Abstract
The effect of neurotrophin-4 (Ntf4) on mouse embryonic (day-14) neural stem cell (mE14-NSC) fate determination and the mechanisms involved were investigated. Using primary mE14-NSCs, immunocytochemistry and molecular-cell biological methods, such as Western-blotting, we characterized the effect of Ntf4 on mE14-NSC differentiation. Obtained in-vitro data revealed an interesting phenomenon of Ntf4 action resulting in enhanced mE14-NSC commitment to progenitor cells of the neuronal lineage. During this process, Ntf4 suppresses the interleukin 6 (Il6) family receptor and the Notch signalling pathways by modulating their specific receptor cleavages. The observed lineage commitment is controlled via an Ntf4-mediated modulation of protein kinase B (PKB/Akt) activity and characterized by a decreased Stat3 (signal transducer and activator of transcription-3) phosphorylation status. These findings suggest that the Ntf4-activated signalling cascade is responsible for initiating a concert among sheddases, kinases, and phosphatases to mediate neurogenesis.
Keywords: Neural stem cells, Neurotrophin, Lif receptor, Phosphatase, Ptpn11, Shp2, TrkB
Introduction
One of the most exciting developments of neurology over the past century is the demonstration of the presence of neural stem cells (NSCs) in the adult mammalian brain. These NSCs are the source of ongoing neurogenesis in the adult mammalian central nervous system (CNS) and are mainly present in the two principle regions of neurogenesis: (1) the subgranular zone (SGZ) of the hippocampus; and (2) the subventricular zone (SVZ) of the lateral ventricle (Curtis et al. 2007). NSCs are capable of unlimited self-renewal and giving rise to neurons [β-tubulin III-positive (Tubb3+)], astrocytes [Gfap+ (glial fibrillary acidic protein)], and oligodendrocytes [CNPase+ (2′,3′-cyclic nucleotide 3′ phosphodiesterase)] (Taupin and Gage 2002). Experimentally, embryonic day 14 (E14) mouse foetal brain is one of the major sources for NSCs. Isolated mE14-NSCs are usually maintained in the presence of epidermal growth factor (Egf) and/or fibroblast growth factor 2 (basic) (Fgf2) in suspension culture in-vitro, where they form spherical cell clusters, termed ‘neural spheres’. The neural spheres exhibit cell type heterogeneity, which includes stem cells, progenitor cells of both glial and neuronal lineage, differentiated cells, and apoptotic cells (Campos 2004).
Accumulating data indicate that the Notch and the interleukin 6 (Il6) receptor family signalling pathways appear to play pivotal roles in neurogliogenesis (Chiba 2006; Chojnacki et al. 2003; Ge et al. 2002; Grandbarbe et al. 2003; Grote and Hannan 2007; Nagao et al. 2007; Yoon and Gaiano 2005). The Notch signalling in NSCs starts from the ligand-induced sequential cleavage by Adam17 (a disintegrin and metalloproteinase domain 17, Tace) in the extracellular domain and a γ-secretase in the transmembrane (TM) domain to produce the Notch-1 intracellular domain (NICD) that translocates into the nucleus to regulate gene transcription in order to promote NSC maintenance, gliogenesis or neurogenesis, respectively (Chiba 2006; Chojnacki et al. 2003; Ge et al. 2002; Grandbarbe et al. 2003; Grote and Hannan 2007; Nagao et al. 2007; Yoon and Gaiano 2005).
Another family of growth factors, the Il6 family, acts hand-in-hand with Notch signalling. Members of the Il6 family bind to a receptor complex of the common Il6 signal transducer gp130 (Il6st) component and a ligand-specific co-receptor to activate the Il6st/Jak/Stat signalling pathway. In this pathway, the Janus-kinase (Jak)-mediated phosphorylated Stat1 (signal transducer and activator of transcription) and Stat3 form either homo- or heterodimers and translocate to the nucleus for target gene transcription activation (Heinrich et al. 1998). Upon stimulation by leukaemia inhibitor factor (Lif), Stat3 activation is sufficient to maintain embryonic stem cells (ES) and NSCs in an undifferentiated status (Matsuda et al. 1999). In contrast, the Jak-Stat signalling pathway selectively enhanced differentiation of NSCs along a glial lineage if stimulated by ciliary neurotrophic factor (Cntf) (Bauer and Patterson 2006; Chojnacki et al. 2003; Nakanishi et al. 2007; Pitman et al. 2004). Thus, while an autoregulatory loop in NSCs may activate Stat1/3 to promote astrogliogenesis, inhibition of the Stat3 pathway may promote neurogenesis (Gu et al. 2005; He et al. 2005; Islam et al. 2009a).
In contrast, members of the neurotrophin (Nt) family were suggested to channel NSC differentiation into a neuronal lineage (Lachyankar et al. 1997). The Nt family contains mainly four members: nerve growth factor (Ngf), brain-derived neurotrophic factor (Bdnf), Nt-3 (Ntf3), and Nt-4 (Ntf4). There are two types of Nt receptors, the Trk (tropomyosin-related kinase) family [Ntrk1 (TrkA), Ntrk2 (TrkB) and Ntrk3 (TrkC)] and the Ngfr (also known as p75NTR). While Ngf binds specifically to Ntrk1 and Ntf3 to Ntrk3, Bdnf and Ntf4 both use Ntrk2 to mediate their specific cellular effects (Reichardt 2006).
However, the actions of Nts are complex, largely because the existence of multiple alternative spliced isoforms of the Ntrk genes in the CNS and an even more complicated signalling mediated by Ngfr (Barker et al. 1993; Baxter et al. 1997; Dubus et al. 2000; Lamballe et al. 1991; Lamballe et al. 1993; Meakin et al. 1997; Ninkina et al. 1997; Radeke et al. 1987; Tacconelli et al. 2004; Tsoulfas et al. 1993; Valenzuela et al. 1993; von Schack et al. 2001). The classical signalling via Ntrk kinase isoforms are the Ras/Mapk, the Plcγ/Pkc and the Pi3k/Akt pathways (Chao 2003; Huang and Reichardt 2001, 2003; Ayyadhury and Heese 2007). The Ngfr on the other hand, can convey both live and death signals (Ibanez 2002; Nykjaer et al. 2005).
The expression of functional Nt receptors (Ntrks) by NSCs suggests that they are responsive to Nt treatment (Islam et al. 2009b). Interestingly, also the Nt receptors, similar to Notch or the Il6 family receptors, are processed by a regulatory intramembrane proteolytic process (RIP) (Althoff et al. 2000; Berezovska et al. 2001; Diaz-Rodriguez et al. 1999; Ebinu and Yankner 2002; Ehebauer et al. 2006; Frade 2005; Gil et al. 2007; Heese and Akatsu 2006; Layton et al. 1992; Lin et al. 2006a; Okochi et al. 2006; Six et al. 2003). Therefore, our study aimed at gaining insights into the effects of Nts on mouse embryonic day-14 neural stem cells (mE14-NSCs) to explore particularly the Ntf4-dependent mechanisms of self-renewal and differentiation. Data (in-vitro) obtained suggest that Ntf4 increased the commitment of mE14-NSCs into neural progenitors with a concurrent reduction of stem cell property.
Materials and Methods
Reagents
All reagents used for experiments were purchased from Sigma-Aldrich (Milwaukee, WI, USA) unless otherwise stated.
Growth Factors
Recombinant Egf and recombinant Ntf4 were purchased from Peprotech (Rocky Hill, NJ, USA).
Antibodies
For Western blotting the following were used: anti-Abcb1b (1:1,000, rabbit polyclonal; ProSci Inc., Poway, CA, USA), anti-phospho-Akt1/2/3 (also known as PKB, protein kinase Bα/β/γ, Serine-473, 1:2,000, rabbit polyclonal; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-Gfap (glial fibrillary acidic protein, 1:2,000, mouse monoclonal; Chemicon, Temecula, CA, USA), anti-Il6st (also known as gp130, 1:3,000; rabbit polyclonal, Upstate, Lake Placid, NY, USA), anti-pMapk1/3 (phosphorylated mitogen-activated protein kinase 1/3 (extracellular-regulated-kinases 2/1), 1:2,000, rabbit polyclonal; Santa Cruz), donkey/sheep polyclonal anti-goat/rabbit/mouse IgG (horseradish-peroxidase-coupled, 1:5,000; Amersham, Piscataway, NJ, USA), anti-Lifr (1:2,000, rabbit polyclonal, Santa Cruz), anti-Nes (Nestin, 1:2,000, mouse monoclonal; Chemicon), anti-Notch1 (1:1,000; goat-polyclonal; Santa Cruz), anti-Pou5f1 (Pou class 5 homeobox 1, also known as Oct-3/4, 1:3000, rabbit polyclonal; Santa Cruz), anti-Stat3 (signal transducers and activators of transcription protein, 1:2,000, rabbit polyclonal; Santa Cruz), anti-phospho-Stat3 (Tyr705, 1:500, mouse monoclonal; Santa Cruz), anti-alpha-tubulin (anti-Tuba1a, 1:2,000, mouse monoclonal; Santa Cruz), neuron-specific anti-β-class-III-tubulin [anti-Tubb3 (also known as Tuj1), 1:2,500, mouse monoclonal IgG; BAbCO, Richmond, CA, USA].
The following were used for immunocytochemistry(ICC)/-histochemistry: neuron-specific anti-β-class-III-tubulin (anti-Tubb3 (Tuj1, 1:500, rabbit polyclonal; Covance, Princeton, NJ, USA), anti-Gfap (1:500, mouse monoclonal; Chemicon), goat serum (Vector Laboratories, Burlingame, CA, USA)].
Animal Material
Experimental methods, including the killing of animals, were in accordance with the International Guiding Principles for Animal Research (WHO) and were approved by the local Institutional Animal Care and Use Committee (NTU-IACUC). Mouse tissues were isolated [C57BL/6J mice from the Animal Facility Centre at the National University (NUS) of Singapore] after humane killing of the animals using approved anaesthetic methods. Mouse brain perfusion and mE14-NSC culture (proliferation and differentiation) were performed as described previously (Islam et al. 2009a, b).
mE14-NSC Culture
Briefly, timed pregnancy female mice (C57BL/6J, NUS, Singapore) were anaesthetized on embryonic day-14 (E14) and foetuses were removed one at a time. Foetuses were killed by rapid decapitation, followed by immediate removal of the brain. Primary cultures were established from the forebrains (SVZ) of the foetuses. Dissociated embryonic tissue was digested with 0.025% trypsin (Invitrogen, Carlsbad, CA, USA) for 10 min, dissociated mechanically, and then passed through a 70-mm nylon mesh (cell strainer, BD Falcon, BD Biosciences, Bedford, MA, USA). After two washes with DMEM-F12 (1:1) (Gibco, Invitrogen), cells were exposed to the mitogen Egf in serum-free conditions. The obtained neurospheres of mE14-NSCs were grown in DMEM/F-12 (1:1) culture medium with 15 mM HEPES-buffer solution (HyClone Laboratories, Inc., Logan, UT, USA) and antibiotics supplemented with B27 supplement (1:50; Invitrogen) in the presence of Egf (5 ng/ml) for three passages (3P, 1P ~4 days). The cells were occasionally cultured in the presence of Egf (5 ng/ml) plus Ntf4 (5–100 ng/ml) for 3P, as indicated in the text. Cells were cultured in non-coated 75 cm2 Nunc (Thermo Fisher Scientific, Roskilde, Denmark) flasks at clonal density (2 × 105 cells/ml), and the medium was changed every 4 days (Islam et al. 2009a).
mE14-NSC Differentiation
Egf-derived neurospheres from E14 cortex tissue were cultured for 3P in Egf [±Ntf4 (100 ng/ml)] and then differentiated by seeding mechanically dissociated (single cell suspension) mE14-NSCs onto poly-L-Lysine (PLL)-coated glass cover-slips in 4-well plates at a density of 5 × 103 cells/ml in Egf-free Neurobasal medium (NB; Gibco, Invitrogen) containing 2 mM glutamax, 2 mM glutamine, and B27 supplement. Cells were incubated in a 37°C incubator for 5 days to observe their differentiation properties (Islam et al. 2009a).
Immunocytochemistry
mE14-NSCs were grown for 3P in Egf (±Ntf4) and then differentiated for 5 days before being fixed with 4% paraformaldehyde for 20 min at room temperature (RT). The cells were rinsed three times with PBS and blocked in PBS containing 0.01% Triton-X-100 (USB Corporation, Cleveland, OH, USA) and 10% normal goat serum (Vector Laboratories) prior to incubation with the different primary antibodies overnight at 4°C. After three washes in PBS, each sample was incubated for 1 h in secondary antibody labelled with FITC (AlexaFluor 568, goat polyclonal anti-rabbit, 1:400, or AlexaFluor 488, goat monoclonal anti-mouse, 1:400; Molecular Probes, Eugene, OR, USA). After three washes, the cover-slips were mounted onto glass slides using DAPI/Antifade glue (Chemicon) and analysed with a Carl Zeiss Live imaging microscope (Axiovert 200; Carl Zeiss, Göttingen, Germany) (Islam et al. 2009a).
Cell Lysis and Protein Extraction
For washing, floating neurospheres were aspirated and collected in 15-ml centrifuge tubes (BD Falcon, BD Biosciences). The cells were washed twice with Ca2+/Mg2+-free PBS (−/−) via centrifugation, and the supernatant was removed after the last wash. Lysis buffer [20 mM Tris–HCl (pH 7.4), 150 mM NaCl, 40 mM NaF, 5 mM EDTA, 1% Triton-X-100, 1 mM sodium orthovanadate, 1% (vol/vol) Nonidet P-40, 0.1% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 10 ng/ml of aprotinin] was added to each respective centrifuge tube, followed by a 5-min incubation on ice. Lysed cells were centrifuged at 10,000×g at 4°C for 10 min. The supernatant containing the protein extract was immediately used for further analysis (Islam et al. 2009a).
Western Blot Analysis
Western blot analyses were performed as described previously (Nehar et al. 2009). Briefly, 20 micrograms of total protein cell lysates were separated by 8–12% resolving SDS-PAGE at 0.02 Ampere (A) of constant current and transferred to a polyvinylidene fluoride (PVDF) membrane (0.22 μm; Amersham) using the ‘semi-dry’ transfer method (BioRad) for 60 min at 0.12 A in buffer containing 25 mM Tris, 192 mM glycine, 20% methanol, and 0.01% (wt/vol) SDS. The membrane was blocked with 5% BSA (BioRad) in Tris-buffered saline (TBS) solution plus 0.1% Tween-20 (TBS-T) or PBS-T for 2 h at RT, washed three times in PBS-T for 10 min each, and incubated with a primary antibody (diluted in 2% BSA in PBS-T) for 1 h at RT. The membranes were then washed as described above, incubated with a HRP-conjugated secondary antibody for 1 h at RT, and developed using the ECL plus Western-blot Detection Reagent (Amersham). X-ray films (Konica Minolta Inc., Tokyo, Japan) were exposed to the membranes before film development in a Kodak X-OMAT 2000 processor (Kodak, Ontario, Canada). For equal protein sample loading, protein quantification was performed with a ‘2D Quant’ kit (Amersham) using at least two independent replicates. BSA was used as a standard for protein quantification. To re-probe the same membrane with another primary antibody, Pierce’s (Pierce Biotechnology, Inc., Rockford, IL, USA) ‘stripping solution’ was used to strip the membranes. In addition, equal sample loading was confirmed using α-tubulin (Tuba1a) as a reference protein. Western blot experiments were performed at least three times for statistical quantification and analysis (n = 3), and representative blots are shown. Values (=relative protein expression) represent the ratio of densitometric scores (GS-800 Calibrated Densitometer and Quantity One quantification analysis software version 4.5.2; BioRad) for the respective Western-blot products [mean ± standard error of mean (SEM)] using the Tuba1a bands as a reference. Student t test was used to evaluate the significance of change between the means of test group and sample group if any comparison was observed.
Results
Ntf4 Inhibits Stem Cell Factors Expression
Based on our previous findings that NSCs express functional Ntrk2 (TrkB) receptors (Islam et al. 2009b), we stimulated mE14-NSCs, grown with Egf, with its specific ligand Ntf4 (Egf ± Ntf4). As indicated in Fig. 1, after three passages gown in Egf plus Ntf4, NSCs show an increased Tubb3 (neuronal marker) expression with Ntf4 in a dose-dependent manner, while Gfap (glial marker) expression was not significantly affected by Ntf4 (Fig. 1). The expression of various stem cell markers (Nestin, Pou5f1, and Abcb1) (Lin et al. 2006b) were all decreased with an increase of the Ntf4 concentration. At higher concentrations, Ntf4 also reduced the level of the intracellular domain of Notch (NICD, ~120 kDa), full-length Lifr-gp190 and full-length gp130 (Il6st). A previous report has shown that the specific Lifr is cleaved upon its activation by Lif and thereby contributing to Lif’s-activated signalling cascade basically conveyed by Il6st (gp130) (Blanchard et al. 2000). An interesting result is that the 45-kDa fragment of Lifr (at very low level in the control lysate (10 ng/ml Egf, no Ntf4) increased tremendously with a concurrent decrease of Lifr-gp190, while the 60-kDa fragment level was rather decreased with an increase of the Ntf4 concentration.
Fig. 1.
Ntf4’s dosage effects on mE14-NSCs. a Western blot analysis of mE14-NSCs grown as indicated in the presence of Egf and Ntf4 for three passages. Molecular weight markers are indicated in kDa. As control alpha-tubulin (Tuba1a) is shown for equal loading. Experiments were repeated three times and representative blots are shown. b and c Quantitative analysis of the Western-blots shown in a. *P < 0.05, comparing Egf [5 ng/ml and Ntf4 (0 ng/ml)] with Egf [5 ng/ml plus Ntf4 (100 ng/ml)]
Ntf4 Promotes Neuronal Differentiation of mE14-NSCs
To confirm the effect observed in our Western blot analysis we applied the ICC technology. The incubation of mE14-NSCs in the presence of Ntf4 produced large amounts of neurons (Tubb3+, red), but only very few glia cells (Gfap+, green) compared with control mE14-NSCs. The final neuron/glia ratio was increased tremendously by Ntf4 (Fig. 2).
Fig. 2.
Ntf4 increases neuronal differentiation in mE14-NSCs. mE14-NSCs were grown (+Egf) in the absence (left) or presence (right) of Ntf4 (100 ng/ml) for three passages before differentiated in NB/B27 for 5 days and ICC was performed with Gfap (green), Tubb3 (red) and DAPI (blue, counterstaining of nuclei). Ntf4-treated cells clearly show a significant higher amount of neurons (red Tubb3+ cells). Experiments were repeated three times and representative pictures are shown. (Color figure online)
Ntf4 Promotes Neurogenesis Through the Inhibition of Stat3
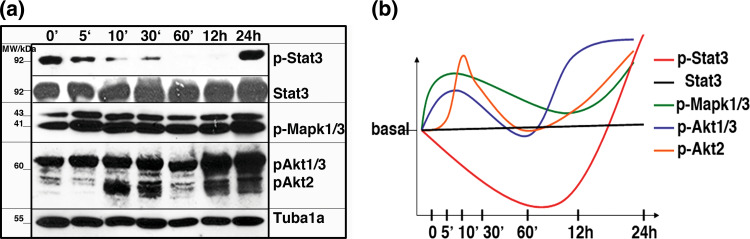
Upon culturing for three passages, mE14-NSCs were stimulated with Ntf4 for the indicated time period (Fig. 3). We could demonstrate that the Stat3 (Tyr705) phosphorylation was immediately down-regulated and reached the bottom level after 12 h, followed by an elevation to a hyper-phosphorylation status at 24 h. However, the total expression of the Stat3 protein was not significantly changed over the entire 24-h time period. Since the processing of the Lifr is also controlled by Mapk1/3 (Blanchard et al. 2000), we took a closer look at this enzyme which is often activated by growth factors including the Nts (Huang and Reichardt 2003). In contrast, Mapk1/3 phosphorylation achieved its first maximum at 5 min and decayed to its basal level before a second moderate phosphorylation at 24 h occurred. The Akt (PKB) action also appeared to have two waves over the entire 24-h period. Akt1/3 (~62 kDa) reached a transient peak of moderate activation ~5 min after Ntf4 stimulation and fell back to basal level after 1 h while the second hyper-activation arrived at 12 h and sustained for additional 12 h (24 h after stimulation). However, the first peak of Akt2 (~56 kDa) phosphorylation turned up at 10 min and decayed to basal level 1 h post Ntf4-stimulation while the second peak of moderate phosphorylation appeared at 12 h and sustained until 24 h. The overall trend of the phosphorylation level changes of all those substrates is represented in the ‘trend-graph’ (Fig. 3b). These results indicate the existence of both transient and sustained signalling mechanisms involved during the mE14-NSC commitment into neuronal progenitor cells upon Ntf4 stimulation.
Fig. 3.
Time-dependent inhibition of Stat3 activity in Ntf4-stimulated mE14-NSCs. a Western blot analysis revealed the changes of the phosphorylation levels of Stat3, Mapk1/3, Akt1/3 (~62 kDa) and Akt2 (~55 kDa) over a period of 24 h with a significant early inhibition of Stat3 activity that, however, returns back even beyond basal levels after 24 h. mE14-NSCs were grown and stimulated as described in “Materials and Methods” for the indicated time. Experiments were repeated three times and representative blots are shown. b A graphic representation showing the trend of phosphorylation level alterations of the substrates analysed in a. (Color figure online)
Discussion
Our in-vitro experiments show that the application of Ntf4 onto mE14-NSC culture decreased the expression of several stem cell property markers and reduced the full-length Lifr and Il6st (gp130) protein levels in mE14-NSCs and thereby increased the neuronal progenitor cell population. This effect was due to an increased shedding of these full-length receptors. Since Mapk1/3 (Erk1/2) is also one of the main signaling components activated by Ntrk receptors (Huang and Reichardt 2003; Islam et al. 2009b), it is possible that the linkage between the Ntf4 signalling pathways and the increased shedding of Lifr and Il6st is the Mapk1/3 cascade. In parallel with the sensitization of Il6st and Lifr for RIP through their phosphorylation, Mapk1/3 may also up-regulate the cleaving activity of Tace because Lifr-gp190 and Il6st cleavages are required for their inhibition in order to facilitate mE14-NSC differentiation (Lin et al. 2006a; Schiemann et al. 1995).
The down-regulation of the NICD signaling pathways by Ntf4 was a result of a change in the proteolytic processing of Notch-1, which could be explained by an inhibited cleavage mediated either by Tace or a γ-secretase, or by further activated cleavage of NICD. A reduced cleavage mechanism by a γ-secretase might be due to the up-regulation of competitive substrates of γ-secretase, such as the β-amyloid precursor protein (APP) (Berezovska et al. 2001).
The rapid decrease of Stat3 phosphorylation implies phosphatase activation upon Ntf4 stimulation. Previous studies confirmed the activation of Ptpn11 (also known as Shp2, SH2 domain-containing protein tyrosine phosphatase-2) by the Ntf4 receptor Ntrk2 (TrkB) as an effector that down-regulates Stat3 action. Therefore, Shp2 might be the responsible mediator of Ntf4 signaling to promote the permission to an initial step of neural differentiation—commitment of mE14-NSCs to neural progenitors. This interpretation leads to a two-step hypothesis of NSC differentiation, exit of self-renewal followed by cell fate determination (Feng 2007; Gu et al. 2005). The later sustained activation of Stat3 and Akt suggests that by the time of 24 h the commitment of mE14-NSCs to progenitors has been completed, and the neural progenitor cells resume a rapid cell cycle. Based on our results obtained a model of Ntf4 action in mE14-NSCs was built as shown in Fig. 4.
Fig. 4.
Proposed Ntf4 signalling during the differentiation of NSCs into neuronal progenitors. Ntf4 activates Erk1/2 (Mapk1/3) and Shp2 resulting (1) in the dephosphorylation and inhibition of Stat3 and (2) the shedding and inhibition of the Il6 family receptors Lifr and gp130 (Il6st), both by substrate sensitization and Tace activation. Concurrently, Ntf4 antagonizes Notch-1 signaling (reduced cleavage) by increasing the shedding of its competitor APP by the γ-secretase. All these Ntf4-initiated effects enhance the permission of NSC commitment towards the neuronal lineage. (Color figure online)
However, further investigations of the concert between the shedding of transmembrane receptors and Nt signaling are needed to provide additional knowledge that can facilitate the development of effective cellular therapies using NSCs for the treatment of neurodegenerative diseases.
Acknowledgments
This study was supported by an A*STAR grant (BMRC/04/1/22/19/360) to K.H.
References
- Althoff K, Reddy P, Voltz N, Rose-John S, Mullberg J (2000) Shedding of interleukin-6 receptor and tumor necrosis factor alpha. Contribution of the stalk sequence to the cleavage pattern of transmembrane proteins. Eur J Biochem 267:2624–2631 [DOI] [PubMed] [Google Scholar]
- Ayyadhury S, Heese K (2007) Neurotrophins—more than neurotrophic. Curr Immunol Rev 3:125–189 [Google Scholar]
- Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM (1993) Tissue-specific alternative splicing generates two isoforms of the trkA receptor. J Biol Chem 268:15150–15157 [PubMed] [Google Scholar]
- Bauer S, Patterson PH (2006) Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci 26:12089–12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter GT, Radeke MJ, Kuo RC, Makrides V, Hinkle B, Hoang R, Medina-Selby A, Coit D, Valenzuela P, Feinstein SC (1997) Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2. J Neurosci 17:2683–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovska O, Jack C, Deng A, Gastineau N, Rebeck GW, Hyman BT (2001) Notch1 and amyloid precursor protein are competitive substrates for presenilin1-dependent gamma-secretase cleavage. J Biol Chem 276:30018–30023 [DOI] [PubMed] [Google Scholar]
- Blanchard F, Duplomb L, Wang Y, Robledo O, Kinzie E, Pitard V, Godard A, Jacques Y, Baumann H (2000) Stimulation of leukemia inhibitory factor receptor degradation by extracellular signal-regulated kinase. J Biol Chem 275:28793–28801 [DOI] [PubMed] [Google Scholar]
- Campos LS (2004) Neurospheres: insights into neural stem cell biology. J Neurosci Res 78:761–769 [DOI] [PubMed] [Google Scholar]
- Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309 [DOI] [PubMed] [Google Scholar]
- Chiba S (2006) Notch signaling in stem cell systems. Stem Cells 24:2437–2447 [DOI] [PubMed] [Google Scholar]
- Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S (2003) Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci 23:1730–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Eriksson PS, Faull RL (2007) Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin Exp Pharmacol Physiol 34:528–532 [DOI] [PubMed] [Google Scholar]
- Diaz-Rodriguez E, Cabrera N, Esparis-Ogando A, Montero JC, Pandiella A (1999) Cleavage of the TrkA neurotrophin receptor by multiple metalloproteases generates signalling-competent truncated forms. Eur J Neurosci 11:1421–1430 [DOI] [PubMed] [Google Scholar]
- Dubus P, Parrens M, El-Mokhtari Y, Ferrer J, Groppi A, Merlio JP (2000) Identification of novel trkA variants with deletions in leucine-rich motifs of the extracellular domain. J Neuroimmunol 107:42–49 [DOI] [PubMed] [Google Scholar]
- Ebinu JO, Yankner BA (2002) A RIP tide in neuronal signal transduction. Neuron 34:499–502 [DOI] [PubMed] [Google Scholar]
- Ehebauer M, Hayward P, Martinez-Arias A (2006) Notch signaling pathway. Sci STKE 2006: cm7 [DOI] [PubMed]
- Feng GS (2007) Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell Res 17:37–41 [DOI] [PubMed] [Google Scholar]
- Frade JM (2005) Nuclear translocation of the p75 neurotrophin receptor cytoplasmic domain in response to neurotrophin binding. J Neurosci 25:1407–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Martinowich K, Wu X, He F, Miyamoto A, Fan G, Weinmaster G, Sun YE (2002) Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. J Neurosci Res 69:848–860 [DOI] [PubMed] [Google Scholar]
- Gil C, Cubi R, Aguilera J (2007) Shedding of the p75NTR neurotrophin receptor is modulated by lipid rafts. FEBS Lett 581:1851–1858 [DOI] [PubMed] [Google Scholar]
- Grandbarbe L, Bouissac J, Rand M, Hrabe de Angelis M, Artavanis-Tsakonas S, Mohier E (2003) Delta-Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development 130:1391–1402 [DOI] [PubMed] [Google Scholar]
- Grote HE, Hannan AJ (2007) Regulators of adult neurogenesis in the healthy and diseased brain. Clin Exp Pharmacol Physiol 34:533–545 [DOI] [PubMed] [Google Scholar]
- Gu F, Hata R, Ma YJ, Tanaka J, Mitsuda N, Kumon Y, Hanakawa Y, Hashimoto K, Nakajima K, Sakanaka M (2005) Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J Neurosci Res 81:163–171 [DOI] [PubMed] [Google Scholar]
- He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, de Vellis J, Sun YE (2005) A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci 8:616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese K, Akatsu H (2006) Alzheimer’s disease—an interactive perspective. Curr Alzheimer Res 3:109–121 [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L (1998) Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334(Pt 2):297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642 [DOI] [PubMed] [Google Scholar]
- Ibanez CF (2002) Jekyll-Hyde neurotrophins: the story of proNGF. Trends Neurosci 25:284–286 [DOI] [PubMed] [Google Scholar]
- Islam O, Gong X, Rose-John S, Heese K (2009a) Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell 20:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam O, Loo TX, Heese K (2009b) Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res 6:42–53 [DOI] [PubMed] [Google Scholar]
- Lachyankar MB, Condon PJ, Quesenberry PJ, Litofsky NS, Recht LD, Ross AH (1997) Embryonic precursor cells that express Trk receptors: induction of different cell fates by NGF, BDNF, NT-3, and CNTF. Exp Neurol 144:350–360 [DOI] [PubMed] [Google Scholar]
- Lamballe F, Klein R, Barbacid M (1991) trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 66:967–979 [DOI] [PubMed] [Google Scholar]
- Lamballe F, Tapley P, Barbacid M (1993) trkC encodes multiple neurotrophin-3 receptors with distinct biological properties and substrate specificities. EMBO J 12:3083–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton MJ, Cross BA, Metcalf D, Ward LD, Simpson RJ, Nicola NA (1992) A major binding protein for leukemia inhibitory factor in normal mouse serum: identification as a soluble form of the cellular receptor. Proc Natl Acad Sci USA 89:8616–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Rose-John S, Grotzinger J, Conrad U, Scheller J (2006a) Functional expression of a biologically active fragment of soluble gp130 as an ELP-fusion protein in transgenic plants: purification via inverse transition cycling. Biochem J 398:577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Islam O, Heese K (2006b) ABC transporters, neural stem cells and neurogenesis—a different perspective. Cell Res 16:857–871 [DOI] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T (1999) STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 18:4261–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin SO, Gryz EA, MacDonald JI (1997) A kinase insert isoform of rat TrkA supports nerve growth factor-dependent cell survival but not neurite outgrowth. J Neurochem 69:954–967 [DOI] [PubMed] [Google Scholar]
- Nagao M, Sugimori M, Nakafuku M (2007) Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol 27:3982–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H (2007) Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci 25:649–658 [DOI] [PubMed] [Google Scholar]
- Nehar S, Mishra M, Heese K (2009) Identification and characterisation of the novel amyloid-beta peptide-induced protein p17. FEBS Lett 583:3247–3253 [DOI] [PubMed] [Google Scholar]
- Ninkina N, Grashchuck M, Buchman VL, Davies AM (1997) TrkB variants with deletions in the leucine-rich motifs of the extracellular domain. J Biol Chem 272:13019–13025 [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE, Petersen CM (2005) p75NTR—live or let die. Curr Opin Neurobiol 15:49–57 [DOI] [PubMed] [Google Scholar]
- Okochi M, Fukumori A, Jiang J, Itoh N, Kimura R, Steiner H, Haass C, Tagami S, Takeda M (2006) Secretion of the Notch-1 Abeta-like peptide during Notch signaling. J Biol Chem 281:7890–7898 [DOI] [PubMed] [Google Scholar]
- Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ (2004) LIF receptor signaling modulates neural stem cell renewal. Mol Cell Neurosci 27:255–266 [DOI] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM (1987) Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature 325:593–597 [DOI] [PubMed] [Google Scholar]
- Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361:1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann WP, Graves LM, Baumann H, Morella KK, Gearing DP, Nielsen MD, Krebs EG, Nathanson NM (1995) Phosphorylation of the human leukemia inhibitory factor (LIF) receptor by mitogen-activated protein kinase and the regulation of LIF receptor function by heterologous receptor activation. Proc Natl Acad Sci USA 92:5361–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six E, Ndiaye D, Laabi Y, Brou C, Gupta-Rossi N, Israel A, Logeat F (2003) The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc Natl Acad Sci USA 100:7638–7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli A, Farina AR, Cappabianca L, Desantis G, Tessitore A, Vetuschi A, Sferra R, Rucci N, Argenti B, Screpanti I, Gulino A, Mackay AR (2004) TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell 6:347–360 [DOI] [PubMed] [Google Scholar]
- Taupin P, Gage FH (2002) Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res 69:745–749 [DOI] [PubMed] [Google Scholar]
- Tsoulfas P, Soppet D, Escandon E, Tessarollo L, Mendoza-Ramirez JL, Rosenthal A, Nikolics K, Parada LF (1993) The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron 10:975–990 [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Maisonpierre PC, Glass DJ, Rojas E, Nunez L, Kong Y, Gies DR, Stitt TN, Ip NY, Yancopoulos GD (1993) Alternative forms of rat TrkC with different functional capabilities. Neuron 10:963–974 [DOI] [PubMed] [Google Scholar]
- von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G (2001) Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci 4:977–978 [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N (2005) Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci 8:709–715 [DOI] [PubMed] [Google Scholar]