed that the arrival of catecholamines to the carbon fiber electrode was still delayed even when the fusion pore was dilated (Albillos et al. 1997; Montesinos et al. 2008). This suggests the direct involvement of the vesicle matrix in the slow release of amines observed once vesicle fusion has taken place.
Some indirect approaches also connect the slow release to the nature of the vesicle’s protein matrix. For instance, secretory vesicles from chromaffin and mast cells behave identically to changes in temperature and ionic composition in spite of their different matrix composition (Pihel et al. 1996). It is likely that the chromaffin matrix of LDCV swells and shrinks as was described in the matrix from mast cells in beige mice (Marszalek et al. 1995). Exocytosis is also largely delayed in the presence of cross-linking agents like glutaraldehyde or formaldehyde that should freeze the dissociation of catecholamines from Cgs (Borges et al. 2000). Moreover, in experiments on chromaffin cells cultured in astrocyte conditioned media, the phenotype of the chromaffin cells switches to a neuronal-like form. Electron microscopy shows many small vesicles that contain little dense material and with amperometry, exocytosis was observed as secretory spikes that were drastically accelerated (Ardiles et al. 2006), suggesting a close relationship between the presence of the vesicular matrix and the slow kinetics of exocytosis.
Partial Versus Full Release of Solutes During Exocytosis
There was a lot of controversy in the 1980s between the supporters of full fusion and partial fusion, this latter hypothesis was led by Bruno Ceccarelli (Wilkinson and Cole 2001). The presence of partial fusion, where all or part of the content is released during a transitory fusion event, has been reported since then in chromaffin and other cell types (Alvarez de Toledo et al. 1993; Ales et al. 1999; Henkel et al. 2000; Lollike et al. 1998).
It has also been shown that vesicular proteins can be retained in secretory vesicles depending on their size, the size of the fusion pore and the duration of the opening state.
Live cell imaging of fluorescently labeled cargo and granule coat proteins showed that the granule can indeed remain seemingly intact despite an open pore to the outside (Taraska et al. 2003; Perrais et al. 2004; Rutter and Tsuboi 2004; Obermuller et al. 2005; Kasai et al. 2006). The size of this dilated pore has been estimated as 7–12 nm by probing the accessibility of the lumen for molecules up to several hundred kDa (Fulop et al. 2005; Liu et al. 2005). Larger peptides can be released through this pore, albeit slowly (Perrais et al. 2004), or with a delay (Barg et al. 2002), which requires a pore size that is larger than the threshold for irreversible fusion. It has therefore been proposed that the initially narrow pore detected by electrical measurements can expand slowly, thereby acting as a filter for the released cargo based on its molecular size (Kasai et al. 2006; Barg et al. 2002). However, even after release of peptides as large as tissue plasminogen activator (tPA-EGFP, ~100 kDa), the granule often remains intact for a prolonged period (min). Two observations indicate that even this expanded pore can close again: the first, by exploiting the pH dependence of a luminal EGFP-label, the resealing of granules was directly demonstrated by periodic acidification of the external medium (Perrais et al. 2004) and the second, when cells are bathed in fluid phase markers (e.g., rhodamine, horseradish peroxidase) during stimulation, the dye becomes trapped in a fraction of the granules (von Grafenstein and Knight 1992, 1993; Lang et al. 1997; Bauer et al. 2004; Obermuller et al. 2005).
These observations received support from data on chromaffin cells obtained by combining whole cell with cell-attached capacitance. These authors showed that weak stimuli evoked release by transitory fusion, whereas strong stimuli switched the release to the full-fusion mode (Elhamdani et al. 2006).
Another crucial datum comes from the comparison between conventional amperometry and patch amperometry. We have observed using the same batch of chromaffin cells that measurements of vesicle cargo using patch amperometry were 3–5 times larger than when using amperometry. As we made the amperometric recordings with a disk electrode placed onto the cell membrane it is unlikely that the lower amounts of amines detected were caused by partial oxidation of the catecholamines released; thus, the most likely explanation is that the suction applied to the membrane to seal the pipette for patch-amperometry recordings forces exocytosis toward the full-fusion mode (Montesinos et al. 2008, 2010).
The reuse of secretory vesicles will cause a progressive loss of proteins forming the matrix. This fact can offer an explanation for the heterogeneous size and density of vesicles observed in electron microscopy.
It is possible to elaborate a hypothesis from these data that contemplates a bi-phasic release of soluble components from the vesicle depending on their association to the matrix and the relative size of the matrix and the fusion pore.
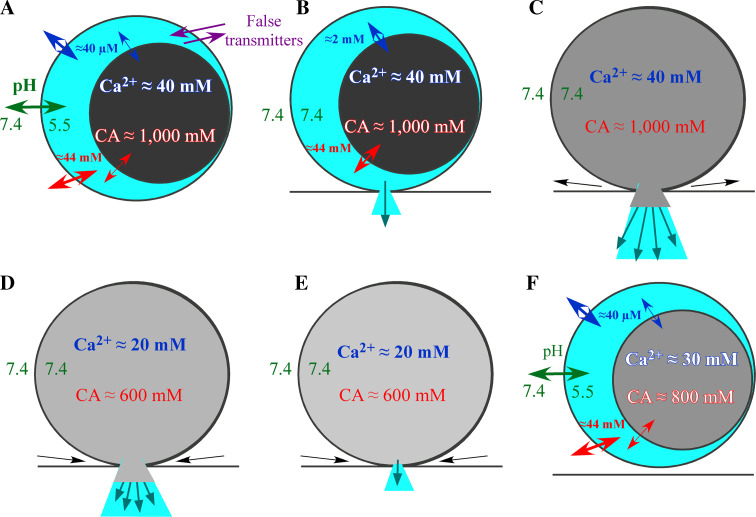
Figure 1 illustrates the proposed model to conciliate all data obtained by monitoring the exocytotic phenomenon. Secretory vesicles from chromaffin cells behave as a bi-compartmental reservoir where most solutes are aggregated to chromogranins forming a matrix. Under resting conditions (a) the halo concentration of catecholamines (CA, in red) and Ca2+ (blue) is held constant with the cytosol due to the pH gradient (green) reached by the H+ pump V-ATPase (not shown). A slower equilibrium also occurs between the matrix and the halo. Permeable weak bases can cross the vesicle membrane and accumulate inside. These substances can displace the natural products and are co-secreted with them. Once the fusion pore is formed (b) the pH gradient collapses and the free catecholamines and Ca2+ start to escape from the vesicle, it causes the pre-spike phenomenon (foot) or the stand-alone foot observed by amperometry and little or nothing affects the major part of these solutes which are fixed to the matrix. The expansion of the fusion pore to form the Ω feature (c) promotes the swelling and expansion of the matrix and the massive release of amines together with a part of the protein content. The loss of vesicular content inverts the situation and the fusion pore starts to collapse, in first place it ends the release of large components like proteins (d) and later to the catecholamines (e). After the fission of the vesicle (f), it rapidly recovers the pH gradient and the concentration of amines and Ca2+ at the halo. However, the loss of chromogranins means a weaker matrix where less amine, Ca2+ and ATP (not shown) are now accumulated. The different dark shades of gray indicate the concentration of chromogranins. The number and length of arrows show the importance of exchanges and flows. The numbers are from bovine chromaffin cells and those for the catecholamines were taken from (Schroeder et al. 1996; Albillos et al. 1992, 1997; Colliver et al. 2000) and those for Ca2+ were from (Moreno et al. 2005; Santodomingo et al. 2008; Yoo et al. 2001; Yoo 2010).
Fig. 1.
Sequential stages of exocytosis of LDCV and its regulation by intravesicular components. Explanation in the text (Color figure online)
In the past several years the mechanisms that govern exocytosis have been deeply studied. However, most of the efforts were put on the fusion machinery present both in vesicular and cell membranes and little in the intravesicular factors. In this brief review, we have tried to summarize some recent experimental evidence that highlight the importance of soluble components that constitute the intravesicular cocktail in the regulation of the exocytotic performance.
Abbreviation
- Cgs
Chromogranins
Footnotes
A commentary to this article can be found at doi:10.1007/s10571-010-9610-0.
References
- Albillos A, Abad F, Garcia AG (1992) Cross-talk between M2 muscarinic and D1 dopamine receptors in the cat adrenal medulla. Biochem Biophys Res Commun 183:1019–1024 [DOI] [PubMed] [Google Scholar]
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M (1997) The exocytotic event in chromaffin cells revealed by patch amperometry. Nature 389:509–512 [DOI] [PubMed] [Google Scholar]
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G (1999) High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol 1:40–44 [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM (1993) Release of secretory products during transient vesicle fusion. Nature 363:554–558 [DOI] [PubMed] [Google Scholar]
- Amatore C, Bouret Y, Travis ER, Wightman RM (2000) Adrenaline release by chromaffin cells: constrained swelling of the vesicle matrix leads to full fusion. Angew Chem Int Ed 39:1952–1955 [DOI] [PubMed] [Google Scholar]
- Ardiles AO, Maripillan J, Lagos VL, Toro R, Mora IG, Villarroel L, Ales E, Borges R, Cardenas AM (2006) A rapid exocytosis mode in chromaffin cells with a neuronal phenotype. J Neurochem 99:29–41 [DOI] [PubMed] [Google Scholar]
- Barg S, Olofsson CS, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renstrom E, Rorsman P (2002) Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron 33:287–299 [DOI] [PubMed] [Google Scholar]
- Bauer RA, Khera RS, Lieber JL, Angleson JK (2004) Recycling of intact dense core vesicles in neurites of NGF-treated PC12 cells. FEBS Lett 571:107–111 [DOI] [PubMed] [Google Scholar]
- Borges R, Machado JD, Alonso C, Brioso MA, Gomez JF (2000) Functional role of chromogranins. The intragranular matrix in the last phase of exocytosis. Adv Exp Med Biol 482:69–81 [DOI] [PubMed] [Google Scholar]
- Borges R, Machado JD, Betancor G, Camacho M (2002) Pharmacological regulation of the late steps of exocytosis. Ann NY Acad Sci 971:184–192 [DOI] [PubMed] [Google Scholar]
- Bulenda D, Gratzl M (1985) Matrix free Ca2+ in isolated chromaffin vesicles. Biochemistry 24:7760–7765 [DOI] [PubMed] [Google Scholar]
- Camacho M, Machado JD, Montesinos MS, Criado M, Borges R (2006) Intragranular pH rapidly modulates exocytosis in adrenal chromaffin cells. J Neurochem 96:324–334 [DOI] [PubMed] [Google Scholar]
- Camacho M, Machado JD, Alvarez J, Borges R (2008) Intravesicular calcium release mediates the motion and exocytosis of secretory organelles: a study with adrenal chromaffin cells. J Biol Chem 283:22383–22389 [DOI] [PubMed] [Google Scholar]
- Colliver TL, Pyott SJ, Achalabun M, Ewing AG (2000) VMAT-mediated changes in quantal size and vesicular volume. J Neurosci 20:5276–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crout JR, Muskus AJ, Trendelenburg U (1962) Effect of tyramine on isolated guinea-pig atria in relation to their noradrenaline stores. Br J Pharmacol Chemother 18:600–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Vera J, Morales YG, Hernandez-Fernaud J, Camacho M, Montesinos MS, Calegari F, Huttner WB, Borges R, Machado JD (2010) Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles. J Neurosci 30:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhamdani A, Azizi F, Artalejo CR (2006) Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci 26:3030–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkensammer G, Fischer-Colbrie R, Winkler H (1985) Biogenesis of chromaffin granules: incorporation of sulfate into chromogranin B and into a proteoglycan. J Neurochem 45:1475–1480 [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH (1997) Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron 19:1271–1283 [DOI] [PubMed] [Google Scholar]
- Fulop T, Radabaugh S, Smith C (2005) Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci 25:7324–7332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt G, Adams RN (1982) Determination of diffusion-coefficients by flow-injection analysis. Anal Chem 54:2618–2620 [Google Scholar]
- Gubernator NG, Zhang H, Staal RG, Mosharov EV, Pereira DB, Yue M, Balsanek V, Vadola PA, Mukherjee B, Edwards RH, Sulzer D, Sames D (2009) Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals. Science 324:1441–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez I, Kisler K, Berberian K, Dernick G, Valero V, Yong MG, Craighead HG, Lindau M (2005) Electrochemical imaging of fusion pore openings by electrochemical detector arrays. Proc Natl Acad Sci USA 102:13879–13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle KB, Reed RK, Pihl KE, Serck-Hanssen G (1985) Osmotic properties of the chromogranins and relation to osmotic pressure in catecholamine storage granules. Acta Physiol Scand 123:21–33 [DOI] [PubMed] [Google Scholar]
- Henkel AW, Meiri H, Horstmann H, Lindau M, Almers W (2000) Rhythmic opening and closing of vesicles during constitutive exo- and endocytosis in chromaffin cells. EMBO J 19:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP, Sagne C, Bedet C, Gasnier B (1998) The vesicular monoamine transporter: from chromaffin granule to brain. Neurochem Int 32:227–246 [DOI] [PubMed] [Google Scholar]
- Kasai H, Kishimoto T, Nemoto T, Hatakeyama H, Liu TT, Takahashi N (2006) Two-photon excitation imaging of exocytosis and endocytosis and determination of their spatial organization. Adv Drug Deliv Rev 58:850–877 [DOI] [PubMed] [Google Scholar]
- Lang T, Wacker I, Steyer J, Kaether C, Wunderlich I, Soldati T, Gerdes HH, Almers W (1997) Ca2+-triggered peptide secretion in single cells imaged with green fluorescent protein and evanescent-wave microscopy. Neuron 18:857–863 [DOI] [PubMed] [Google Scholar]
- Liu TT, Kishimoto T, Hatakeyama H, Nemoto T, Takahashi N, Kasai H (2005) Exocytosis and endocytosis of small vesicles in PC12 cells studied with TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis. J Physiol 568:917–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollike K, Borregaard N, Lindau M (1998) Capacitance flickers and pseudoflickers of small granules, measured in the cell-attached configuration. Biophys J 75:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado JD, Segura F, Brioso MA, Borges R (2000) Nitric oxide modulates a late step of exocytosis. J Biol Chem 275:20274–20279 [DOI] [PubMed] [Google Scholar]
- Machado JD, Morales A, Gomez JF, Borges R (2001) cAMP modulates exocytotic kinetics and increases quantal size in chromaffin cells. Mol Pharmacol 60:514–520 [PubMed] [Google Scholar]
- Machado JD, Alonso C, Morales A, Gomez JF, Borges R (2002a) Nongenomic regulation of the kinetics of exocytosis by estrogens. J Pharmacol Exp Ther 301:631–637 [DOI] [PubMed] [Google Scholar]
- Machado JD, Gomez JF, Betancor G, Camacho M, Brioso MA, Borges R (2002b) Hydralazine reduces the quantal size of secretory events by displacement of catecholamines from adrenomedullary chromaffin secretory vesicles. Circ Res 91:830–836 [DOI] [PubMed] [Google Scholar]
- Machado JD, Camacho M, Alvarez J, Borges R (2009) On the role of intravesicular calcium in the motion and exocytosis of secretory organelles. Commun Integr Biol 2:71–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek PE, Markin VS, Tanaka T, Kawaguchi H, Fernandez JM (1995) The secretory granule matrix-electrolyte interface: a homologue of the p-n rectifying junction. Biophys J 69:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MS, Machado JD, Camacho M, Diaz J, Morales YG, Alvarez de la Rosa D, Carmona E, Castaneyra A, Viveros OH, O’Connor DT, Mahata SK, Borges R (2008) The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci 28:3350–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos MS, Camacho M, Machado JD, Viveros OH, Beltran B, Borges R (2010) The quantal secretion of catecholamines is impaired by the accumulation of beta-adrenoceptor antagonists into chromaffin cell vesicles. Br J Pharmacol 159:1548–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Lobaton CD, Santodomingo J, Vay L, Hernandez-SanMiguel E, Rizzuto R, Montero M, Alvarez J (2005) Calcium dynamics in catecholamine-containing secretory vesicles. Cell Calcium 37:555–564 [DOI] [PubMed] [Google Scholar]
- Mundorf ML, Hochstetler SE, Wightman RM (1999) Amine weak bases disrupt vesicular storage and promote exocytosis in chromaffin cells. J Neurochem 73:2397–2405 [DOI] [PubMed] [Google Scholar]
- Nelson N, Harvey WR (1999) Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev 79:361–385 [DOI] [PubMed] [Google Scholar]
- Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S (2005) Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci 118:4271–4282 [DOI] [PubMed] [Google Scholar]
- Perrais D, Kleppe IC, Taraska JW, Almers W (2004) Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J Physiol 560:413–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippu A, Schumann HJ (1965) Effect of alpha-methyldopa, alpha-methyldopamine, and alpha-methyl-norepinephrine on the norepinephrine content of the isolated heart. Life Sci 4:2039–2046 [DOI] [PubMed] [Google Scholar]
- Pihel K, Travis ER, Borges R, Wightman RM (1996) Exocytotic release from individual granules exhibits similar properties at mast and chromaffin cells. Biophys J 71:1633–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter GA, Tsuboi T (2004) Kiss and run exocytosis of dense core secretory vesicles. Neuroreport 15:79–81 [DOI] [PubMed] [Google Scholar]
- Santodomingo J, Vay L, Camacho M, Hernandez-Sanmiguel E, Fonteriz RI, Lobaton CD, Montero M, Moreno A, Alvarez J (2008) Calcium dynamics in bovine adrenal medulla chromaffin cell secretory granules. Eur J Neurosci 28:1265–1274 [DOI] [PubMed] [Google Scholar]
- Schroeder TJ, Borges R, Finnegan JM, Pihel K, Amatore C, Wightman RM (1996) Temporally resolved, independent stages of individual exocytotic secretion events. Biophys J 70:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S (1993) Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem 60:527–535 [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433 [DOI] [PubMed] [Google Scholar]
- Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W (2003) Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci USA 100:2070–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grafenstein H, Knight DE (1992) Membrane recapture and early triggered secretion from the newly formed endocytotic compartment in bovine chromaffin cells. J Physiol 453:15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grafenstein H, Knight DE (1993) Triggered exocytosis and endocytosis have different requirements for calcium and nucleotides in permeabilized bovine chromaffin cells. J Membr Biol 134:1–13 [DOI] [PubMed] [Google Scholar]
- Wilkinson RS, Cole JC (2001) Resolving the Heuser-Ceccarelli debate. Trends Neurosci 24:195–197 [DOI] [PubMed] [Google Scholar]
- Winkler H, Westhead E (1980) The molecular organization of adrenal chromaffin granules. Neuroscience 5:1803–1823 [DOI] [PubMed] [Google Scholar]
- Yoo SH (2010) Secretory granules in inositol 1,4,5-trisphosphate-dependent Ca2+ signaling in the cytoplasm of neuroendocrine cells. FASEB J 24:653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Oh YS, Kang MK, Huh YH, So SH, Park HS, Park HY (2001) Localization of three types of the inositol 1,4,5-trisphosphate receptor/Ca(2+) channel in the secretory granules and coupling with the Ca(2+) storage proteins chromogranins A and B. J Biol Chem 276:45806–45812 [DOI] [PubMed] [Google Scholar]