Abstract
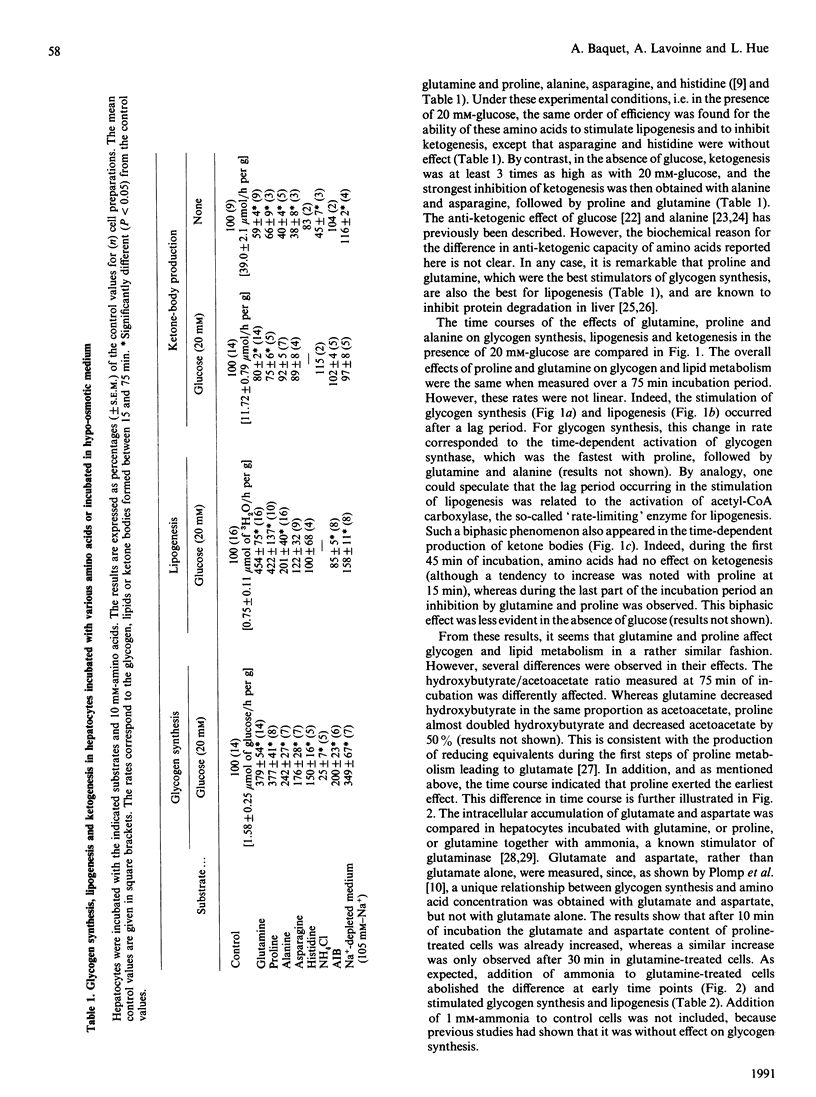
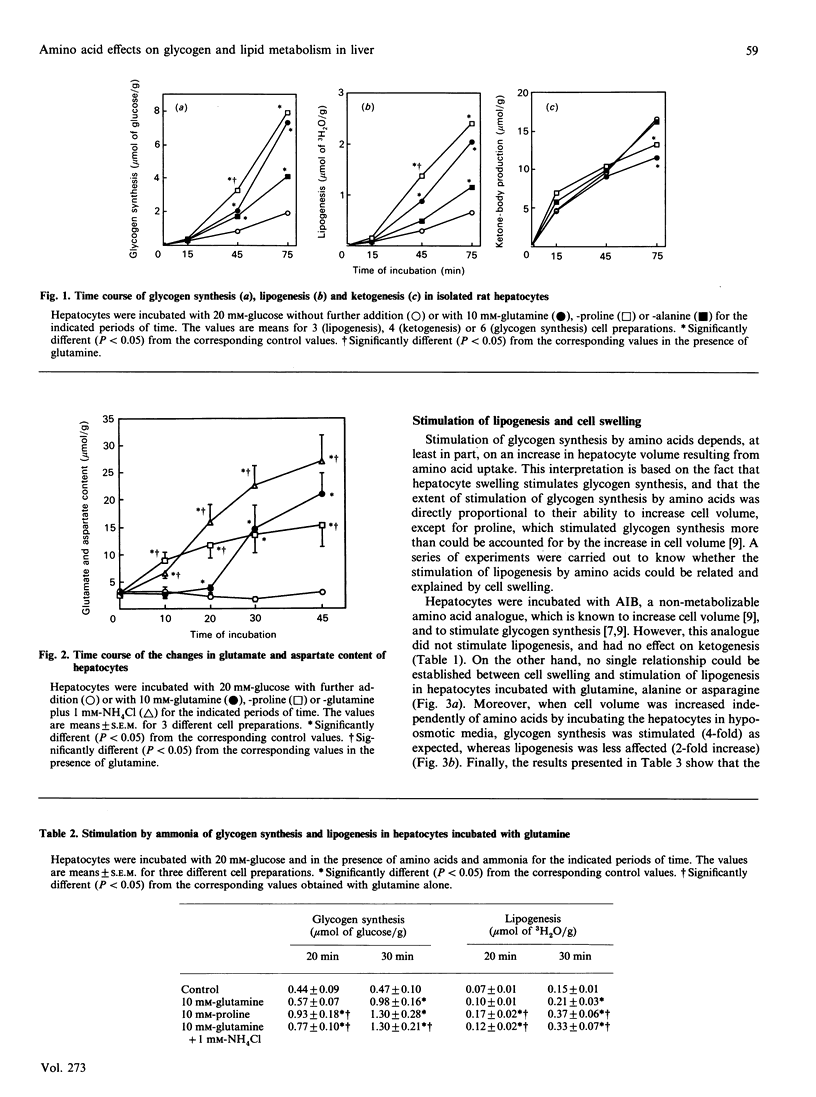
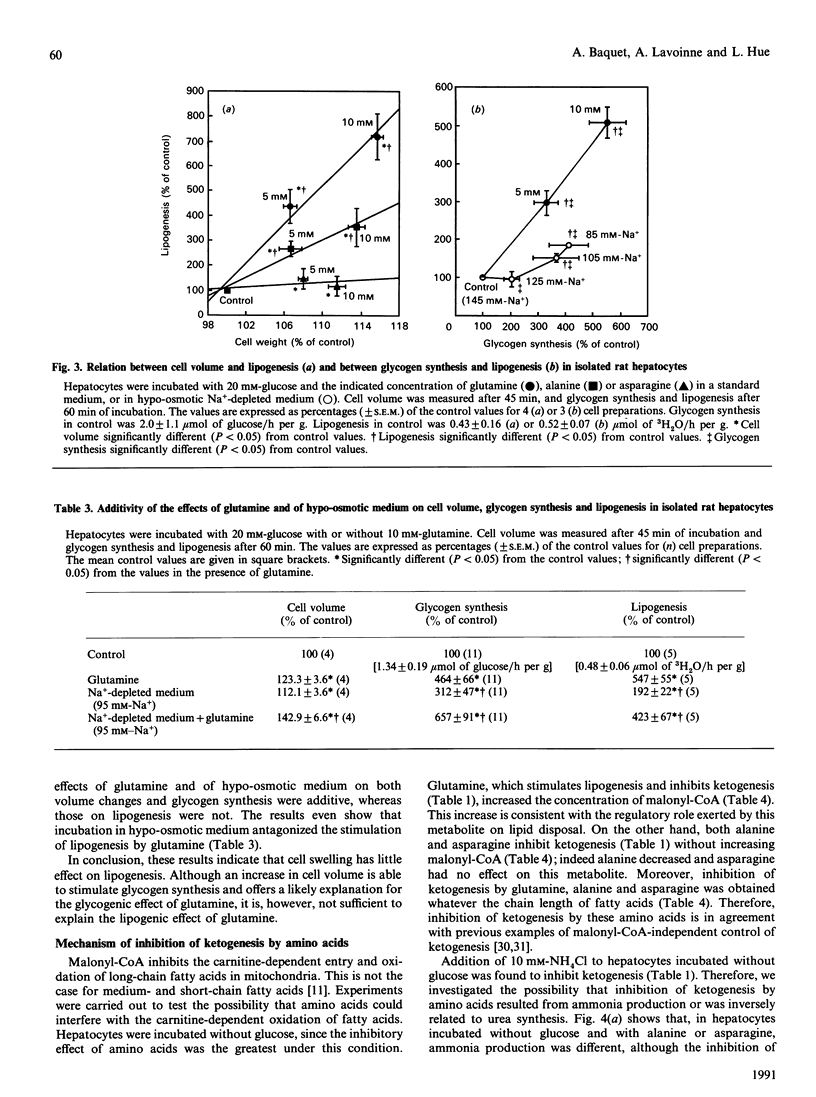
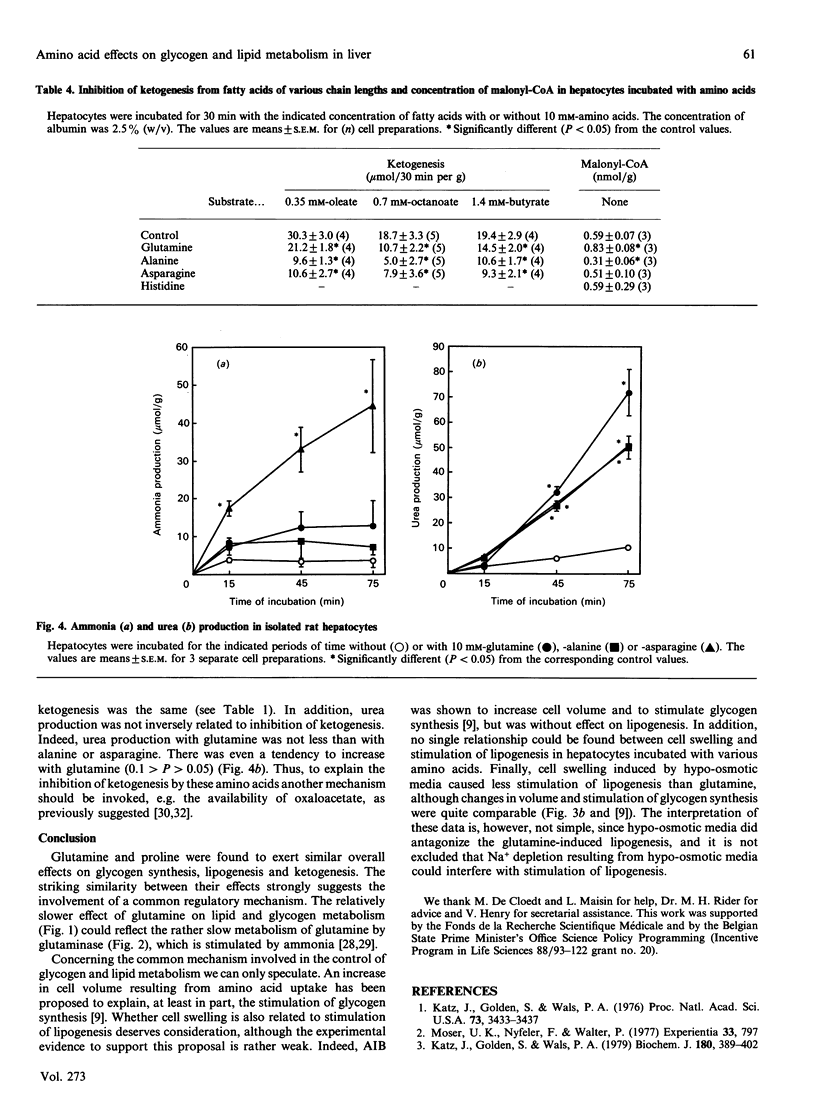
Several amino acids were found to stimulate glycogen synthesis and lipogenesis, and to inhibit ketogenesis in isolated rat hepatocytes. When hepatocytes were incubated in the presence of 20 mM-glucose, the amino acids could be classified in decreasing order of efficiency as follows: glutamine and proline, alanine, aminoisobutyric acid, asparagine and histidine for stimulation of glycogen synthesis; glutamine, proline and alanine for stimulation of lipogenesis; proline and glutamine for inhibition of ketogenesis. The study of the time course revealed that the rates were not linear and were preceded by a lag period. In all conditions studied, glutamine and proline were found to have similar quantitative effects on glycogen synthesis and lipid metabolism. However, their effects differ qualitatively. Indeed, the effects of proline on glycogen synthesis, lipogenesis and glutamate and aspartate content were faster. Moreover, proline increased the hydroxybutyrate/acetoacetate ratio, whereas glutamine did not change it. Incubation of hepatocytes with aminoisobutyric acid or under hypo-osmotic conditions, which increased cell volume and mimicked the amino acid-induced stimulation of glycogen synthesis, had little effect on lipogenesis. In hepatocytes incubated without glucose, ketogenesis was inhibited, in decreasing order of efficiency, by alanine, asparagine, glutamine and proline. Under these conditions, glutamine increased, alanine decreased and asparagine did not affect the concentration of malonyl-CoA. This indicates that the latter cannot be responsible for the inhibition of ketogenesis by alanine and asparagine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baquet A., Hue L., Meijer A. J., van Woerkom G. M., Plomp P. J. Swelling of rat hepatocytes stimulates glycogen synthesis. J Biol Chem. 1990 Jan 15;265(2):955–959. [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. H., Plomp P. J., Leverve X. M., Meijer A. J. A combination of intracellular leucine with either glutamate or aspartate inhibits autophagic proteolysis in isolated rat hepatocytes. Eur J Biochem. 1989 May 15;181(3):717–720. doi: 10.1111/j.1432-1033.1989.tb14782.x. [DOI] [PubMed] [Google Scholar]
- Chen K. S., Lardy H. A. Multiple requirements for glycogen synthesis by hepatocytes isolated from fasted rats. J Biol Chem. 1985 Nov 25;260(27):14683–14688. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Decaux J. F., Ferré P., Robin D., Robin P., Girard J. Decreased hepatic fatty acid oxidation at weaning in the rat is not linked to a variation of malonyl-CoA concentration. J Biol Chem. 1988 Mar 5;263(7):3284–3289. [PubMed] [Google Scholar]
- EVANS W. H., MUELLER P. S. EFFECTS OF PALMITATE ON THE METABOLISM OF LEUKOCYTES FROM GUINEA PIG EXUDATE. J Lipid Res. 1963 Jan;4:39–45. [PubMed] [Google Scholar]
- Hensgens H. E., Meijer A. J., Williamson J. R., Gimpel J. A., Tager J. M. Prolone metabolism in isolated rat liver cells. Biochem J. 1978 Mar 15;170(3):699–707. doi: 10.1042/bj1700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Felíu J. E., Hers H. G. Control of gluconeogenesis and of enzymes of glycogen metabolism in isolated rat hepatocytes. A parallel study of the effect of phenylephrine and of glucagon. Biochem J. 1978 Dec 15;176(3):791–797. doi: 10.1042/bj1760791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., McGivan J. D. The effect of ammonium chloride and glucagon on the metabolism of glutamine in isolated liver cells from starved rats. Biochim Biophys Acta. 1978 Sep 21;543(1):16–28. doi: 10.1016/0304-4165(78)90450-6. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., McGivan J. D. The effects of ammonium chloride and bicarbonate on the activity of glutaminase in isolated liver mitochondria. Biochem J. 1978 Dec 15;176(3):837–844. doi: 10.1042/bj1760837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Stimulation of hepatic glycogen synthesis by amino acids. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3433–3437. doi: 10.1073/pnas.73.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The regulation of the release of ketone bodies by the liver. Adv Enzyme Regul. 1966;4:339–354. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- Lavoinne A., Baquet A., Hue L. Stimulation of glycogen synthesis and lipogenesis by glutamine in isolated rat hepatocytes. Biochem J. 1987 Dec 1;248(2):429–437. doi: 10.1042/bj2480429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C. Purification and crystallization of rat liver fatty acid synthetase. Arch Biochem Biophys. 1981 Jul;209(2):613–619. doi: 10.1016/0003-9861(81)90320-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- Mortimore G. E., Wert J. J., Jr, Adams C. E. Modulation of the amino acid control of hepatic protein degradation by caloric deprivation. Two modes of alanine co-regulation. J Biol Chem. 1988 Dec 25;263(36):19545–19551. [PubMed] [Google Scholar]
- Nosadini R., Datta H., Hodson A., Alberti K. G. A possible mechanism for the anti-ketogenic action of alanine in the rat. Biochem J. 1980 Aug 15;190(2):323–332. doi: 10.1042/bj1900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp P. J., Boon L., Caro L. H., van Woekom G. M., Meijer A. J. Stimulation of glycogen synthesis in hepatocytes by added amino acids is related to the total intracellular content of amino acids. Eur J Biochem. 1990 Jul 20;191(1):237–243. doi: 10.1111/j.1432-1033.1990.tb19115.x. [DOI] [PubMed] [Google Scholar]
- Riou J. P., Beylot M., Laville M., De Parscau L., Delinger J., Sautot G., Mornex R. Antiketogenic effect of glucose per se in vivo in man and in vitro in isolated rat liver cells. Metabolism. 1986 Jul;35(7):608–613. doi: 10.1016/0026-0495(86)90165-4. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Possible role for carbamyl phosphate in the control of liver glycogen synthesis. Biochem Biophys Res Commun. 1985 Jul 16;130(1):229–233. doi: 10.1016/0006-291x(85)90406-1. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Kientsch-Engel R. I., Wieland O. H. Role of free oxaloacetate in ketogenesis. Derivation from the direct measurement of mitochondrial [3-hydroxybutyrate]/[acetoacetate] ratio in hepatocytes. Eur J Biochem. 1982 Jan;121(3):493–499. doi: 10.1111/j.1432-1033.1982.tb05814.x. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I. Effects of L-alanine on ketogenesis in vitro. Biochim Biophys Acta. 1982 Aug 6;717(2):385–386. doi: 10.1016/0304-4165(82)90193-3. [DOI] [PubMed] [Google Scholar]


