Abstract
Case:
We describe treatment of severe multilevel congenital thoracic fusion in a 3-year-old girl with Apert Syndrome by posterior element excision, posterior column osteotomies, and gradual distraction with magnetically controlled growing rods (MCGR) with 3-year follow-up. We also describe short-term follow-up with similar management in an 8-year-old patient with a congenitally fused thoracic spine from Jarcho-Levin syndrome.
Conclusion:
Posterior element resection and targeted posterior column osteotomies combined with gradual distraction with MCGR offers a promising treatment course for children with severe thoracic insufficiency syndrome derived from congenital fusions.
Keywords: pediatric, thoracic insufficiency syndrome, TIS, MCGR, magnetically controlled growing rod, laminectomy, osteotomy, Apert syndrome, Jarcho-Levin syndrome, posterior release, posterior column release, congenital fusion, congenital failure of segmentation, failure of segmentation, thorax
Thoracic insufficiency syndrome (TIS) describes a collection of disorders which prevent the thorax from adequately supporting normal respiration and lung growth1. Common causes of TIS include scoliosis/kyphosis, skeletal dysplasia, neuromuscular dysfunctions, and other afflictions, which affect the chest wall, lungs, or respiratory muscles1-3. Severe cases of TIS are often lethal if left untreated, as the child’s own growth drives increasing demands for which the impacted lungs are unable to meet1,2,4. While definitive spinal fusion can be successful in treating cases of TIS associated with deformity, the procedure limits the future growth, potentially exacerbating the underlying issue.5
Rib and spine-based growth friendly constructs, including magnetically controlled growing rods (MCGRs), can be used to increase the spine length and correct the deformities causing TIS1. While traditional growing rod constructs require subsequent operations for each expansion, MCGRs can be expanded without the need for an operation and can be performed more frequently, resulting in fewer infectious complications and anesthesia exposures4,6. Although MCGRs are most often indicated for managing scoliosis, there is little literature demonstrating their utility in treating other causes of TIS7. Here we describe the treatment of 2 cases of severe posterior element fusion of the thoracic spine using a novel surgical strategy of posterior release and gradual lengthening with MCGRs.
The patients were informed that data concerning their cases would be submitted for publication, and they provided consent.
Case Reports
Patient 1
A 3-year-old girl with Apert Syndrome and severe TIS due to the failure of segmentation throughout the thoracic spine, progressive lordotic deformity, and tracheal sleeve had been admitted to our hospital since the age of 2 months. She successfully underwent a slide tracheoplasty but remained ventilator dependent with limited thoracic growth, worsening thoracic lordosis, and compression of the main stem bronchus. On examination, she was ventilator dependent, nonambulatory, with craniofacial and hand features of Apert syndrome. Radiographs and 3D computed tomography (CT) (Figs. 1 and 2) demonstrated the segmentation anomalies and a T1-T12 length of 8 cm. Thoracic lordosis measured 60° (Fig. 3). Owing to the patient's young age, developmental ability, and ventilator dependency, spirometry measures were not obtainable. She underwent posterior element release and insertion of magnetic growing rods as described. After T2-T10 laminectomy and posterior column osteotomies at T3, T9, and T10, (which included complete bilateral open foraminotomies and facetectomies) (Fig. 4), 4.5 × 70 mm actuator MCGRs were placed bilaterally from T1 to L2, with 4.5-mm screws at T1, T3, L1, and L2, using computer navigation. Osteotomy levels were determined practically by space available, with the goal to create at least 2 to 3 levels of separation of the posterior tether to allow the distraction and growth of the spine. These were essentially Ponte-type osteotomies. Bone wax and bovine collagen–dural substitute were placed in the osteotomy sites to help prevent reconsolidation of the fusion mass. Neuromonitoring was temporarily lost after the insertion of the right T1 screw, so a rib hook was used at that level instead. Postoperatively, the patient began 10 days of lengthening, striving for 1 mm per day. These lengthenings were performed awake in the pediatric intensive care unit. The rods were then expanded 2 mm per month over the next 3 months, until the patient was discharged from the hospital, acquiring approximately 1.7 cm of distraction bilaterally as confirmed by ultrasound.
Fig. 1.
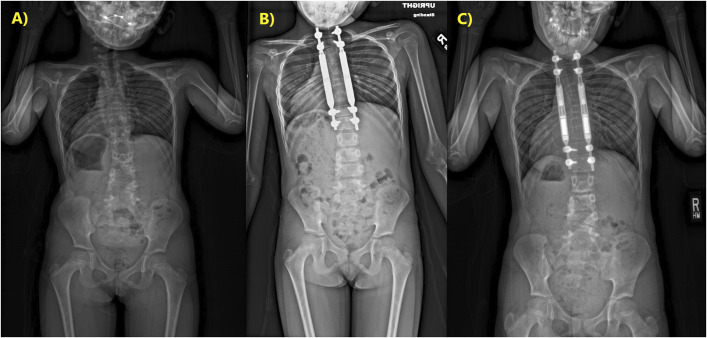
Fig. 1-A Preindex PA radiograph of Patient 1 at the age of 3 years, demonstrating the congenital fusion and short thorax. Fig. 1-B 5-month postoperative PA radiograph of Patient 1. Fig. 1-C 3.5-year (most recent) postoperative PA radiograph of Patient 1.
Fig. 2.
Fig. 2-A Preoperative lateral radiograph of Patient 1 at the age of 3 years. Fig. 2-B 5-month postoperative lateral radiograph of Patient 1. Fig. 2-C 3.5-year (most recent) postoperative lateral radiograph of Patient 1.
Fig. 3.
Fig. 3-A Preoperative and (Fig. 3-B) postoperative sagittal CT scans of the spine of Patient 1. The preoperative CT scan demonstrates the extensive posterior spinal fusion and thoracic lordosis. The immediate postoperative CT scan demonstrates the extensive laminectomy, straightening of her thoracic lordosis, and lengthening from 98 mm to 112 mm. Fig. 3-C Preoperative 3D reconstruction conveys the extent and severity of the posterior fusion.
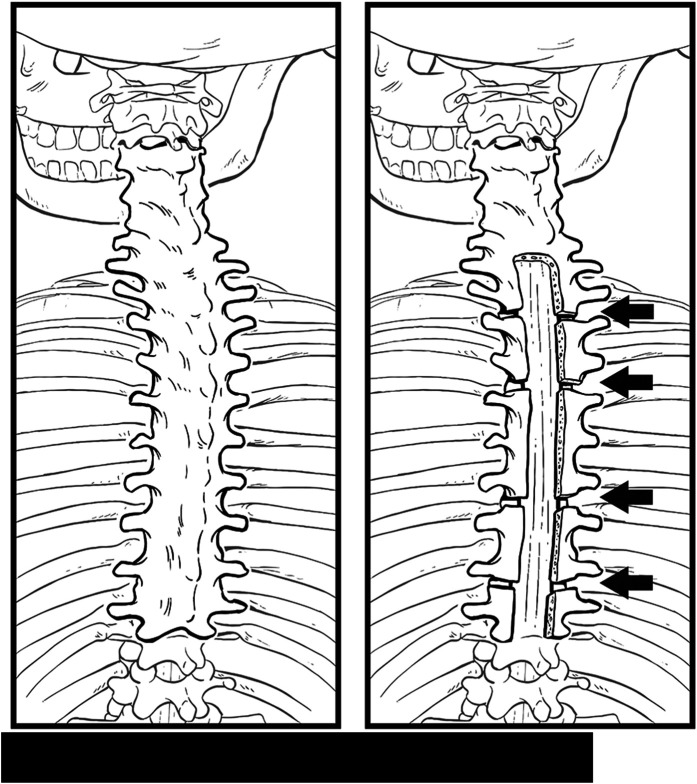
Fig. 4.
Illustration demonstrating extensive preoperative posterior element fusion and postoperative resection of lamina with posterior column osteotomies at select levels determined by available space.
She returned to the clinic at 3-month intervals for lengthening of 3 mm. After outgrowing her rods, she underwent a rod exchange to 90-mm actuators just under 2 years after her index magnetic growing rod placement. By then, radiographs demonstrated 3 cm of additional length, with ultrasound showing 2.7 cm of total MCGR distraction at an average gain of 0.90 mm/month since her first outpatient distraction. She has continued her lengthening, achieving a total T1-T12 length radiographically of 14.7 cm (net 6.7 cm length gain) and a thoracic lordosis of 3° at 3.5 years postoperatively. Ultrasound revealed 1.4 cm of MCGR distraction of the new rods, at a rate of 0.70 mm/month postexchange. Her ventilator support has not changed since the index procedure; however, her overall activity level has subjectively improved dramatically.
Patient 2
An 8-year-old girl with Jarcho-Levin syndrome; congenital fusion of her spine from C1-C6, T3-T11, T12-L1; and partial right-side fusion of T2-T3 (Figs. 5 and 6) had been followed for 4 years. Throughout this time, she had little growth, reaching a T1-T12 length of 11 cm and advancing thoracic lordosis measuring 43°. This resulted in significant short stature and progressively worsening restrictive lung disease and TIS, with a preoperative forced vital capacity (FVC) of 0.81 L, 65% predicted based on arm span. Her physical examination findings were otherwise unremarkable. Encouraged by the results of Patient 1, this patient underwent posterior release by laminectomy from T1-L1, posterior column osteotomies of the fused thoracic spine at T4-T5 and T9-T10, and implantation of MCGR from T1-L1. The procedure occurred uneventfully. She remained in the hospital for daily 1-mm lengthening starting on postoperative day 2. On postoperative day 6, she was discharged home in a stable condition, with 4.5 mm of achieved MCGR distraction as confirmed by ultrasound. She then underwent weekly 3-mm lengthening for the first 2 weeks, transitioning to planned 2-mm lengthening every 3 months. At her most recent radiographic evaluation, 1.5 years later, her T1-T12 length was 13.2 cm (net 2.2 cm length gain) and her lordosis had improved to 2°. Total MCGR distraction was 1.2 cm bilaterally (average distraction 0.39 mm/month since first outpatient distraction) as confirmed by ultrasound. One-year postoperative spirometry showed FVC of 0.84 L, 60% predicted. Her FVC has increased from 0.64 in 2020 to 0.81 in 2023. %FVC improved from 56% in 2020 to 65% in 2023.
Fig. 5.
Fig. 5-A Preoperative PA radiograph of Patient 2 at the age of 8 years. Fig. 5-B 1-month postoperative PA radiograph of Patient 2. Fig. 5-C 1.5-year (most recent) PA radiograph of Patient 2.
Fig. 6.
Fig. 6-A Preoperative lateral radiograph of Patient 2 at the age of 8 years. Fig. 6-B 1-month postoperative lateral radiograph of Patient 2. Fig. 6-C 1.5-year (most recent) lateral radiograph of Patient 2.
Discussion
In this report, we describe posterior spinal release and gradual distraction with MCGRs in 2 patients with severe congenital posterior element fusion. Treatment of these unusual, life-threatening deformities has not to our knowledge been described. Early onset spine deformity is associated with increased mortality8. The cause of death appears to be primarily cardiopulmonary, with an annual mortality rate of 25 deaths per 100,000 patients with EOS, at an average age of death of 10.6 years9. For those who are treated operatively the average age was 12.3 years, and for those without surgery, the average age was 7.0 years.
Spine growth is a key contributor to thoracic volume. Normal thoracic spine length at birth is 12 cm and increases to 18 cm by the age of 5 years, with a normal adult thoracic spine length of 24 to 26 cm4,10. Severe congenital fusion limits the thoracic spinal growth (in these cases locked in at 8 cm and 11 cm of length, respectively) and, consequently, thoracic volume. Along with the secondary lordotic deformity, the condition of these patients is expected to result in decreased pulmonary function, increased central airway compression and obstructive airway disease, and increased risk of cardiopulmonary complications and death5,11,12.
Thoracic insufficiency as originally described by Campbell was focused on chest wall growth and treatment thereof to prevent cardiopulmonary failure13. These 2 patients were selected based on existing, severe, and restrictive lung disease and worsening thoracic insufficiency due to tethered spine growth. For patient 1, we were unable to perform spirometry due to her young age and the presence of a tracheostomy, but she was already ventilator dependent. Patient 2 had a measurable worsening of restrictive lung disease on spirometry, again with little demonstrable growth potential due to posterior column spine tethering.
For patient 1, her pulmonary disease was considered terminal, so we accepted the risk that growth might not be possible. Given the success of patient 1 and patient 2's worsening condition, we had confidence that this technique could be successful, and so proceeded with appropriate informed consent of the family. For both of these patients, mobility of the anterior spine was not formally assessed, but given the presence of rudimentary disks, we were hopeful that distraction-driven growth would be possible. A complete fusion block of the anterior spine may be a relative contraindication; however, the growth of fused segments with distraction techniques has been described7.
While the short-term success of this procedure is encouraging, the long-term outcomes remain in question. Given the limited follow-up, we cannot say with certainty that we will avoid either the “law of diminishing returns” or autofusion. As described, we attempted to use multiple combined strategies to avoid the autofusion. At the time of revision, the osteotomy sights were not exposed, but repeat CT scan (not shown) would suggest that the autofusion had not occurred. Owing to the novelty of this approach, there are only a few cases reported in the literature on similar procedures7. The importance of establishing further research into this treatment course and its long-term outcomes is paramount to understanding the true benefit and utility of this method in the treatment of severe TIS.
Conclusion
Severe congenital spine fusion causing TIS is a complex and challenging pathology to treat. Here, we presented 2 cases of posterior release and gradual lengthening using magnetically controlled growing rods with a methodology that demonstrated a safe and effective strategy.
Acknowledgments
Note: The authors would like to thank Natalie Maharaj for her assistance in preparing this manuscript.
Footnotes
Investigation performed at Children's Hospital Colorado and Seattle Children's Hospital, Seattle, Washington
All of the following authors contributed significantly to the development of this manuscript through data analysis, manuscript preparation, or analysis and editing of final manuscript.
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSCC/C476).
References
- 1.Mayer O, Campbell R, Cahill P, Redding G. Thoracic insufficiency syndrome. Curr Probl Pediatr Adolesc Health Care. 2016;46(3):72-97. [DOI] [PubMed] [Google Scholar]
- 2.Redding GJ. Clinical issues for pediatric pulmonologists managing children with thoracic insufficiency syndrome. Front Pediatr. 2020;8:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell RM, Jr, Smith MD, Mayes TC, Mangos JA, Willey-Courand DB, Kose N, Pinero RF, Alder ME, Duong HL, Surber JL. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85(3):399-408. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RM, Jr, Smith MD. Thoracic insufficiency syndrome and exotic scoliosis. J Bone Joint Surg Am. 2007;89(suppl 1):108-22. [DOI] [PubMed] [Google Scholar]
- 5.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90(6):1272-81. [DOI] [PubMed] [Google Scholar]
- 6.Cheung JPY, Cheung KM. Current status of the magnetically controlled growing rod in treatment of early-onset scoliosis: what we know after a decade of experience. J Orthop Surg (Hong Kong). 2019;27(3):2309499019886945. [DOI] [PubMed] [Google Scholar]
- 7.Kwan KYH, Cheung JPY, Yiu KKL, Cheung KMC. Ten year follow-up of Jarcho-Levin syndrome with thoracic insufficiency treated by VEPTR and MCGR VEPTR hybrid. Eur Spine J. 2018;27(suppl 3):287-91. [DOI] [PubMed] [Google Scholar]
- 8.Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976). 1992;17(9):1091-6. [DOI] [PubMed] [Google Scholar]
- 9.Guzek RH, Murphy R, Hardesty CK, Emans JB, Garg S, Smith JT, Roye BD, Glotzbecker MP, Sturm PF, Snyder BD, Poon SC, Poe-Kochert C, Anari JB, Pediatric Spine Study Group PSSG. Mortality in early-onset scoliosis during the growth-friendly surgery era. J Pediatr Orthop. 2022;42(3):131-7. [DOI] [PubMed] [Google Scholar]
- 10.Dimeglio A. Le rachis en croissance: scoliose, taille assise et puberté Alain Dimeglio, François Bonnel. Paris Berlin Heidelberg: Springer-Verlag; 1990. [Google Scholar]
- 11.Karol LA. The natural history of early-onset scoliosis. J Pediatr Orthop. 2019;39(6, suppl 1):S38-s43. [DOI] [PubMed] [Google Scholar]
- 12.Vitale MG, Matsumoto H, Bye MR, Gomez JA, Booker WA, Hyman JE, Roye DP, Jr. A retrospective cohort study of pulmonary function, radiographic measures, and quality of life in children with congenital scoliosis: an evaluation of patient outcomes after early spinal fusion. Spine (Phila Pa 1976). 2008;33(11):1242-9. [DOI] [PubMed] [Google Scholar]
- 13.Campbell RM, Jr, Hell-Vocke AK. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am. 2003;85(3):409-20. [DOI] [PubMed] [Google Scholar]