Abstract
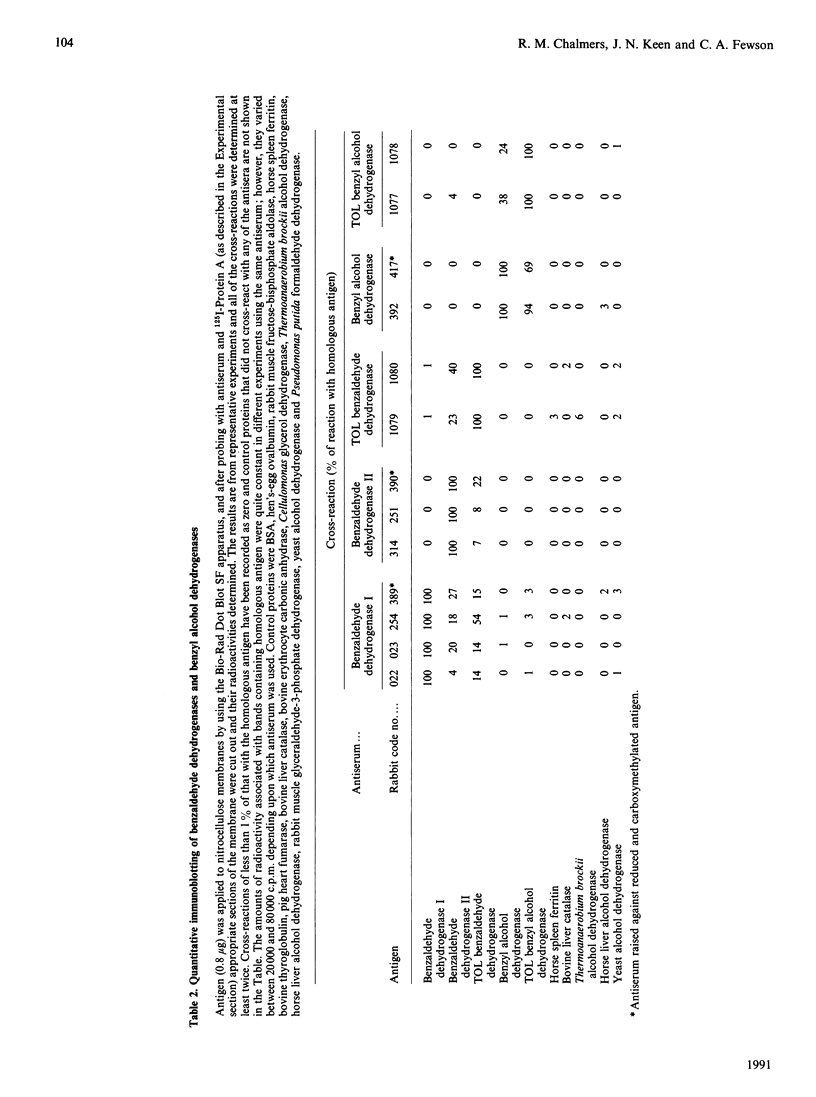
1. N-Terminal sequences were determined for benzyl alcohol dehydrogenase, benzaldehyde dehydrogenase I and benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus N.C.I.B. 8250, benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase encoded by the TOL plasmid pWW53 in Pseudomonas putida MT53 and yeast K(+)-activated aldehyde dehydrogenase. Comprehensive details of the sequence determinations have been deposited as Supplementary Publication SUP 50161 (5 pages) at the British Library Document Supply Centre, Boston Spa. Wetherby. West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1991) 273. 5. The extent of sequence similarity suggests that the benzyl alcohol dehydrogenases are related to each other and also to established members of the family of long-chain Zn2(+)-dependent alcohol dehydrogenases. Benzaldehyde dehydrogenase II from Acinetobacter appears to be related to the Pseudomonas TOL-plasmid-encoded benzaldehyde dehydrogenase. The yeast K(+)-activated aldehyde dehydrogenase has similarity of sequence with the mammalian liver cytoplasmic class of aldehyde dehydrogenases but not with any of the Acinetobacter or Pseudomonas enzymes. 2. Antisera were raised in rabbits against the three Acinetobacter enzymes and both of the Pseudomonas enzymes, and the extents of the cross-reactions were determined by immunoprecipitation assays with native antigens and by immunoblotting with SDS-denatured antigens. Cross-reactions were detected between the alcohol dehydrogenases and also among the aldehyde dehydrogenases. This confirms the interpretation of the N-terminal sequence comparisons and also indicates that benzaldehyde dehydrogenase I from Acinetobacter may be related to the other two benzaldehyde dehydrogenases. 3. The amino acid compositions of the Acinetobacter and the Pseudomonas enzymes were determined and the numbers of amino acid residues per subunit were calculated to be: benzyl alcohol dehydrogenase and TOL-plasmid-encoded benzyl alcohol dehydrogenase, 381; benzaldehyde dehydrogenase I and benzaldehyde dehydrogenase II, 525; TOL-plasmid-encoded benzaldehyde dehydrogenase, 538.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N. Immunochemical resemblance between human leukemia and hen egg-ehite lysozyme and their reduced carboxymethyl derivatives. J Mol Biol. 1971 Oct 14;61(1):237–250. doi: 10.1016/0022-2836(71)90220-8. [DOI] [PubMed] [Google Scholar]
- Arnon R., Neurath H. An immunological approach to the study of evolution of trypsins. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1323–1328. doi: 10.1073/pnas.64.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Betts G. F. Rapid purification and properties of potassium-activated aldehyde dehydrogenase from Saccharomyces cerevisiae. Biochem J. 1978 Sep 1;173(3):773–786. doi: 10.1042/bj1730773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen J., Kolb E., Harris J. I. Amino acid sequence homology in alcohol dehydrogenase. FEBS Lett. 1973 Jun 15;33(1):1–3. doi: 10.1016/0014-5793(73)80144-9. [DOI] [PubMed] [Google Scholar]
- Burlage R. S., Hooper S. W., Sayler G. S. The TOL (pWW0) catabolic plasmid. Appl Environ Microbiol. 1989 Jun;55(6):1323–1328. doi: 10.1128/aem.55.6.1323-1328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassan M., Parsot C., Cohen G. N., Patte J. C. Nucleotide sequence of lysC gene encoding the lysine-sensitive aspartokinase III of Escherichia coli K12. Evolutionary pathway leading to three isofunctional enzymes. J Biol Chem. 1986 Jan 25;261(3):1052–1057. [PubMed] [Google Scholar]
- Chalmers R. M., Fewson C. A. Purification and characterization of benzaldehyde dehydrogenase I from Acinetobacter calcoaceticus. Biochem J. 1989 Nov 1;263(3):913–919. doi: 10.1042/bj2630913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. How reliably do amino acid composition comparisons predict sequence similarities between proteins? J Theor Biol. 1979 Feb 21;76(4):369–386. doi: 10.1016/0022-5193(79)90007-9. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Harayama S., Rekik M., Wubbolts M., Rose K., Leppik R. A., Timmis K. N. Characterization of five genes in the upper-pathway operon of TOL plasmid pWW0 from Pseudomonas putida and identification of the gene products. J Bacteriol. 1989 Sep;171(9):5048–5055. doi: 10.1128/jb.171.9.5048-5055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck R., Woenckhaus C., Harris J. J., Runswick M. J. Identification of the amino acid residue modified in Bacillus stearothermophilus alcohol dehydrogenase by the NAD+ analogue 4-(3-bromoacetylpyridinio)butyldiphosphoadenosine. Eur J Biochem. 1979 Jan 2;93(1):57–64. doi: 10.1111/j.1432-1033.1979.tb12794.x. [DOI] [PubMed] [Google Scholar]
- Jendrossek D., Steinbüchel A., Schlegel H. G. Alcohol dehydrogenase gene from Alcaligenes eutrophus: subcloning, heterologous expression in Escherichia coli, sequencing, and location of Tn5 insertions. J Bacteriol. 1988 Nov;170(11):5248–5256. doi: 10.1128/jb.170.11.5248-5256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- Julià P., Pareś X., Jörnvall H. Rat liver alcohol dehydrogenase of class III. Primary structure, functional consequences and relationships to other alcohol dehydrogenases. Eur J Biochem. 1988 Feb 15;172(1):73–83. doi: 10.1111/j.1432-1033.1988.tb13857.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. Differences in thiol groups and multiple forms of rat liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1096–1101. doi: 10.1016/0006-291x(73)90577-9. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Persson B., Jeffery J. Characteristics of alcohol/polyol dehydrogenases. The zinc-containing long-chain alcohol dehydrogenases. Eur J Biochem. 1987 Sep 1;167(2):195–201. doi: 10.1111/j.1432-1033.1987.tb13323.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., von Bahr-Lindström H., Jany K. D., Ulmer W., Fröschle M. Extended superfamily of short alcohol-polyol-sugar dehydrogenases: structural similarities between glucose and ribitol dehydrogenases. FEBS Lett. 1984 Jan 9;165(2):190–196. doi: 10.1016/0014-5793(84)80167-2. [DOI] [PubMed] [Google Scholar]
- Katinka M., Cossart P., Sibilli L., Saint-Girons I., Chalvignac M. A., Le Bras G., Cohen G. N., Yaniv M. Nucleotide sequence of the thrA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5730–5733. doi: 10.1073/pnas.77.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Metabolism of mandelate and related compounds by bacterium NCIB 8250. J Gen Microbiol. 1968 Sep;53(2):259–273. doi: 10.1099/00221287-53-2-259. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. Evidence from peptide 'maps' for the identity of the subunits. Biochem J. 1978 Feb 1;169(2):441–444. doi: 10.1042/bj1690441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh R. W., Fewson C. A. Benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus. Purification and preliminary characterization. Biochem J. 1988 Mar 15;250(3):743–751. doi: 10.1042/bj2500743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh R. W., Fewson C. A. Benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus. Substrate specificities and inhibition studies. Biochem J. 1988 Oct 15;255(2):653–661. [PMC free article] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G., Hamilton I. D., Fewson C. A., Wilkins M. B. Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J. 1986 Oct 1;239(1):213–220. doi: 10.1042/bj2390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz M., Burstein Y. Amino acid sequence of alcohol dehydrogenase from the thermophilic bacterium Thermoanaerobium brockii. Biochemistry. 1989 Aug 8;28(16):6549–6555. doi: 10.1021/bi00442a004. [DOI] [PubMed] [Google Scholar]
- Pickett M., Gwynne D. I., Buxton F. P., Elliott R., Davies R. W., Lockington R. A., Scazzocchio C., Sealy-Lewis H. M. Cloning and characterization of the aldA gene of Aspergillus nidulans. Gene. 1987;51(2-3):217–226. doi: 10.1016/0378-1119(87)90310-6. [DOI] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. II. Comparison of precipitin and micro-complement fixation results. J Biol Chem. 1971 Nov 25;246(22):7010–7017. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Youngleson J. S., Jones W. A., Jones D. T., Woods D. R. Molecular analysis and nucleotide sequence of the adh1 gene encoding an NADPH-dependent butanol dehydrogenase in the Gram-positive anaerobe Clostridium acetobutylicum. Gene. 1989 May 30;78(2):355–364. doi: 10.1016/0378-1119(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Zakin M. M., Duchange N., Ferrara P., Cohen G. N. Nucleotide sequence of the metL gene of Escherichia coli. Its product, the bifunctional aspartokinase ii-homoserine dehydrogenase II, and the bifunctional product of the thrA gene, aspartokinase I-homoserine dehydrogenase I, derive from a common ancestor. J Biol Chem. 1983 Mar 10;258(5):3028–3031. [PubMed] [Google Scholar]
- von Bahr-Lindström H., Hempel J., Jörnvall H. The cytoplasmic isoenzyme of horse liver aldehyde dehydrogenase. Relationship to the corresponding human isoenzyme. Eur J Biochem. 1984 May 15;141(1):37–42. doi: 10.1111/j.1432-1033.1984.tb08152.x. [DOI] [PubMed] [Google Scholar]


