Abstract
The wrapping of intracellular mature vaccinia virions by modified trans-Golgi or endosomal cisternae to form intracellular enveloped virions is dependent on at least two viral proteins encoded by the B5R and F13L open reading frames. B5R is a type I integral membrane glycoprotein, whereas F13L is an unglycosylated, palmitylated protein with a motif that is conserved in a superfamily of phospholipid-metabolizing enzymes. Microscopic visualization of the F13L protein was achieved by fusing it to the enhanced green fluorescent protein (GFP). F13L-GFP was functional when expressed by a recombinant vaccinia virus in which it replaced the wild-type F13L gene or by transfection of uninfected cells with a plasmid vector followed by infection with an F13L deletion mutant. In uninfected or infected cells, F13L-GFP was associated with Golgi cisternae and post-Golgi vesicles containing the LAMP 2 late endosomal-lysosomal marker. Association of F13L-GFP with vesicles was dependent on an intact phospholipase catalytic motif and sites of palmitylation. The B5R protein was also associated with LAMP2-containing vesicles when F13L-GFP was coexpressed, but was largely restricted to Golgi cisternae in the absence of F13L-GFP or when the F13L moiety was mutated. We suggest that the F13L protein, like its human phospholipase D homolog, regulates vesicle formation and that this process is involved in intracellular enveloped virion membrane formation.
Poxviruses are large, enveloped DNA viruses that replicate within the cytoplasm of vertebrate or invertebrate cells (38). Vaccinia virus, a member of the Orthopoxvirus genus, is the best-characterized poxvirus and has served as the vaccine for smallpox and as a widely used expression vector (37). The double-stranded DNA genome of vaccinia virus contains nearly 200,000 bp and approximately 200 functional open reading frames (ORFs) (17). The initial steps in vaccinia virus morphogenesis involve the formation of crescent membranes that enclose granular viroplasm to form spherical immature virions, which develop into brick-shaped, infectious intracellular mature virions (IMV) (8, 20, 27, 36, 57). Some of the IMV are wrapped with an additional double membrane derived from trans-Golgi or late endosomal cisternae (25, 53, 61) to form the intracellular enveloped virions (IEV). The microtubule cytoskeleton is involved in the formation and movement of both IMV and IEV (43, 51, 66). At the cell periphery, the outermost viral membrane fuses with the plasma membrane to form extracellular virions. The particles called cell-associated enveloped virions (CEV) that remain attached to the cell and those called extracellular enveloped virions (EEV) that are shed into the medium promote cell-to-cell and long-range spread, respectively (4, 41). Cell-to-cell spread is facilitated by the attachment of actin tails to the IEV or CEV, leading to the formation of virus-tipped microvilli (7, 15, 58, 68).
The membrane-wrapping events that form IEV have been the subject of numerous investigations. Proteins encoded by seven viral genes have been identified as specific components of IEV, CEV, or EEV membranes. Five of these, A33R, A34R, A36R, A56R, and B5R, are glycoproteins, whereas F12L and F13L are unglycosylated (11, 13, 28, 40, 42, 48, 54, 64, 72). Recent studies indicated that the A36R protein is present only in the outer IEV membrane, which is not retained on extracellular virions (64). Deletion of the gene encoding any of the seven proteins except A56R produces a small-plaque phenotype. The membrane-wrapping step is inhibited in cells infected with F13L and B5R deletion mutants (3, 14, 67), whereas later steps, including actin tail formation, are blocked when A33R, A34R, or A36R is not expressed (46, 50, 68, 71). Physical interactions among the A33R, A34R, A36R, and B5R proteins have been reported (49, 70).
The two proteins, B5R and F13L, required for membrane wrapping are very different structurally and functionally. B5R is a 42-kDa type I integral membrane component of the EEV (13, 28). Several studies with infected cells showed that removal or replacement of either the lumenal domain or the cytoplasmic tail of B5R had no effect on the incorporation of the mutated protein into EEV (23, 29, 33, 35). In the absence of other viral proteins, the B5R protein was targeted to Golgi cisternae (29, 34, 65). Under these conditions, Golgi membrane localization was dependent on the transmembrane domain and enhanced by plasma membrane retrieval signals in the cytoplasmic tail (65).
The 42-kDa F13L protein, sometimes called p37 because of its apparent mobility as a 37-kDa protein on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), is the most abundant component of the EEV envelope (25, 26). The resistance of EEV-associated F13L protein to protease digestion suggested that it was present on the inner surface of the EEV envelope (54). In infected cells, the F13L protein was localized on trans-Golgi and IEV membranes (25, 53). The protein is modified by palmitylation of cysteines 185 and 186, which mediates hydrophobicity and membrane association (22, 24, 25, 54). Nonpalmitylated F13L protein, expressed by a transfection-infection protocol, exhibited diffuse cytoplasmic staining (21). There was juxtanuclear and diffuse cytoplasmic F13L immunostaining in a stably transfected rabbit kidney cell line that was capable of rescuing an F13L deletion mutant (5), but only diffuse immunostaining when F13L was expressed by a Semliki Forest virus vector (34). In both cases, the authors attributed poor intracellular localization of the F13L protein to a partial defect in palmitylation or the absence of other viral proteins.
The role of the F13L protein in membrane wrapping is not understood. However, F13L contains variant HKD (His-Lys-Asp) motifs that are conserved in a superfamily of phospholipases and phospholipid synthases (30, 44, 60), and there has been a report of lipase activity associated with recombinant F13L protein (1). Furthermore, mutant vaccinia viruses with substitutions of either the conserved Lys or Asp exhibited wrapping defects that inhibited IEV formation (47, 60). These results are intriguing because phospholipase D regulates the budding of vesicles from trans-Golgi membranes (2, 6, 16, 31, 55, 56, 59).
In the present study we investigated the localization of the F13L and B5R proteins in post-Golgi vesicles. Visualization of the F13L protein was achieved by fusing it to the enhanced green fluorescent protein (GFP). GFP is particularly suitable as a fluorescent reporter because of its rapid folding and compact structure (63). GFP linked to either the vesicular stomatitis virus G protein or the vaccinia virus B5R protein did not interfere with their normal membrane localization and transport in transfected cells (45, 65). Moreover, a recombinant virus in which B5R-GFP replaced B5R had a normal phenotype (66).
Similarly, we show here that F13L-GFP is functional whether expressed by a recombinant vaccinia virus in which it replaced the wild-type F13L gene or by transfection of uninfected cells with a plasmid vector followed by infection with an F13L deletion mutant. In both uninfected and infected cells, we found that F13L-GFP containing an intact phospholipase motif was associated with Golgi cisternae and vesicles containing a late endosomal-lysosomal marker and induced vesicle colocalization of the B5R glycoprotein.
MATERIALS AND METHODS
Cells and viruses.
HeLa, RK-13, and BSC-1 cells were grown in Dulbecco's modified Eagle's medium or Eagle's modified medium supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. The mutant vF13LΔ, previously referred to as vRB12 (3), has most of the F13L gene replaced with the Escherichia coli xanthine guanine phosphoribosyltransferase (gpt) gene. Recombinant vF13L-GFP, constructed in this study, contains the GFP coding sequence at the C terminus of the F13L ORF. For virus titration and analysis of plaque size, infected BSC-1 monolayers were fixed and stained with 0.1% crystal violet in 20% ethanol.
Plasmid construction.
The oligonucleotide primers used to generate PCR products are listed below with restriction endonuclease cleavage sites in italics and the F13L start codon, termination codon, and point mutations in bold: F13L-LF-F, TATTATTATCGGTTTACGGATGAAAAAT; F13L-LF-R, TTCAAATGTTGTTAAATGATCGGATCT; F13L-RF-F, GCGGCCGCTTAAAAATTTAAAAAAAAGAAAATAGAGACG; F13L-RF-R, GGATCCCGGTAACACATCAATTTCGGG; F13L-UF, TATAGAAATAGACGAAGATGACATCATGG; F13L-DF, GCCTTCTTCATTTCGTGCCAA; F13L-463, CCGCTGGCCACAGATCTTCGTAG; GFP-265, CTCCTGGACGTCGCCTTCGGG; F13L-K314R-F, CAGAATAATACAAGATTGTTGATAGTCGACGACG AA; F13L-K314R-R, GACTATCAACAATCTTGTATTATTCTGAAT; F13L-D319E-F, TTGTTGATAGTCGAAGACGAATATGTTCAT; F13L-D319E-R, AACATATTCGTCTTCGACTATCAACAATTTTGT; F13L-1, ATGTGGCCATTTGCATCGGTA CCT; F13L-C185S-F, GCGGCTTCTTGTTTGCCAGTTAGCAC; F13L-C185S-R, GCAAACAAGAAGCCGCAGAGCA; F13L-C186S-F, GCGGCTTGTTCTTTGCCAGTTAGCAC; F13L-C186S-R, GCAAAGAACAAGCCGCAGAGCA; and F13L-937, AACATATTCGTCGTCGACTATCAACAATTTTGT. Platinum PCR supermix and PCR Supermix High Fidelity (Gibco) were used to amplify DNA fragments. PCR products were separated by electrophoresis in a 0.8 to 1.2% low- or high-melting-point agarose (Gibco) gel. Portions of gel with the desired DNA fragments were excised and eluted with 10 mM Tris (pH 8.0) using a Qiaex II gel extraction kit (Qiagen).
PCR was used to add an NcoI site to the 3′ end of the F13L ORF in plasmid pSG5-F13L (29) so that an NcoI-BamHI fragment containing GFP from plasmid pCDM8 could be ligated in frame to produce pSG5-F13LGFP (abbreviated as pF13L-GFP).
For making the recombinant virus expressing F13L-GFP, DNA segments of approximately 600 bp on either side of the F13L ORF were amplified by PCR using the F13L-LF-F/F13L-LF-R and F13L-RF-F/F13L-RF-R primer pairs and vaccinia virus WR genomic DNA as the template. PCR fragments were cloned into plasmid pGEM-T-easy and sequenced. EcoRI-KpnI left-flank DNA fragment, NotI-BamHI right-flank DNA fragment from pGEM-T-easy, and KpnI-NotI F13L-GFP fragment from plasmid pF13L-GFP were inserted at the EcoRI and BamHI sites of plasmid pGEM7 (Promega) by four-fragment ligation, yielding plasmid pGF13L. The presence of all the three DNA fragments in plasmid pGF13L was confirmed by a series of restriction endonuclease digestions. Point mutations in the F13L coding sequence were introduced by standard two-stage PCR. To construct pF13L(K314R)-GFP, in which Lys was replaced with Arg, and pF13L(D319E)-GFP, in which Asp was replaced with Glu, PCR fragments containing a one-base mutation for each construct were amplified from plasmid pF13L-GFP using primer pairs F13L-463/F13L-K314R-R and F13L-K314R-F/GFP-265, and pairs F13L-463/F13L-D319E-R and F13L-D319E-F/GFP-265. These fragments were joined by a second PCR using primer pair F13L-463/GFP-265 and cloned in plasmid pGEM-T-Easy, and the mutation was confirmed by sequencing. Plasmid pF13L-GFP had more than one NcoI site, so it was digested with restriction enzymes NotI and SpeI to release a 1,157-bp fragment that was subsequently digested with NcoI to release a 423-bp SpeI-NcoI fragment and a 734-bp NcoI-NotI GFP coding sequence. SpeI-NcoI fragments containing the point mutation from pGEM-T-Easy and NcoI-NotI GFP fragment were ligated together with plasmid pF13L-GFP previously cleaved with SpeI and NotI. Similarly, point mutations F13L(C185S)-GFP and F13L(C186S)-GFP, in which the Cys residues at positions 185 and 186, respectively, were changed to Ser, were introduced by PCR. The PCR fragments were amplified from pF13LGFP using primer pairs F13L-1/F13L-C185S-R and F13L-C185S-F/F13L-937 and pairs F13L-1/F13L-C186S-R and F13L-C186S-F/F13L-937 and joined by a second PCR using primer pair F13L-1/F13L-937. Second PCR products were digested with KpnI and SpeI to release a 674-bp fragment and cloned in pF13L-GFP that had been cleaved with KpnI and SpeI. The final constructs were sequenced to confirm mutations using primer F13L-937. PCR fragments cloned in plasmid pGEM-T-Easy were sequenced using M13 forward and reverse primers (Promega).
Restriction digestion of PCR fragments and plasmids was routinely carried out at 37°C for ∼20 h and 3 to 4 h, respectively. Ligations were done at 4°C overnight. All plasmids for cloning and sequencing were prepared using the Wizard DNA purification system (Promega).
Construction of vF13L-GFP.
HeLa cells were infected with vF13LΔ and immediately transfected with plasmid pGF13L. After 3 days at 37°C, cells were harvested, and the lysate was analyzed by plaque assay on BSC-1 cells. After 2 days, the plates were examined with a fluorescence microscope, and large green plaques were picked, plaque purified, and amplified as described (12).
Antibodies.
A Golgi sampler kit containing mouse monoclonal antibodies (MAbs) to marker proteins of cis- and trans-Golgi compartments and EEA1 was purchased from Transduction Laboratories. Rabbit anti-β-COP polyclonal antibody was purchased from Affinity Bioreagent Inc. Secondary-antibody conjugates were purchased from Jackson ImmunoResearch Laboratories. Rabbit polyclonal antibodies recognizing full-length GFP and mouse anti-GFP MAb were purchased from Clonetech Laboratories and Covance Co., respectively. The 192C rat MAb against B5R has been described (53). Mouse anti-LAMP2 MAb was a kind gift from Thomas August, Johns Hopkins School of Medicine, Baltimore, Md.
Transfection and infection.
HeLa cells were transfected with plasmids using Lipofectamine (LF) 2000 (Gibco-BRL) according to the instructions of the manufacturer. Briefly, cells were grown on glass coverslips (22 by 22 mm) till they reached 80% confluence. Routinely, 10 μg of LF 2000 and 2 μg of DNA were diluted separately in Opti-MEM I medium (Gibco-BRL), mixed, and incubated at room temperature for 20 min. The LF 2000-DNA complex was added to the cells; after 5 h at 37°C, the overlay was replaced with fresh Opti-MEM I, and the incubation was continued for a total of 24 h. Plasmid DNA for transfection was prepared using Qiagen plasmid midipreparation kit. For infection, virus stocks were diluted in the culture medium with 2.5% FBS and added to the cell monolayers in the wells or coverslips. After 2 h of incubation at 37°C, the virus inoculum was replaced with fresh culture medium (2.5% FBS) and incubated for a further 17 to 18 h.
Immunoblotting and immunoprecipitation.
For immunoblotting, transfected or virus-infected HeLa cells were harvested and washed once with phosphate-buffered saline (PBS). The cells were resuspended in sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 0.02% [wt/vol] bromophenol blue, 10% [vol/vol] glycerol, and 5% [vol/vol] β-mercaptoethanol) and heated for 10 min at 95°C. Proteins were resolved by electrophoresis in an SDS–12% polyacrylamide gel and transferred to a nitrocellulose membrane. Protein-free sites of the membrane were blocked by incubation overnight with 3% (wt/vol) nonfat milk protein in PBS. The membrane was incubated with mouse anti-GFP MAb, followed by horseradish peroxidase-conjugated anti-mouse immunoglobulin (Ig) antibody, both diluted in 1% nonfat milk for 1 h at room temperature with constant shaking. The membrane was washed extensively with PBS containing 0.05% (wt/vol) Tween 20 before incubation with each antibody and developed with a chemiluminescence kit (Pierce) according to the manufacturer's procedure.
For immunoprecipitation, transfected or infected HeLa cells were pulse labeled with 200 or 50 μCi of [35S]methionine per ml, respectively, for 2 h at 37°C. Cells were harvested and lysed in PBS with three freeze-thaw cycles. An equal volume of 2× radioimmunoprecipitation assay (RIPA) buffer (1× RIPA buffer is 1% [wt/vol] sodium deoxycholate, 1% [vol/vol] Triton X-100, 0.2% [wt/vol] SDS, 150 mM sodium chloride, 50 mM Tris-HCl [pH 7.4], and 1 mM phenylmethylsulfonyl fluoride) was added to the cell lysate and incubated on ice for 15 min. The samples were then heated at 70°C for 2 min and centrifuged at 10,000 × g for 5 min at 4°C. The supernatant was collected, diluted to 0.5 ml with water, and incubated with 2.5 μl of rabbit polyclonal anti-GFP antibody for 2 h on ice. Protein G-Sepharose (40 μl) was added and incubated at 4°C for 18 h with gentle shaking. The beads were washed with 1× RIPA buffer, and proteins were resolved as described by SDS-PAGE and visualized by fluorography followed by autoradiography.
Confocal microscopy.
At 24 h after transfection, the cells were fixed with cold 4% paraformaldehyde in PBS at room temperature for 20 min and then permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature. The permeabilized cells were incubated with primary antibodies diluted in 10% FBS in PBS for 1 h, followed by secondary antibody diluted in 10% FBS in PBS for 30 min at room temperature. For double staining of the proteins, cells were stained separately with each antibody to minimize the cross-reactivity. For staining actin filaments, the cells were fixed with 3% paraformaldehyde in CSB (10 mM MES [morpholineethanesulfonic acid, pH 6.1], 150 mM sodium chloride, 5 mM EGTA, 5 mM glucose, 5 mM MgCl2 · 6H2O), permeabilized, and incubated with phalloidin-rhodamine (Molecular Probes) in PBS for 30 min at room temperature. Golgi apparatus was visualized by staining with mouse anti-p115 MAb unless otherwise stated. Stained cells were washed extensively with PBS, and coverslips were mounted in 20% glycerol and sealed with rubber cement. In some experiments, 10 μg of brefeldin A (BFA) (Sigma) per ml was added to the cells at 24 h after transfection, and the cells were incubated for an additional 30 min at 37°C and stained as described above. Fluorescence was examined with a Leica TCS NT inverted confocal microscope, and images were overlaid by using Adobe Photoshop version 5.0.2.
Immunoelectron microscopy.
RK-13 cells were infected with vF13L-GFP at a multiplicity of 10 and incubated for 22 h. The cells were fixed and prepared for immunoelectron microscopy as described (69). Briefly, cryosections were incubated with rabbit anti-GFP polyclonal antibody followed by protein A conjugated to 10-nm colloidal gold. Stained cryosections were viewed using a Philips CM 100 transmission electron microscope.
RESULTS
Localization and function of F13L-GFP fusion protein during vaccinia virus infection.
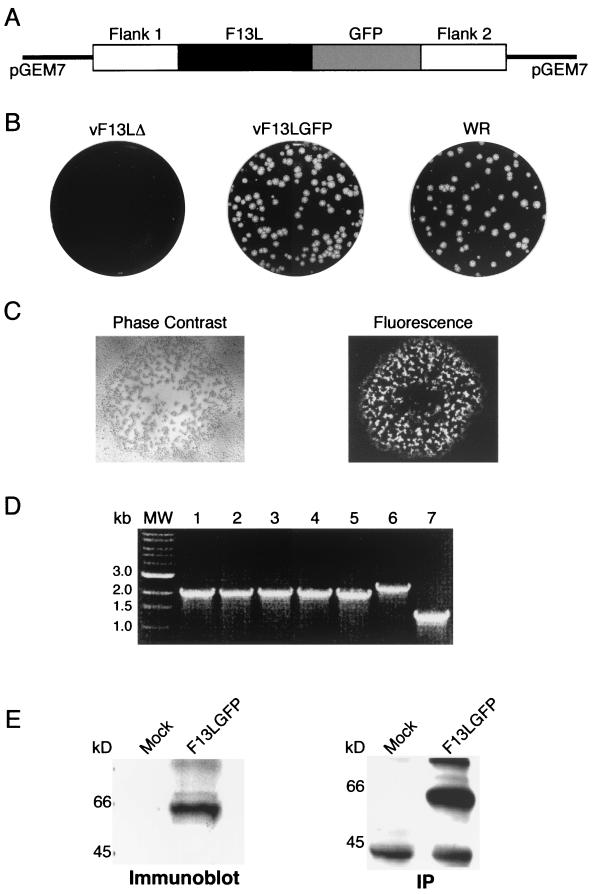
Initial experiments were designed to determine whether the attachment of GFP to F13L would perturb the function of the viral protein. We considered that the rescue of a mutant vaccinia virus with a deleted F13L gene would demonstrate that the F13L-GFP protein functioned properly. To insert the F13L-GFP gene into the vaccinia virus genome by homologous recombination, we constructed a plasmid containing the F13L gene and flanking DNA in which the GFP coding sequence was appended to the C terminus of the F13L ORF, leaving the viral transcriptional regulatory sequences unaltered (Fig. 1A). HeLa cells were infected with vF13LΔ, a mutant vaccinia virus that contains gpt in place of the deleted F13L gene (3), and transfected with the plasmid carrying the F13L-GFP chimera. The plaques exhibiting green fluorescence were similar in size to those of wild-type virus and much larger than those of vF13LΔ (Fig. 1B and C). Of five such plaques picked, each was shown to contain the appropriate 1.9-kbp F13L-GFP ORF by PCR instead of the slightly larger product containing the gpt gene of the deletion mutant (Fig. 1D). One of these recombinant viruses, named vF13L-GFP, was plaque picked additional times and amplified to give titers similar to that of wild-type vaccinia virus. Expression of the F13L-GFP protein was demonstrated by infecting HeLa cells with vF13L-GFP and analyzing the lysate by SDS-PAGE and Western blotting or by metabolic labeling followed by SDS-PAGE and autoradiography. A major band with a predicted mass of 64 kDa reacted with antibody to GFP (Fig. 1E).
FIG. 1.
Construction and characterization of a recombinant vaccinia virus that expresses GFP-tagged F13L protein. (A) Diagram of a portion of the plasmid transfer vector used for recombination. The GFP coding sequence was appended in frame to the C-terminal amino acid of the F13L ORF. Flank 1 and flank 2 represent approximately 600 bp each of viral DNA on either side of the F13L ORF. (B) Crystal violet-stained plaques of the F13L deletion mutant vF13LΔ, vF13L-GFP, and wild-type vaccinia virus strain WR prepared with a methylcellulose overlay. (C) Visualization of a vF13L-GFP plaque by phase contrast and fluorescence microscopy. (D) Agarose gel electrophoresis of PCR products from cells infected with virus from five different recombinant plaques (lanes 1 to 5) and genomic DNA of vF13LΔ (lane 6) and wild-type vaccinia virus (lane 7). Oligonucleotide primers flanked the F13L gene. Lane MW, size markers. (E) SDS-PAGE immunoblot and autoradiogram of immunoprecipitate of F13L-GFP protein. HeLa cells were mock infected or infected with vF13L-GFP and harvested after 24 h. The immunoblot was probed with an anti-GFP mouse MAb. For immunoprecipitation (IP), the mock-infected or infected cells were labeled with [35S]methionine for 2 h, and the lysate was incubated with rabbit anti-GFP polyclonal antibodies, followed by protein A beads.
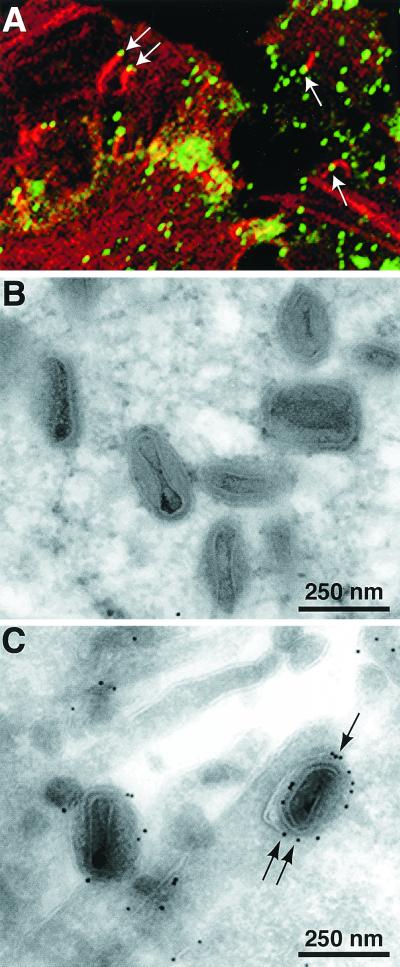
The formation of large plaques by vF13L-GFP suggested that IEV, actin tails, and CEV had formed. This was confirmed by microscopy. We visualized green fluorescent particles in the peripheral regions of HeLa cells infected with vF13L-GFP. Some of the particles were shown to have actin tails by staining with phalloidin-rhodamine, indicating that they were IEV or CEV (Fig. 2A). Direct evidence of IEV and CEV formation and the incorporation of the F13L-GFP fusion protein into their membranes was obtained by immunoelectron microscopy. Thin sections of vF13L-GFP-infected RK-13 cells were labeled with rabbit anti-GFP polyclonal antibody followed by a protein A-gold conjugate. Gold grains corresponding to GFP were present on the outer membranes of IEV (Fig. 2C), whereas no gold grains were detected on IMV (Fig. 2B). Thus, the formation of normal-size plaques, actin tails, IEV, and CEV and the association of F13L-GFP with wrapped virions indicated that the chimeric protein was functional.
FIG. 2.
Visualization of F13L-GFP by confocal or electron microscopy in cells infected with vF13L-GFP. (A) HeLa cells were infected with 1 PFU of vF13L-GFP per cell. After 18 h, the cells were fixed, permeabilized, stained with rhodamine-phalloidin, and examined by confocal microscopy. Arrows point to green fluorescent particles at the tips of red actin tails. RK13 cells were infected with 10 PFU of vF13L-GFP virus per cell. After 22 h, the cells were fixed, cryosectioned, and probed with rabbit anti-GFP polyclonal antibodies followed by protein A conjugated to 10-nm colloidal gold particles. Electron micrographs show unlabeled IMV (B) and labeled IEV (C). Arrows point to gold grains.
Colocalization of F13L-GFP and B5R envelope proteins.
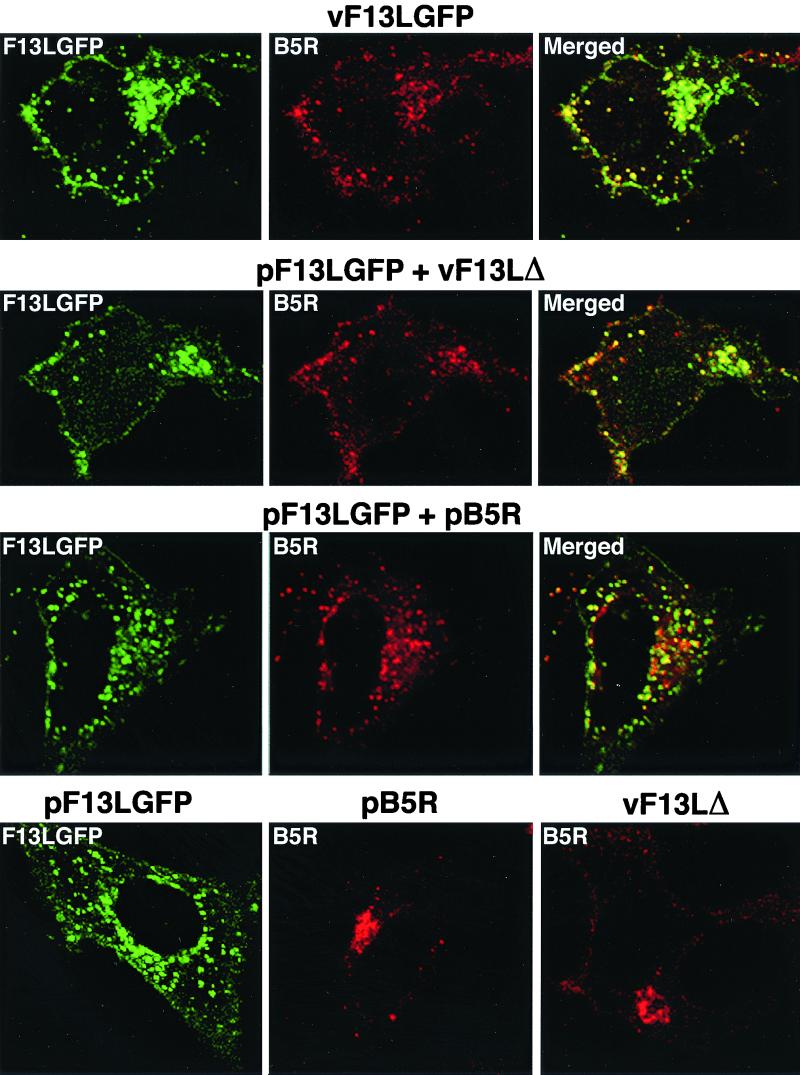
To facilitate further experiments, we also constructed a plasmid expression vector that contained the F13L-GFP ORF regulated by a simian virus 40 promoter instead of a vaccinia virus promoter. Expression of the 64-kDa fusion protein in uninfected cells was detected by immunoblotting and immunoprecipitation (data not shown). HeLa cells were transfected with pF13L-GFP and 24 h later infected with the vaccinia virus F13L deletion mutant vF13LΔ to visualize the intracellular location of the chimeric protein expressed in this manner. The B5R envelope protein colocalized with F13L-GFP in punctate structures in the periphery of the cell and in a juxtanuclear region (Fig. 3, Second row). Moreover, the pattern was similar to that obtained when cells were infected with vF13L-GFP (Fig. 3, first row). In contrast, the B5R protein was predominantly in the juxtanuclear region when vF13LΔ-infected cells were not transfected with pF13L-GFP (Fig. 3, fourth row, last panel).
FIG. 3.
Colocalization of F13L-GFP and B5R protein in infected or transfected cells. First row, HeLa cells were infected with vF13L-GFP (1 PFU/cell) for 18 h and then fixed, permeabilized, and stained with anti-B5R MAb followed by rhodamine-conjugated anti-rat Ig antibody and examined by confocal microscopy. Second row, HeLa cells were transfected with plasmid pF13L-GFP; after 24 h, the cells were infected with vF13LΔ (5 PFU/cell) and stained and examined by confocal microscopy as in the first row. Third row, HeLa cells were cotransfected with pF13L-GFP and pB5R. After 24 h, the cells were fixed, permeabilized, and stained. Fourth row, HeLa cells were transfected with pF13L-GFP alone (left) or pB5R alone (middle) or infected with vF13LΔ alone (right) and stained and examined by confocal microscopy. Green, GFP; red, rhodamine; yellow, overlap of green and red.
The above experiment demonstrated that expression of F13L was required for the localization of the B5R protein in peripheral punctate structures. However, many other viral proteins may also have been required, since expression occurred in the context of a viral infection. To evaluate this possibility, HeLa cells were cotransfected with pF13L-GFP and a plasmid expressing B5R (pB5R) but were not subsequently infected (Fig. 3, third row). Colocalization of B5R and F13L-GFP occurred in the absence of other viral proteins (Fig. 3, third row). As expected from previous studies, B5R was largely in the juxtanuclear region when expressed in uninfected cells without F13L-GFP (Fig. 3, fourth row, center panel). The location of the F13L-GFP protein in juxtanuclear and punctate structures, however, was unaffected by the absence of the B5R protein (Fig. 3, fourth row, first panel). Taken together, these data suggest that the F13L-GFP was intrinsically capable of localizing in peripheral punctate as well as in juxtanuclear structures and that F13L-GFP induced colocalization of the B5R protein.
Intracellular localization of F13L-GFP in the absence of other viral proteins.
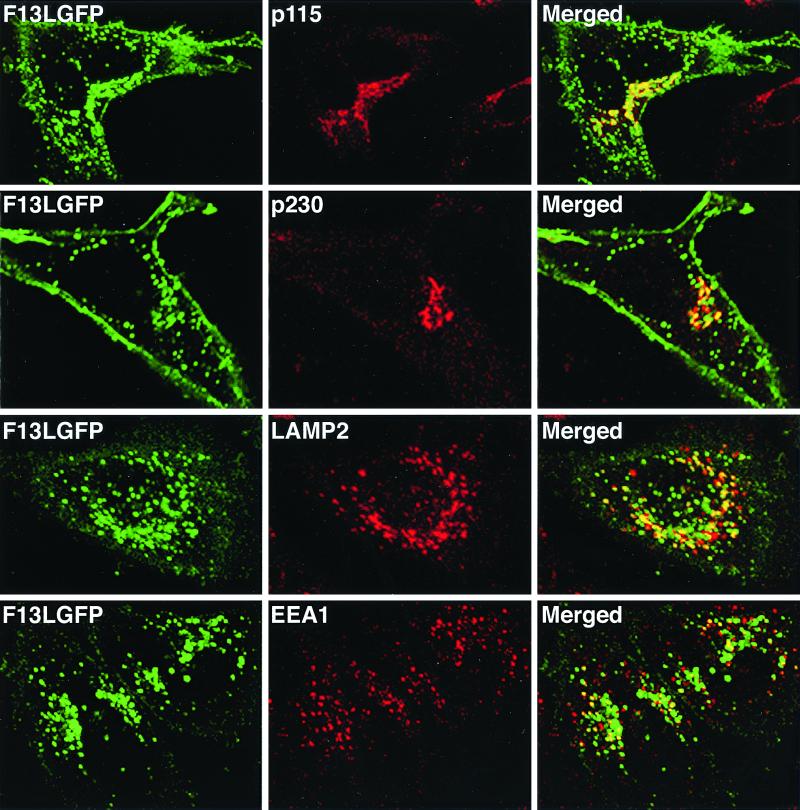
A variety of MAbs were used to characterize the cellular compartments containing F13L-GFP. The fluorescent F13L-GFP in the juxtanuclear region overlapped the cis-Golgi proteins p115 (Fig. 4) and GM130 (data not shown) as well as p230, a peripheral membrane protein associated with the cytoplasmic face of the trans-Golgi cisternae (Fig. 4). Much of the F13L-GFP, however, was in punctate structures. To identify these structures, we stained the transfected cells with MAbs to LAMP2, a type 1 integral membrane protein that is associated with late endosomes and lysosomes, and EEA1, a marker for early endosomes. There was more overlap of green fluorescence with LAMP2 than EEA1 (Fig. 4), suggesting a greater association of F13L-GFP with late endosomal-lysosomal vesicles than early endosomes.
FIG. 4.
Colocalization of F13L-GFP with cellular markers in transfected cells. HeLa cells were transfected with plasmid pF13LGFP for 24 h and then fixed, permeabilized, and stained with the indicated MAbs. Transfected HeLa cells were stained with mouse anti-p115, anti-p230, LAMP2, or EEA1 MAbs followed by rhodamine-conjugated anti-mouse Ig antibody. Stained cells were then examined by confocal microscopy. Green, GFP; red, rhodamine; yellow, overlap of green and red.
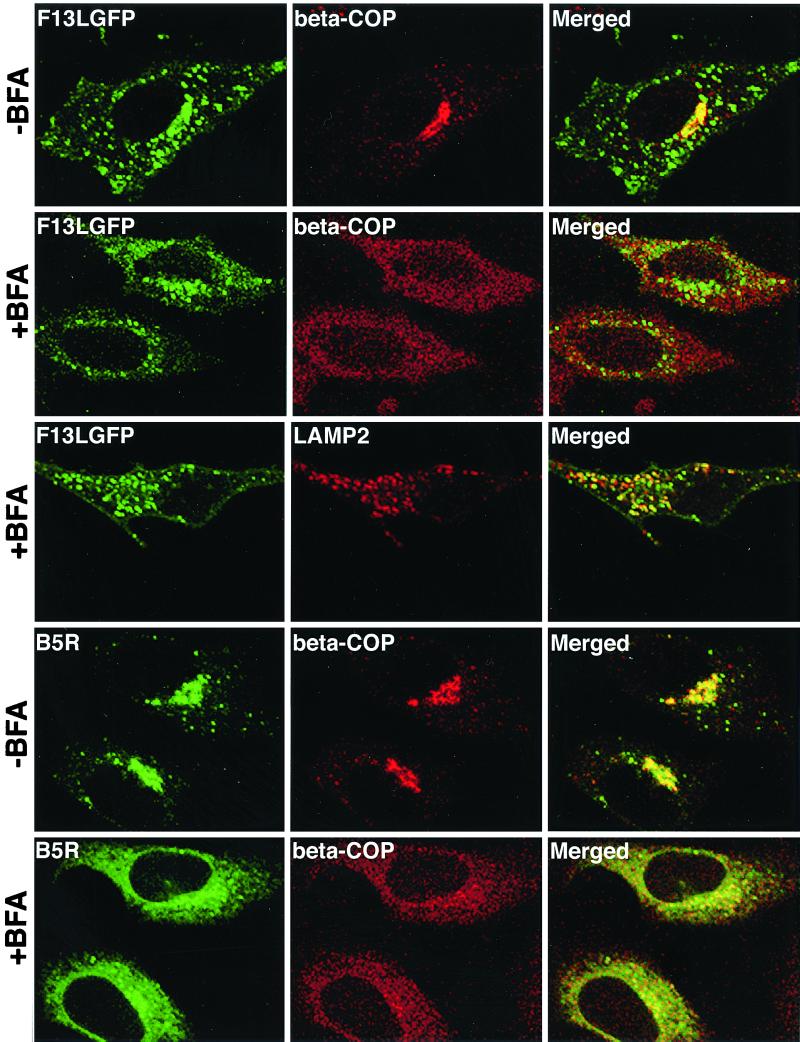
BFA, a fungal metabolite that disrupts the structure and function of the Golgi cisternae (9, 32), was used to further investigate the intracellular locations of F13L-GFP. HeLa cells were transfected with pF13L-GFP for 24 h and then treated for 30 min with BFA. The cells were stained with an MAb to the coatomer protein β-COP, which rapidly dissociates from the Golgi apparatus after BFA treatment (10). In the absence of BFA, the MAb to β-COP stained the Golgi complex as well as endoplasmic reticulum-to-Golgi vesicles (Fig. 5, first row). Some overlap of β-COP with F13L-GFP in the Golgi region was noted (Fig. 5, first row), consistent with the data above. After BFA treatment, the β-COP was diffusely distributed in the cytoplasm, whereas there was persistence of vesicles containing F13L-GFP (Fig. 5, second row). Comparison of the F13L-GFP in the absence (first row) and presence (second row) of BFA, however, did show increased diffuse staining under the latter conditions, resulting from the dissociation of some F13L-GFP from Golgi membranes. Most strikingly, BFA did not affect the colocalization of F13L-GFP with LAMP2 (Fig. 5, third row), consistent with the association of F13L-GFP with endosomes or lysosomes. As another control, cells were transfected with a plasmid expressing B5R alone. As expected, the B5R protein colocalized with β-COP in the absence of BFA and was dispersed after BFA treatment (Fig. 5, last two rows).
FIG. 5.
Effect of BFA on the localization of F13L-GFP and B5R protein in transfected cells. HeLa cells were transfected with pF13L-GFP or pB5R for 24 h. One set of transfected cells was treated with BFA (10 μg/ml) for 30 min. The cells were then fixed, permeabilized, and stained with rabbit anti-β-COP polyclonal antibody followed by Texas red-conjugated anti-rabbit Ig antibody or with LAMP2 MAb followed by rhodamine-conjugated anti-mouse Ig antibody. Those cells that had been transfected with pB5R were then stained with rat anti-B5R MAb followed by fluorescein isothiocyanate (FITC)-conjugated anti-rat Ig antibody. Cells were analyzed by confocal microscopy. Green, GFP or FITC; red, Texas red or rhodamine; yellow, overlap of green and red.
Effect of F13L-GFP on intracellular localization of B5R protein.
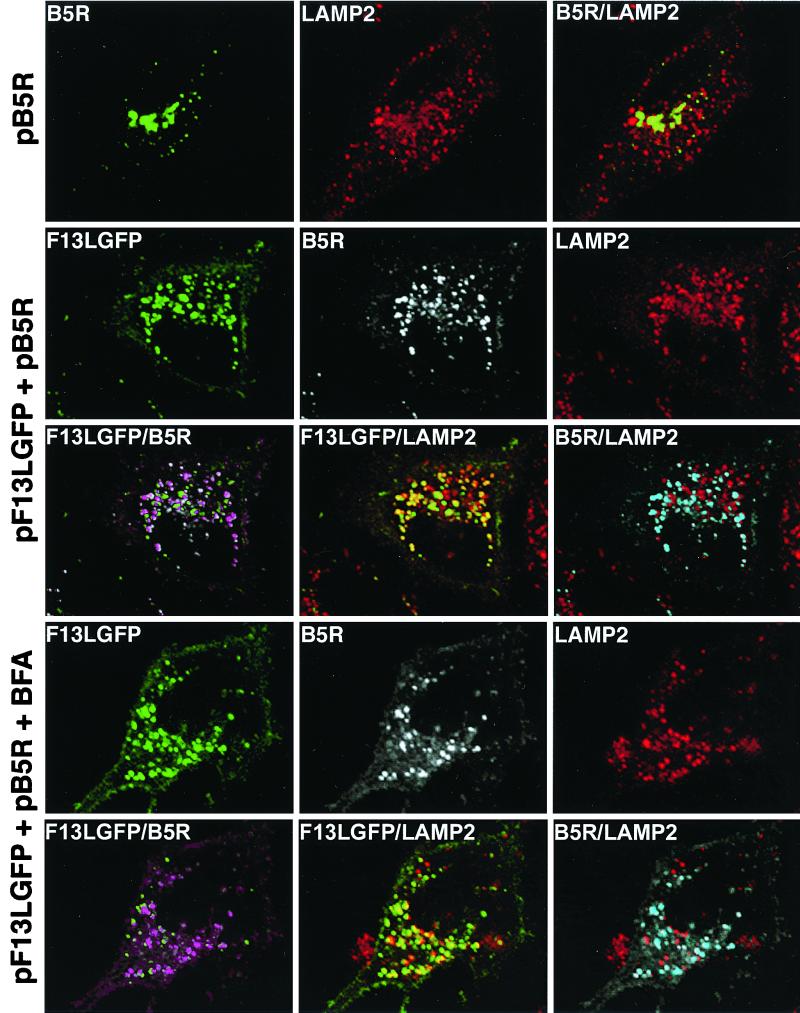
Additional experiments were carried out to determine whether B5R, when coexpressed with F13L-GFP, also colocalized in BFA-resistant LAMP2-containing vesicles. First we showed that when B5R was expressed alone, there was no colocalization with LAMP2 (Fig. 6, first row). When B5R and F13L-GFP were coexpressed, however, B5R was present in vesicles associated with F13L-GFP and LAMP2 (Fig. 6, second and third rows). Furthermore, this association was resistant to BFA (Fig. 6, fourth and fifth rows). These observations indicated that F13L-GFP was responsible for alterations in the intracellular localization of B5R.
FIG. 6.
Colocalization of B5R protein with LAMP2 in cells expressing F13L-GFP. HeLa cells were transfected with pB5R alone or cotransfected with pF13L-GFP and pB5R for 24 h. One set of cotransfected cells was treated with BFA for 30 min. Cells were fixed, stained, and examined as described in the legend to Fig. 5. Unmerged and merged images are labeled with the name of a single protein or the names of two proteins, respectively. First row: green, FITC; red, rhodamine; yellow, overlap of green and red. Other rows: green, GFP; red, rhodamine; white, indodicarbocyanine (Cy5); yellow, overlap of green and red; purple, overlap of green and white; blue, overlap of white and red.
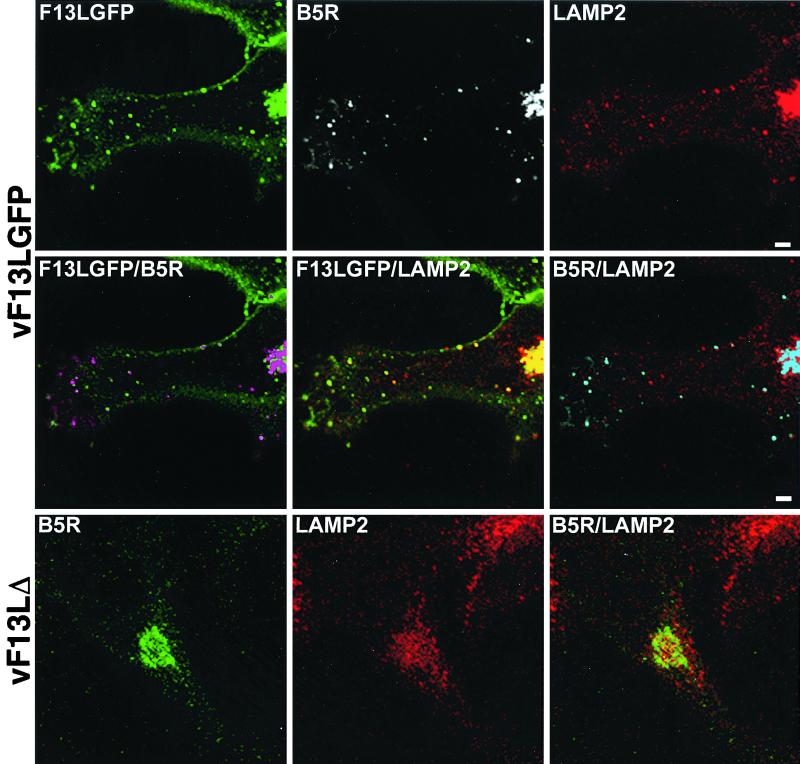
Additional studies were designed to see if colocalization of F13L-GFP, B5R, and LAMP2 occurred during a productive vaccinia virus infection. We therefore stained vF13L-GFP-infected cells with anti-LAMP2 and anti-B5R antibodies. Although staining was less intense under these conditions, there was colocalization of F13L-GFP, B5R, and LAMP2 in punctate structures that could be endosomal vesicles or IEV membranes (Fig. 7, first and second rows). No colocalization of B5R and LAMP2 in peripheral punctate structures occurred when F13L was not expressed in cells infected with vF13LΔ (Fig. 7, third row). Thus, F13L was required for colocalization of B5R with LAMP2-containing vesicles in both transfected and infected cells.
FIG. 7.
Colocalization of F13L-GFP, B5R, and LAMP2 in vF13L-GFP-infected cells. HeLa cells were infected with 1 PFU of vF13L-GFP or vF13LΔ per cell for 18 h and then fixed, permeabilized, and stained with mouse anti-LAMP2 MAb followed by rhodamine-conjugated anti-mouse Ig antibody. The cells were then stained with rat anti-B5R MAb followed by Cy5-conjugated anti-rat Ig antibody and examined by confocal microscopy. Unmerged and merged images are labeled with the name of a single protein or the names of two proteins, respectively. First and second rows: green, GFP; white, Cy5; red, rhodamine; purple, overlap of green and white; yellow, overlap of green and red; blue, overlap of white and red. Third row: green, Cy5; red, rhodamine; yellow, overlap of green and red.
Effect of mutations in phospholipase catalytic site motif of F13L-GFP.
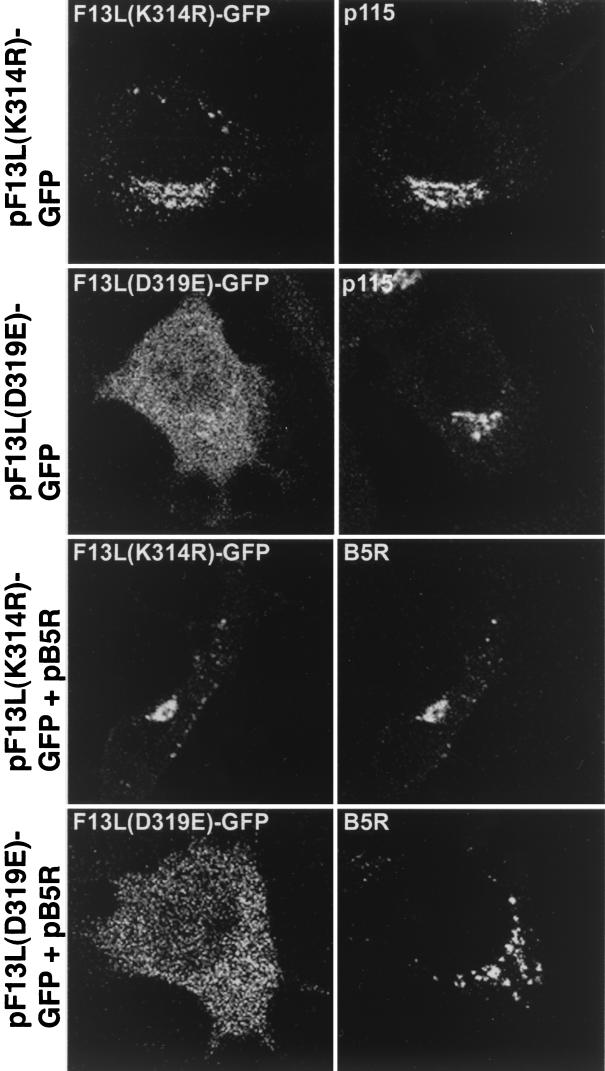
We suspected that the localization of the F13L protein in endosome-like vesicles might require phospholipase activity. To evaluate this possibility, we constructed expression plasmids with single amino acid substitutions in the conserved active-site phospholipase motif of F13L-GFP. Previous studies had shown that vaccinia virus mutants with F13L(K314R) or F13K(D319E) substitutions in the putative phospholipase catalytic site behaved like F13L deletion mutants (47, 60). In those studies, the F13L(K314R) protein was still palmitylated, whereas the F13K(D319E) protein was insoluble and difficult to analyze. We found that after transfection, F13L(K314R)-GFP localized predominantly in the juxtanuclear Golgi complex, with relatively little fluorescence associated with vesicles or the plasma membrane (Fig. 8, first row). F13L(D319E)-GFP, however, was distributed throughout the cytoplasm (Fig. 8, second row), suggesting a general defect in membrane association that was correlated with decreased partitioning into the Triton X-114 detergent phase (data not shown). Western blots similar to the one in Fig. 1E confirmed that the GFP moiety was still associated with the F13L fusion protein (data not shown). Additional experiments indicated that the B5R protein also remained predominantly in the juxtanuclear Golgi region when coexpressed with mutated F13L-GFP (Fig. 8, third and fourth rows). Therefore, the putative catalytic site of F13L was important for localization of the F13L-GFP and B5R protein.
FIG. 8.
Effect of amino acid substitutions in the phospholipase active-site motif of F13L-GFP on intracellular localization. HeLa cells were transfected with pF13L(K314R)-GFP or pF13L(D319E)-GFP or cotransfected with those plasmids and pB5R. After 24 h, the cells were fixed, permeabilized, and stained with mouse anti-p115 MAb followed by rhodamine-conjugated anti-mouse Ig antibody or rat anti-B5R MAb followed by rhodamine-conjugated anti-rat Ig antibody.
Effect of mutations in palmitylation site of F13L-GFP.
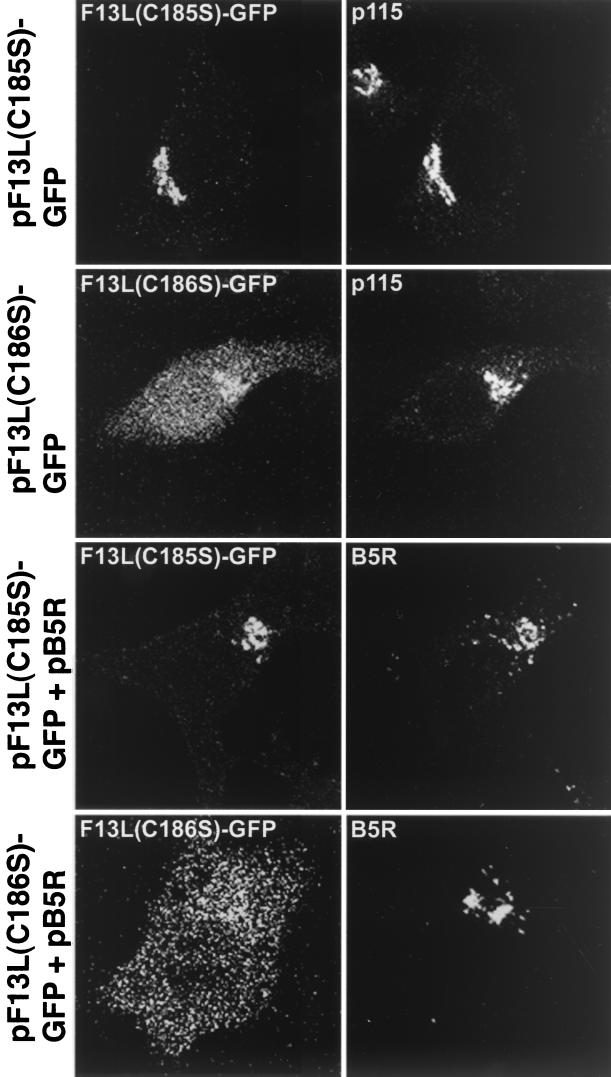
Palmitylation of the F13L protein occurs at cysteines 185 and 186, and mutation of the two abrogated Golgi localization of F13L in virus-infected cells (22). We constructed plasmids that express F13L-GFPs containing a single C185S or C186S mutation. When transfected alone, F13L(C185S)-GFP was localized primarily in the Golgi complex while F13L(C186S)GFP was distributed throughout the cell (Fig. 9, first and second rows). The F13L(C186S)-GFP was expressed to a lower level, as shown by Western blotting, and exhibited decreased partitioning in the Triton X-114 detergent phase compared to the unmutated F13L-GFP (data not shown). The B5R protein remained predominantly in the juxtanuclear region when coexpressed with either of these mutants (Fig. 9, third and fourth rows).
FIG. 9.
Effect of amino acid substitutions of the palmitylation sites of F13L-GFP on intracellular localization. HeLa cells were transfected with pF13L(C185S)-GFP or pF13L(C186S)-GFP or cotransfected with those plasmids and pB5R for 24 h. Cells were fixed, permeabilized, stained, and examined by confocal microscopy as described for Fig. 8.
DISCUSSION
The present study concerns the two EEV proteins F13L and B5R, which are required for the membrane wrapping of IMV to form IEV. To visualize the F13L protein by confocal microscopy, we expressed an F13L-GFP fusion protein from a recombinant vaccinia virus or a plasmid vector. The fluorescent fusion protein was incorporated into IEV and CEV membranes and rescued a well-characterized F13L deletion mutant that made tiny plaques and had a defect in wrapping IMV (3). Having demonstrated the proper function of the F13L-GFP protein in the context of a virus infection, we analyzed its distribution in the absence of other viral proteins. Strong fluorescence was obtained in the juxtanuclear region, more peripheral punctate structures, and the plasma membrane. A similar distribution was found using an influenza virus hemagglutinin (HA) epitope-tagged F13L protein (M. Husain, unpublished data). We found that F13L-GFP in the juxtanuclear region colocalized with the cis- and and trans-Golgi cisternal membrane markers p115, GM130, and p230. Many of the punctate structures colocalized with LAMP2, a protein that enters the endosomal pathway at the trans-Golgi network and is found in late endosomes and lysosomes (18, 19). There was less colocalization of F13L-GFP with EEA1, an early endosomal marker (39), than with LAMP2. As expected for endosomal localization, the fluorescent F13L-GFP/LAMP2-associated vesicles were resistant to BFA addition.
The colocalization of the B5R protein with BFA-resistant LAMP2 vesicles when B5R was coexpressed with F13L-GFP was a notable finding. When the B5R protein was expressed alone, it was mostly associated with Golgi cisternal markers and was sensitive to BFA, as anticipated from previous studies (65). How could the F13L protein alter the localization of the B5R protein? Previous efforts to detect physical association of the F13L protein with the B5R or other viral proteins by immunoprecipitation were unsuccessful using a variety of lysis conditions (54). Similarly, we could not detect associations by coimmunprecipitation (M. Husain, unpublished data). An alternative possibility is that the F13L protein regulates vesicle formation through its phospholipase activity, analogous to that reported for phospholipase D (2, 6, 16, 31, 55, 56, 59). Phospholipase D catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid and choline, which induces coatomer binding and vesicle formation at the trans-Golgi network. By a similar mechanism, inclusion of the B5R protein into post-Golgi vesicles might depend on phospholipase activity of F13L rather than a direct physical association between the two viral proteins. Support for such a mechanism comes from mutagenesis. Mutation of the conserved Lys-314 in the phospholipase D active-site motif to Arg did not affect the Golgi membrane localization of the F13L protein but inhibited its association and the association of the B5R protein with post-Golgi vesicles. This result could suggest that the F13L protein, like B5R, is targeted to the Golgi cisternae but requires phospholipase activity to generate or associate with post-Golgi vesicles. The F13L-GFP protein with the conserved Asp-319 changed to Glu was distributed diffusely in the cytoplasm, suggesting that the mutation affected palmitylation. Mutation to serine of either of the two Cys residues that become palmitylated also inhibited the association of F13L-GFP protein and the B5R protein with post-Golgi vesicles. Golgi localization still occurred with the Cys-185 mutant but not with the Cys-186 mutant. Importantly, mutations in both the catalytic motif and the palmitylation sites blocked IEV formation in infected cells (21, 47, 60). That the F13L protein actually induces vesicle formation or blocks vesicle recycling is suggested by the eventual dispersal of much of the Golgi cisternae by 48 h after transfection with the F13L-GFP expression vector (M. Husain, unpublished data). A similar disruption of the Golgi complex occurs late during a productive vaccinia virus infection and has been attributed to the exhaustive use of Golgi membranes for wrapping IMV (25). Further studies on the lipase activity of the F13L protein are needed. Although phospholipase A and C activities of a recombinant F13L were reported (1), phospolipase D activity remains to be demonstrated (60).
The F13L protein lacks a signal peptide or a membrane-spanning domain, and membrane association is dependent on palmitylation (21, 54). Presumably, F13L-GFP associates with the cytoplasmic face of the Golgi and endosomal vesicles. The fact that single-base substitutions of F13L-GFP can largely restrict it to the Golgi cisternae argues against a trivial explanation for the endosomal location. These static studies, however, do not reveal whether F13L-GFP goes directly to endosomal vesicles, arrives there via the trans-Golgi membrane during vesicle formation, and is internalized by endocytic receptors from the plasma membrane or by some combination of these mechanisms. The F13L protein has several tyrosine- and dileucine-based motifs that could be involved in internalization from endocytic receptors or targeting to endosomal compartments (52, 62). Efforts to determine the roles of these motifs in the localization of F13L-GFP are in progress.
The majority of experiments in this study were carried out using a simple transfection system in order to dissect out possible roles for the F13L protein. The vesicles that have been characterized here contain only two viral proteins and cannot be equivalent to the cisternal structures that envelop IMV in infected cells. Nevertheless, there are some correlates between the transfection and infection systems. For example, in both, the absence of the F13L protein results in retention of the B5R protein in Golgi cisternae. Moreover, a similar situation occurs when the F13L protein is mutated in the phospholipase motif or the palmityl acceptor cysteines. In addition, using confocal microscopy, we detected colocalization of F13L-GFP, B5R protein, and LAMP2 in punctate structures in the cytoplasm of infected cells. Further studies will be needed to confirm or reject the hypothesis that the F13L protein has an enzymatic role in inducing or modifying vesicles that form the IEV membrane.
ACKNOWLEDGMENTS
We thank members of the Laboratory of Viral Diseases for interest and assistance. In particular, Brian Ward provided pSG5-F13LGFP and made many useful suggestions regarding confocal microscopy, Andrea Weisberg carried out electron microscopy, and Norman Cooper provided tissue culture cells. Much of the work was carried out in the NIAID imaging facility with the expert guidance of Owen Schwartz. Brian Ward and Jonathan Yewdell made helpful comments regarding the preparation of the manuscript.
REFERENCES
- 1.Baek S-H, Kwak J-Y, Lee S H, Lee T, Ryu S H, Ublinger D J, Lambeth J D. Lipase activities of p37, the major envelope protein of vaccinia virus. J Biol Chem. 1997;272:32042–32049. doi: 10.1074/jbc.272.51.32042. [DOI] [PubMed] [Google Scholar]
- 2.Bednarek S Y, Orici L, Schekman R. Traffic COPS and the formation of vesicle coats. Trends Cell Biol. 1996;6:468–473. doi: 10.1016/0962-8924(96)84943-9. [DOI] [PubMed] [Google Scholar]
- 3.Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J Virol. 1991;65:5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco R, Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J Virol. 1992;66:4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrego B, Lorenzo M M, Blasco R. Complementation of P37 (F13L gene) knock-out in vaccinia virus by a cell line expressing the gene constitutively. J Gen Virol. 1999;80:425–432. doi: 10.1099/0022-1317-80-2-425. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y G, Siddhanta A, Austin C D, Hammond S M, Sung T C, Frohman M A, Morris A J, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 8.Dales S, Siminovitch L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doms R W, Russ G, Yewdell J W. Brefeldin A redistributes resident and itinerant Golgi proteins to the enodplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donaldson J G, Lippincott-Schwartz J, Bloom G S, Kreis T E, Klausner R D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990;111:2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan S A, Smith G L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992;66:1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earl P L, Moss B. Generation of recombinant vaccinia viruses. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: Greene Publishing Associates & Wiley Interscience; 1991. pp. 16.17.1–16.17.16. [Google Scholar]
- 13.Engelstad M, Howard S T, Smith G L. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992;188:801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 14.Engelstad M, Smith G L. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology. 1993;194:627–637. doi: 10.1006/viro.1993.1302. [DOI] [PubMed] [Google Scholar]
- 15.Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 16.Frohman M A, Morris A J. Rho is only ARF the story: phospholipid signalling. Curr Biol. 1996;6:945–947. doi: 10.1016/s0960-9822(02)00634-6. [DOI] [PubMed] [Google Scholar]
- 17.Goebel S J, Johnson G P, Perkus M E, Davis S W, Winslow J P, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 18.Gough N R, Fambrough D M. Different steady state subcellular distributions of the three splice variants of lysosome-associated membrane protein LAMP-2 are determined largely by the COOH-terminal amino acid residue. J Cell Biol. 1997;137:1161–1169. doi: 10.1083/jcb.137.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green S A, Zimmer K P, Griffiths G, Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987;105:1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimley P M, Rosenblum E N, Mims S J, Moss B. Interruption by rifampin of an early stage in vaccinia virus morphogenesis: accumulation of membranes which are precursors of virus envelopes. J Virol. 1970;6:519–533. doi: 10.1128/jvi.6.4.519-533.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosenbach D W, Hruby D E. Analysis of a vaccinia virus mutant expressing a nonpalmitylated form of p37, a mediator of virion envelopment. J Virol. 1998;72:5108–5120. doi: 10.1128/jvi.72.6.5108-5120.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosenbach D W, Ulaeto D O, Hruby D E. Palmitylation of the vaccinia virus 37-kDa major envelope antigen. Identification of a conserved acceptor motif and biological relevance. J Biol Chem. 1997;272:1956–1964. doi: 10.1074/jbc.272.3.1956. [DOI] [PubMed] [Google Scholar]
- 23.Herrera E, del Mar Lorenzo M, Blasco R, Isaacs S N. Functional analysis of vaccinia virus B5R protein: essential role in virus envelopment is independent of a large portion of the extracellular domain. J Virol. 1998;72:294–302. doi: 10.1128/jvi.72.1.294-302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiller G, Eibl H, Weber K. Characterization of intracellular and extracellular vaccinia virus variants: N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine interferes with cytoplasmic virus dissemination and release. J Virol. 1981;39:903–913. doi: 10.1128/jvi.39.3.903-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiller G, Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J Virol. 1985;55:651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirt P, Hiller G, Wittek R. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J Virol. 1986;58:757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollinshead M, Vanderplasschen A, Smith G L, Vaux D J. Vaccinia virus intracellular mature virions contain only one lipid membrane. J Virol. 1999;73:1503–1517. doi: 10.1128/jvi.73.2.1503-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isaacs S N, Wolffe E J, Payne L G, Moss B. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J Virol. 1992;66:7217–7224. doi: 10.1128/jvi.66.12.7217-7224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz E, Wolffe E J, Moss B. The cytoplasmic and transmembrane domains of the vaccinia virus B5R protein target a chimeric human immunodeficiency virus type 1 glycoprotein to the outer envelope of nascent vaccinia virions. J Virol. 1997;71:3178–3187. doi: 10.1128/jvi.71.4.3178-3187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koonin E V. A duplicated catalytic motif in a new superfamily of phosphohydrolases and phospholipid synthases that includes poxvirus envelope proteins. Trends Biochem Sci. 1996;21:242–243. [PubMed] [Google Scholar]
- 31.Ktistakis N T, Brown H A, Waters M G, Sternweis P C, Roth M G. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippincott-Schwartz J, Yuan L C, Bonifacino J S, Klausner R D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenzo M D, Herrera E, Blasco R, Isaacs S N. Functional analysis of vaccinia virus B5R protein: role of the cytoplasmic tail. Virology. 1998;252:450–457. doi: 10.1006/viro.1998.9483. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo M M, Galindo I, Griffiths G, Blasco R. Intracellular localization of vaccinia virus extracellular enveloped virus envelope proteins individually expressed using a Semliki Forest virus replicon. J Virol. 2000;74:10535–10550. doi: 10.1128/jvi.74.22.10535-10550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew E, Sanderson C M, Hollinshead M, Smith G L. The extracellular domain of vaccinia virus protein B5R affects plaque phenotype, extracellular enveloped virus release, and intracellular actin tail formation. J Virol. 1998;72:2429–2438. doi: 10.1128/jvi.72.3.2429-2438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan C, Ellison S, Rose H, Moore D. Structure and development of viruses observed in the electron microscope. II. Vaccinia and fowl pox viruses. J Exp Med. 1954;100:301–310. doi: 10.1084/jem.100.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2637–2671. [Google Scholar]
- 39.Mu F T, Callaghan J M, Steele-Mortimer O, Stenmark H, Parton R G, Campbell P L, McCluskey J, Yeo J P, Tock E P, Toh B H. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 40.Parkinson J E, Smith G L. Vaccinia virus gene A36R encodes a Mr 43–50 K protein on the surface of extracellular enveloped virus. Virology. 1994;204:376–390. doi: 10.1006/viro.1994.1542. [DOI] [PubMed] [Google Scholar]
- 41.Payne L G. Significance of extracellular virus in the in vitro and in vivo dissemination of vaccinia virus. J Gen Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 42.Payne L G, Norrby E. Presence of hemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976;32:63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- 43.Ploubidou A, Moreau V, Ashman K, Reckmann I, Gonzalez C, Way M. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 2000;19:3932–3944. doi: 10.1093/emboj/19.15.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponting C P, Kerr I D. A novel family of phospholipase D homologues that includes phospholipid synthases and putative endonucleases: identification of duplicated repeats and potential active site residues. Protein Sci. 1996;5:914–922. doi: 10.1002/pro.5560050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Presley J F, Cole N B, Schrer T A, Hirschberg K, Zaal K J M, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 46.Roper R, Wolffe E J, Weisberg A, Moss B. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J Virol. 1998;72:4192–4204. doi: 10.1128/jvi.72.5.4192-4204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roper R L, Moss B. Envelope formation is blocked by mutation of a sequence related to the HKD phospholipid metabolism motif in the vaccinia virus F13L protein. J Virol. 1999;73:1108–1117. doi: 10.1128/jvi.73.2.1108-1117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roper R L, Payne L G, Moss B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J Virol. 1996;70:3753–3762. doi: 10.1128/jvi.70.6.3753-3762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rottger S, Frischknecht F, Reckmann I, Smith G L, Way M. Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J Virol. 1999;73:2863–2875. doi: 10.1128/jvi.73.4.2863-2875.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanderson C M, Frischknecht F, Way M, Hollinshead M, Smith G L. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low-pH-induced cell-cell fusion. J Gen Virol. 1998;79:1415–1425. doi: 10.1099/0022-1317-79-6-1415. [DOI] [PubMed] [Google Scholar]
- 51.Sanderson C M, Hollinshead M, Smith G L. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J Gen Virol. 2000;81:47–58. doi: 10.1099/0022-1317-81-1-47. [DOI] [PubMed] [Google Scholar]
- 52.Sandoval I V, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 53.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmutz C, Rindisbacher L, Galmiche M C, Wittek R. Biochemical analysis of the major vaccinia virus envelope antigen. Virology. 1995;213:19–27. doi: 10.1006/viro.1995.1542. [DOI] [PubMed] [Google Scholar]
- 55.Seaman M N J. Phospholipase D in vesicle budding. Trends Cell Biol. 1996;6:473. doi: 10.1016/0962-8924(96)84944-0. [DOI] [PubMed] [Google Scholar]
- 56.Simon J P, Ivanov I E, Adesnik M, Sabatini D D. The production of post-Golgi vesicles requires a protein kinase C-like molecule, but not its phosphorylating activity. J Cell Biol. 1996;135:355–370. doi: 10.1083/jcb.135.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sodeik B, Doms R W, Ericsson M, Hiller G, Machamer C E, van't Hof W, van Meer G, Moss B, Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stokes G V. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J Virol. 1976;18:636–643. doi: 10.1128/jvi.18.2.636-643.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stutchfield J, Cockcroft S. Correlation between secretion and phospholipase D activation in differentiated HL60 cells. Biochem J. 1993;293:649–655. doi: 10.1042/bj2930649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung T-C, Roper R L, Zhang Y, Rudge S A, Temel R, Hammond S M, Morris A J, Moss B, Engebrecht J, Frohman M A. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 1997;16:4519–4530. doi: 10.1093/emboj/16.15.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 62.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 63.Tsien R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 64.van Eijl H, Hollinshead M, Smith G L. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology. 2000;271:26–36. doi: 10.1006/viro.2000.0260. [DOI] [PubMed] [Google Scholar]
- 65.Ward B M, Moss B. Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein. J Virol. 2000;74:3771–3780. doi: 10.1128/jvi.74.8.3771-3780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward B M, Moss B. Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera. J Virol. 2001;75:4802–4813. doi: 10.1128/JVI.75.10.4802-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolffe E J, Isaacs S N, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993;67:4732–4741. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolffe E J, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997;71:3904–3915. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolffe E J, Moore D M, Peters P J, Moss B. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J Virol. 1996;70:2797–2808. doi: 10.1128/jvi.70.5.2797-2808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolffe E J, Weisberg A, Moss B. The vaccinia virus A33R protein provides a chaperone function for viral membrane localization and tyrosine phosphorylation of the A36R protein. J Virol. 2001;75:303–310. doi: 10.1128/JVI.75.1.303-310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolffe E J, Weisberg A S, Moss B. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology. 1998;244:20–26. doi: 10.1006/viro.1998.9103. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W H, Wilcock D, Smith G L. Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J Virol. 2000;74:11654–11662. doi: 10.1128/jvi.74.24.11654-11662.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]