Abstract
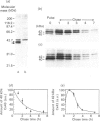
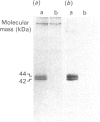
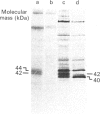
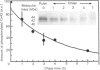
Cultured cardiomyocytes were used to study the turnover and post-translational modification of connexin43 (Cx43), a major gap junction protein in neonatal cardiac myocytes. Immunoprecipitation of [35S]Met-labelled lysates with anti-Cx43 antibodies followed by analysis using SDS/PAGE and fluorography revealed two bands, one at 40 kDa and the other at 42 kDa. Alkaline phosphatase treatment of [35S]Met-labelled Cx43 eliminated the band at 42 kDa, suggesting that it represented a phosphorylated form of the protein. This was confirmed by [32P]P1 incorporation into the 42 kDa band, but not into the band at 40 kDa. In addition, another alkaline phosphatase-sensitive phosphorylated form of Cx43 was identified at 44 kDa. In pulse-chase experiments, the half-life of Cx43 in cardiomyocytes was determined to be 1-2 h. Furthermore, the turnover rate of phosphate groups on Cx43 was found to be experimentally defined by the half-life of the protein. The observation that phosphate groups can remain with the protein throughout its life is consistent with the finding that in isolated adult rat heart gap junction plaques, Cx43 is primarily phosphorylated. We postulate that the rapid turnover of Cx43 and its multiple sites of phosphorylation play important roles in the regulation of cell-cell communication via gap junctions.
Full text
PDF
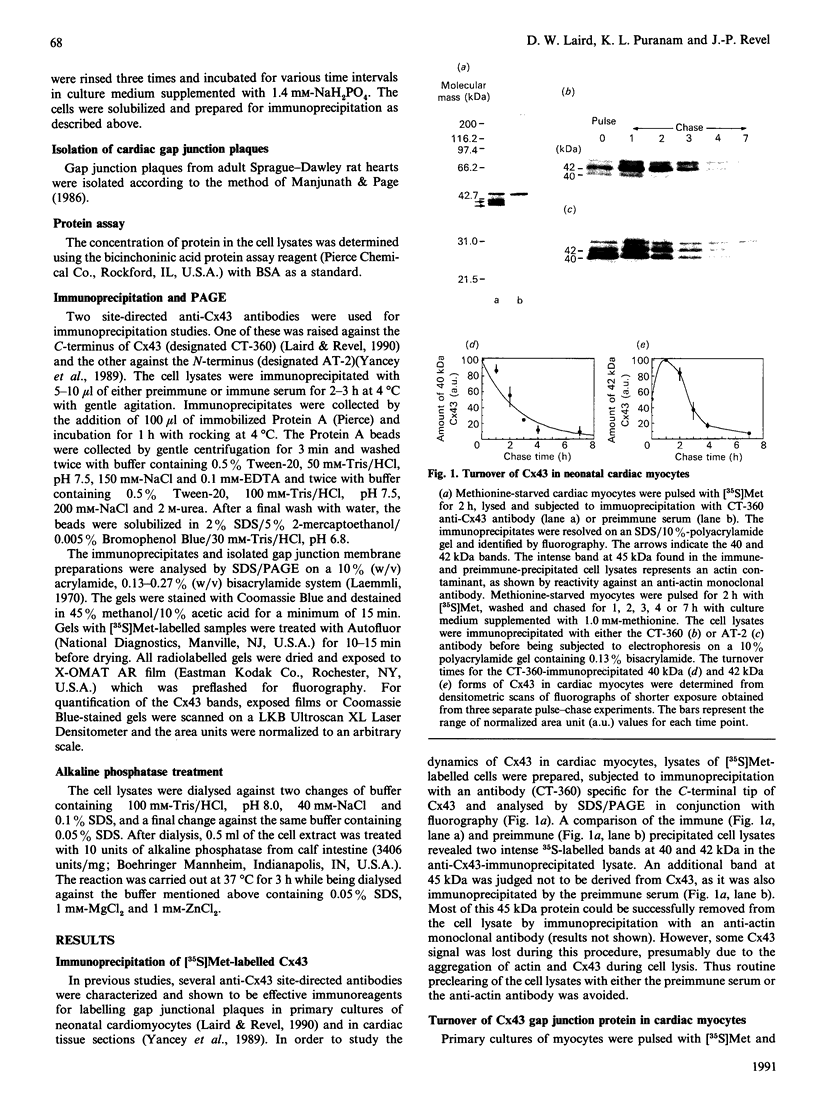

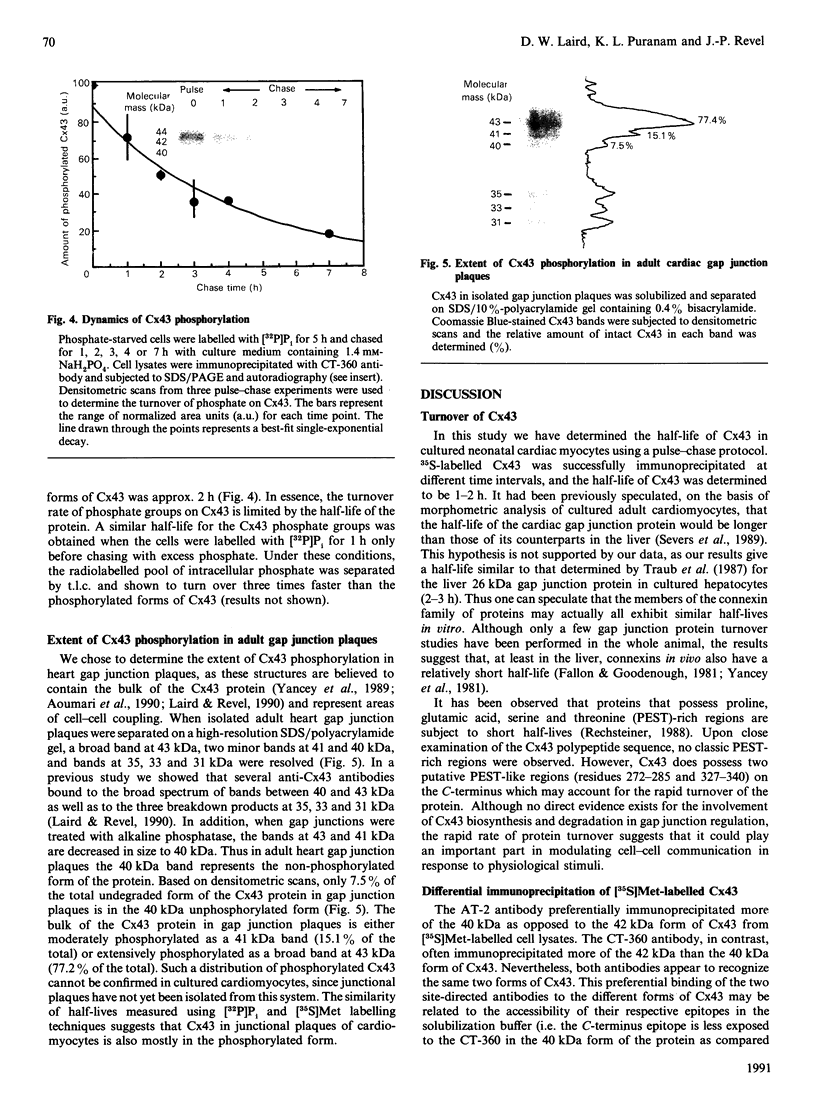


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azarnia R., Dahl G., Loewenstein W. R. Cell junction and cycle AMP: III. Promotion of junctional membrane permeability and junctional membrane particles in a junction-deficient cell type. J Membr Biol. 1981;63(1-2):133–146. doi: 10.1007/BF01969454. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Kistler J., Paul D. L., Goodenough D. A. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989 Feb;108(2):595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow D. S., Beyer E. C., Paul D. L., Kobe S. S., Lau A. F. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990 Apr;10(4):1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R., Yancey S. B., Traub O., Willecke K., Revel J. P. Major loss of the 28-kD protein of gap junction in proliferating hepatocytes. J Cell Biol. 1987 Oct;105(4):1925–1934. doi: 10.1083/jcb.105.4.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Sasaki Y., Shiba Y., Kanno Y., Yamasaki H. Tumor promoters cause a rapid and reversible inhibition of the formation and maintenance of electrical cell coupling in culture. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5628–5632. doi: 10.1073/pnas.78.9.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Rahman S. Gap junctions and intercellular communication: topology of the major junctional protein of rat liver. Biochem Soc Trans. 1989 Dec;17(6):983–985. doi: 10.1042/bst0170983. [DOI] [PubMed] [Google Scholar]
- Fallon R. F., Goodenough D. A. Five-hour half-life of mouse liver gap-junction protein. J Cell Biol. 1981 Aug;90(2):521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. J., Knowles S. E., Ballard F. J., Murray A. W. Rapid and reversible inhibition of junctional communication by tumor promoters in a mouse cell line. Cancer Res. 1983 Aug;43(8):3614–3618. [PubMed] [Google Scholar]
- Flagg-Newton J. L., Dahl G., Loewenstein W. R. Cell junction and cyclic AMP: 1. Upregulation of junctional membrane permeability and junctional membrane particles by administration of cyclic nucleotide or phosphodiesterase inhibitor. J Membr Biol. 1981;63(1-2):105–121. doi: 10.1007/BF01969452. [DOI] [PubMed] [Google Scholar]
- Ginzberg R. D., Gilula N. B. Modulation of cell junctions during differentiation of the chicken otocyst sensory epithelium. Dev Biol. 1979 Jan;68(1):110–129. doi: 10.1016/0012-1606(79)90247-1. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Paul D. L., Jesaitis L. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J Cell Biol. 1988 Nov;107(5):1817–1824. doi: 10.1083/jcb.107.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Spray D. C., Bennett M. V. Reduction of gap junctional conductance by microinjection of antibodies against the 27-kDa liver gap junction polypeptide. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2412–2416. doi: 10.1073/pnas.82.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y., Enomoto T., Shiba Y., Yamasaki H. Protective effect of cAMP on tumor promoter-mediated inhibition of cell-cell communication. Exp Cell Res. 1984 May;152(1):31–37. doi: 10.1016/0014-4827(84)90227-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird D. W., Revel J. P. Biochemical and immunochemical analysis of the arrangement of connexin43 in rat heart gap junction membranes. J Cell Sci. 1990 Sep;97(Pt 1):109–117. doi: 10.1242/jcs.97.1.109. [DOI] [PubMed] [Google Scholar]
- Larsen W. J., Wert S. E. Roles of cell junctions in gametogenesis and in early embryonic development. Tissue Cell. 1988;20(6):809–848. doi: 10.1016/0040-8166(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Manjunath C. K., Nicholson B. J., Teplow D., Hood L., Page E., Revel J. P. The cardiac gap junction protein (Mr 47,000) has a tissue-specific cytoplasmic domain of Mr 17,000 at its carboxy-terminus. Biochem Biophys Res Commun. 1987 Jan 15;142(1):228–234. doi: 10.1016/0006-291x(87)90475-x. [DOI] [PubMed] [Google Scholar]
- Manjunath C. K., Page E. Rat heart gap junctions as disulfide-bonded connexon multimers: their depolymerization and solubilization in deoxycholate. J Membr Biol. 1986;90(1):43–57. doi: 10.1007/BF01869685. [DOI] [PubMed] [Google Scholar]
- Milks L. C., Kumar N. M., Houghten R., Unwin N., Gilula N. B. Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J. 1988 Oct;7(10):2967–2975. doi: 10.1002/j.1460-2075.1988.tb03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. J., Parker R. A., Gibson D. M. Phosphorylation and degradation of HMG CoA reductase. Adv Enzyme Regul. 1989;28:65–77. doi: 10.1016/0065-2571(89)90064-2. [DOI] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A., Dahl G., Loewenstein W. R. Hormonal regulation of cell junction permeability: upregulation by catecholamine and prostaglandin E1. J Membr Biol. 1982;70(3):239–251. doi: 10.1007/BF01870566. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Regulation of enzyme levels by proteolysis: the role of pest regions. Adv Enzyme Regul. 1988;27:135–151. doi: 10.1016/0065-2571(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs N. J., Shovel K. S., Slade A. M., Powell T., Twist V. W., Green C. R. Fate of gap junctions in isolated adult mammalian cardiomyocytes. Circ Res. 1989 Jul;65(1):22–42. doi: 10.1161/01.res.65.1.22. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Gregory W. A., Watanabe T., Dermietzel R., Hertzberg E. L., Reid L., Bennett M. V., Spray D. C. cAMP delays disappearance of gap junctions between pairs of rat hepatocytes in primary culture. Am J Physiol. 1989 Jul;257(1 Pt 1):C1–11. doi: 10.1152/ajpcell.1989.257.1.1-a. [DOI] [PubMed] [Google Scholar]
- Takeda A., Hashimoto E., Yamamura H., Shimazu T. Phosphorylation of liver gap junction protein by protein kinase C. FEBS Lett. 1987 Jan 5;210(2):169–172. doi: 10.1016/0014-5793(87)81330-3. [DOI] [PubMed] [Google Scholar]
- Traub O., Look J., Paul D., Willecke K. Cyclic adenosine monophosphate stimulates biosynthesis and phosphorylation of the 26 kDa gap junction protein in cultured mouse hepatocytes. Eur J Cell Biol. 1987 Feb;43(1):48–54. [PubMed] [Google Scholar]
- Wiener E. C., Loewenstein W. R. Correction of cell-cell communication defect by introduction of a protein kinase into mutant cells. 1983 Sep 29-Oct 5Nature. 305(5933):433–435. doi: 10.1038/305433a0. [DOI] [PubMed] [Google Scholar]
- Yancey S. B., Edens J. E., Trosko J. E., Chang C. C., Revel J. P. Decreased incidence of gap junctions between Chinese hamster V-79 cells upon exposure to the tumor promoter 12-O-tetradecanoyl phorbol-13-acetate. Exp Cell Res. 1982 Jun;139(2):329–340. doi: 10.1016/0014-4827(82)90257-9. [DOI] [PubMed] [Google Scholar]
- Yancey S. B., John S. A., Lal R., Austin B. J., Revel J. P. The 43-kD polypeptide of heart gap junctions: immunolocalization, topology, and functional domains. J Cell Biol. 1989 Jun;108(6):2241–2254. doi: 10.1083/jcb.108.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey S. B., Nicholson B. J., Revel J. P. The dynamic state of liver gap junctions. J Supramol Struct Cell Biochem. 1981;16(3):221–232. doi: 10.1002/jsscb.1981.380160303. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer D. B., Green C. R., Evans W. H., Gilula N. B. Topological analysis of the major protein in isolated intact rat liver gap junctions and gap junction-derived single membrane structures. J Biol Chem. 1987 Jun 5;262(16):7751–7763. [PubMed] [Google Scholar]
- el Aoumari A., Fromaget C., Dupont E., Reggio H., Durbec P., Briand J. P., Böller K., Kreitman B., Gros D. Conservation of a cytoplasmic carboxy-terminal domain of connexin 43, a gap junctional protein, in mammal heart and brain. J Membr Biol. 1990 May;115(3):229–240. doi: 10.1007/BF01868638. [DOI] [PubMed] [Google Scholar]