Abstract
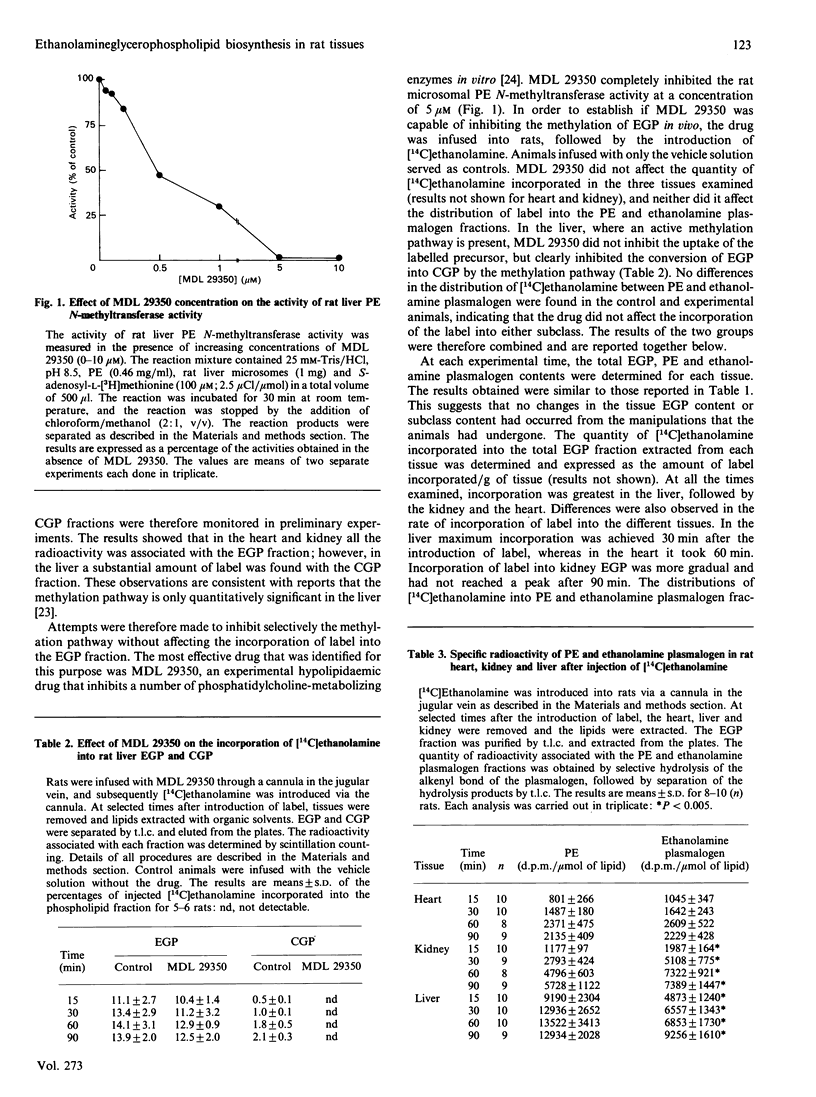
Studies with mammalian cell lines have led to suggestions that mammalian tissues may derive all of their phosphatidylethanolamine (PE) from the decarboxylation of phosphatidylserine (PS), and also that the physiological significance of the CDP-ethanolamine pathway was the synthesis of ethanolamine plasmalogen. We have therefore investigated the biosynthesis of PE and ethanolamine plasmalogen via the CDP-ethanolamine and decarboxylation pathways in vivo in three rat tissues (heart, kidney and liver), which differ in ethanolamine plasmalogen content. In all three tissues [14C]ethanolamine was incorporated into both PE and ethanolamine plasmalogen, whereas [3H]serine was incorporated into only PS and PE fractions. When [14C]ethanolamine was introduced into the animals, the specific radioactivity of ethanolamine plasmalogen in the kidney was always greater than that of the PE fraction; in the heart the specific radioactivity of the ethanolamine plasmalogen fraction was similar to that of the PE fraction, whereas in the liver the specific radioactivity of the PE fraction was always greater than that of the ethanolamine plasmalogen fraction. The results obtained in this study indicate that: (1) the CDP-ethanolamine pathway is utilized for the synthesis of both PE and ethanolamine plasmalogen in all three tissues; (2) the decarboxylation pathway is utilized solely for the synthesis of PE; (3) serine plasmalogens are not formed by base-exchange reactions; (4) the relative utilization of the CDP-ethanolamine pathway for the synthesis of PE and ethanolamine plasmalogen varies among tissues. Our studies also revealed that the hypolipidaemic drug MDL 29350 is a potent inhibitor of PE N-methyltransferase activity in vitro and in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur G., Mock T., Zaborniak C., Choy P. C. The distribution and acyl composition of plasmalogens in guinea pig heart. Lipids. 1985 Oct;20(10):693–698. doi: 10.1007/BF02534389. [DOI] [PubMed] [Google Scholar]
- Arvidson G. A. Structural and metabolic heterogeneity of rat liver glycerophosphatides. Eur J Biochem. 1968 May;4(4):478–486. doi: 10.1111/j.1432-1033.1968.tb00237.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- GOTTFRIED E. L., RAPPORT M. M. The biochemistry of plasmalogens. I. Isolation and characterization of phosphatidal choline, a pure native plasmalogen. J Biol Chem. 1962 Feb;237:329–333. [PubMed] [Google Scholar]
- Hirata F., Strittmatter W. J., Axelrod J. beta-Adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci U S A. 1979 Jan;76(1):368–372. doi: 10.1073/pnas.76.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer J. N. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980 Dec;58(12):1370–1380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- Kuge O., Nishijima M., Akamatsu Y. Phosphatidylserine biosynthesis in cultured Chinese hamster ovary cells. II. Isolation and characterization of phosphatidylserine auxotrophs. J Biol Chem. 1986 May 5;261(13):5790–5794. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pajares M. A., Alemany S., Varela I., Marin Cao D., Mato J. M. Purification and photoaffinity labelling of lipid methyltransferase from rat liver. Biochem J. 1984 Oct 1;223(1):61–66. doi: 10.1042/bj2230061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Kritchevsky D., Baumann W. J. Hypolipidemic drugs are inhibitors of phosphatidylcholine synthesis. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6890–6893. doi: 10.1073/pnas.79.22.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Simon G., Kritchevsky G. Species variations in phospholipid class distribution of organs. I. Kidney, liver and spleen. Lipids. 1969 Nov;4(6):599–606. doi: 10.1007/BF02531047. [DOI] [PubMed] [Google Scholar]
- Simon G., Rouser G. Species variations in phospholipid class distribution of organs. II. Heart and skeletal muscle. Lipids. 1969 Nov;4(6):607–614. doi: 10.1007/BF02531048. [DOI] [PubMed] [Google Scholar]
- Sundler R. Biosynthesis of rat liver phosphatidylethanolamines from intraportally injected ethanolamine. Biochim Biophys Acta. 1973 May 24;306(2):218–226. doi: 10.1016/0005-2760(73)90227-0. [DOI] [PubMed] [Google Scholar]
- Tijburg L. B., Geelen M. J., Van Golde L. M. Biosynthesis of phosphatidylethanolamine via the CDP-ethanolamine route is an important pathway in isolated rat hepatocytes. Biochem Biophys Res Commun. 1989 May 15;160(3):1275–1280. doi: 10.1016/s0006-291x(89)80141-x. [DOI] [PubMed] [Google Scholar]
- Vance J. E. Compartmentalization and differential labeling of phospholipids of rat liver subcellular membranes. Biochim Biophys Acta. 1988 Nov 4;963(1):10–20. doi: 10.1016/0005-2760(88)90332-3. [DOI] [PubMed] [Google Scholar]
- Vance J. E. Compartmentalization of phospholipids for lipoprotein assembly on the basis of molecular species and biosynthetic origin. Biochim Biophys Acta. 1988 Nov 4;963(1):70–81. doi: 10.1016/0005-2760(88)90339-6. [DOI] [PubMed] [Google Scholar]
- Vance J. E. The use of newly synthesized phospholipids for assembly into secreted hepatic lipoproteins. Biochim Biophys Acta. 1989 Nov 6;1006(1):59–69. doi: 10.1016/0005-2760(89)90323-8. [DOI] [PubMed] [Google Scholar]
- Vance J. E., Vance D. E. A deazaadenosine-insensitive methylation of phosphatidylethanolamine is involved in lipoprotein secretion. FEBS Lett. 1986 Aug 18;204(2):243–246. doi: 10.1016/0014-5793(86)80820-1. [DOI] [PubMed] [Google Scholar]
- Vance J. E., Vance D. E. Specific pools of phospholipids are used for lipoprotein secretion by cultured rat hepatocytes. J Biol Chem. 1986 Apr 5;261(10):4486–4491. [PubMed] [Google Scholar]
- Voelker D. R., Frazier J. L. Isolation and characterization of a Chinese hamster ovary cell line requiring ethanolamine or phosphatidylserine for growth and exhibiting defective phosphatidylserine synthase activity. J Biol Chem. 1986 Jan 25;261(3):1002–1008. [PubMed] [Google Scholar]
- Voelker D. R. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavin E., Zeigler B. P. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Serine incorporation into serine phosphoglycerides: base exchange and decarboxylation patterns. J Biol Chem. 1977 Jan 10;252(1):260–267. [PubMed] [Google Scholar]
- Yeung S. K., Kuksis A. Utilization of L-serine in the in vivo biosynthesis of glycerophospholipids by rat liver. Lipids. 1976 Jul;11(7):498–505. doi: 10.1007/BF02532893. [DOI] [PubMed] [Google Scholar]
- Yorek M. A., Rosario R. T., Dudley D. T., Spector A. A. The utilization of ethanolamine and serine for ethanolamine phosphoglyceride synthesis by human Y79 retinoblastoma cells. J Biol Chem. 1985 Mar 10;260(5):2930–2936. [PubMed] [Google Scholar]
- Zelinski T. A., Choy P. C. Phosphatidylethanolamine biosynthesis in isolated hamster heart. Can J Biochem. 1982 Aug;60(8):817–823. doi: 10.1139/o82-102. [DOI] [PubMed] [Google Scholar]


