Abstract
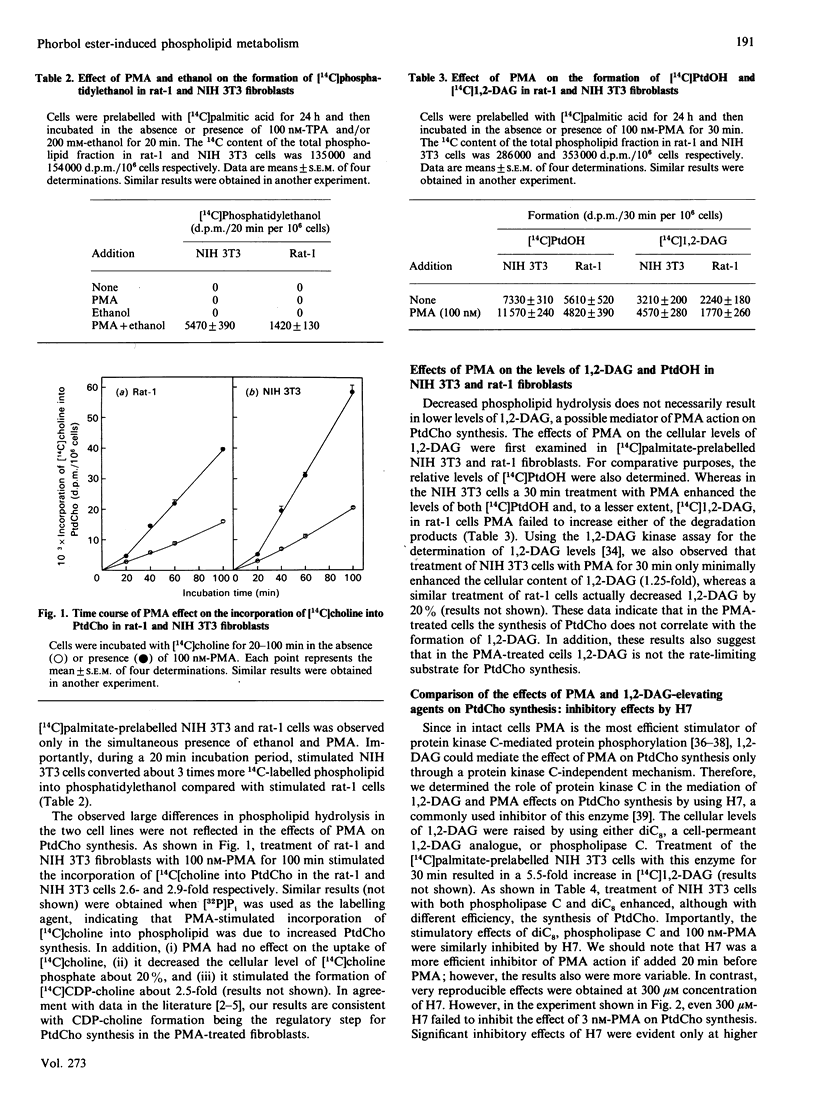
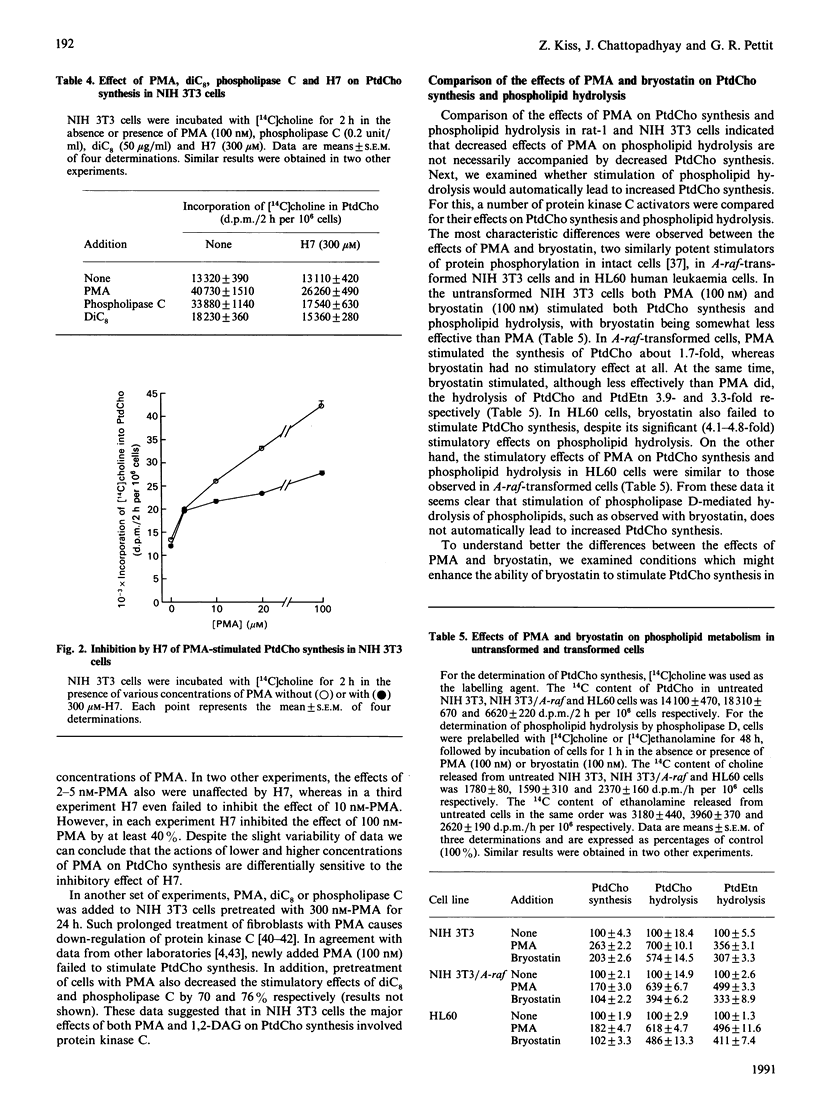
The aim of this study was to clarify the relationship between the stimulatory effects of protein kinase C activators, including phorbol 12-myristate 13-acetate (PMA) and bryostatin, on the hydrolysis of phosphatidylcholine (PtdCho) and phosphatidylethanolamine (PtdEtn) and on PtdCho synthesis. The cell lines used were selected because of their differential responses to protein kinase C activators and included rat-1 fibroblasts, untransformed and A-raf-transformed NIH 3T3 fibroblasts and human HL60 leukaemia cells. Exposure of rat-1 and NIH 3T3 fibroblasts to 100 nM-PMA stimulated phospholipase D-mediated hydrolysis of phospholipids about 2- and 6-fold respectively. In contrast, 100 nM-PMA had similar (2.5-3.0-fold) stimulatory effects on PtdCho synthesis in these cell lines. In the untransformed NIH 3T3 cells, both PMA and bryostatin stimulated both phospholipid hydrolysis and PtdCho synthesis, with 100 nM-bryostatin being somewhat less potent than 100 nM-TPA. In contrast, in A-raf-transformed NIH 3T3 cells or in HL60 cells, only TPA, but not bryostatin, stimulated PtdCho synthesis. In these transformed cells, bryostatin had 3-fold, or higher, stimulatory effects on phospholipid hydrolysis. Addition of ionomycin, a Ca2(+)-elevating agent, partially restored the stimulatory effect of bryostatin on PtdCho synthesis, but it failed to modify the effect of bryostatin on phospholipid hydrolysis. These data indicate that increased phospholipid hydrolysis is not necessarily associated with increased PtdCho synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck T. W., Huleihel M., Gunnell M., Bonner T. I., Rapp U. R. The complete coding sequence of the human A-raf-1 oncogene and transforming activity of a human A-raf carrying retrovirus. Nucleic Acids Res. 1987 Jan 26;15(2):595–609. doi: 10.1093/nar/15.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Cook H. W., Vance D. E. Evaluation of possible mechanisms of phorbol ester stimulation of phosphatidylcholine synthesis in HeLa cells. Can J Biochem Cell Biol. 1985 Feb;63(2):145–151. doi: 10.1139/o85-021. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R., Vance D. E. Translocation of CTP: phosphocholine cytidylyltransferase from cytosol to membranes in HeLa cells: stimulation by fatty acid, fatty alcohol, mono- and diacylglycerol. Biochim Biophys Acta. 1987 May 13;919(1):26–36. doi: 10.1016/0005-2760(87)90214-1. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Waite M., Wykle R. L. A novel mechanism of diglyceride formation. 12-O-tetradecanoylphorbol-13-acetate stimulates the cyclic breakdown and resynthesis of phosphatidylcholine. J Biol Chem. 1986 Jul 15;261(20):9128–9132. [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Grove R. I., Schimmel S. D. Effects of 12-O-tetradecanoylphorbol 13-acetate on glycerolipid metabolism in cultured myoblasts. Biochim Biophys Acta. 1982 May 13;711(2):272–280. doi: 10.1016/0005-2760(82)90036-4. [DOI] [PubMed] [Google Scholar]
- Guy G. R., Murray A. W. Tumor promoter stimulation of phosphatidylcholine turnover in HeLa cells. Cancer Res. 1982 May;42(5):1980–1985. [PubMed] [Google Scholar]
- Hennings H., Blumberg P. M., Pettit G. R., Herald C. L., Shores R., Yuspa S. H. Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 1987 Sep;8(9):1343–1346. doi: 10.1093/carcin/8.9.1343. [DOI] [PubMed] [Google Scholar]
- Hess A. D., Silanskis M. K., Esa A. H., Pettit G. R., May W. S. Activation of human T lymphocytes by bryostatin. J Immunol. 1988 Nov 15;141(10):3263–3269. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hovis J. G., Stumpo D. J., Halsey D. L., Blackshear P. J. Effects of mitogens on ornithine decarboxylase activity and messenger RNA levels in normal and protein kinase C-deficient NIH-3T3 fibroblasts. J Biol Chem. 1986 Aug 5;261(22):10380–10386. [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. ATP stimulates the hydrolysis of phosphatidylethanolamine in NIH 3T3 cells. Potentiating effects of guanosine triphosphates and sphingosine. J Biol Chem. 1990 May 5;265(13):7345–7350. [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. Phorbol ester stimulates the hydrolysis of phosphatidylethanolamine in leukemic HL-60, NIH 3T3, and baby hamster kidney cells. J Biol Chem. 1989 Jan 25;264(3):1483–1487. [PubMed] [Google Scholar]
- Kiss Z., Deli E., Kuo J. F. Cyclic AMP-like effects of polyamines on phosphatidylcholine synthesis and protein phosphorylation in human promyelocytic leukemia HL60 cells. Comparison with the effects of phorbol ester. FEBS Lett. 1987 Mar 23;213(2):365–371. doi: 10.1016/0014-5793(87)81523-5. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Deli E., Shoji M., Koeffler H. P., Pettit G. R., Vogler W. R., Kuo J. F. Differential effects of various protein kinase C activators on protein phosphorylation in human acute myeloblastic leukemia cell line KG-1 and its phorbol ester-resistant subline KG-1a. Cancer Res. 1987 Mar 1;47(5):1302–1307. [PubMed] [Google Scholar]
- Kiss Z., Farkas T. The effect of isoproterenol on the metabolism of phosphatidylinositol by rat heart in vitro. Biochem Pharmacol. 1975 May 1;24(9):999–1002. doi: 10.1016/0006-2952(75)90435-9. [DOI] [PubMed] [Google Scholar]
- Kiss Z. Possible phospholipid precursor for phosphatidylserine in rat heart. Eur J Biochem. 1976 Aug 16;67(2):557–561. doi: 10.1111/j.1432-1033.1976.tb10721.x. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Steinberg R. A. Phorbol ester-mediated protein phosphorylations in S49 mouse lymphoma cells. Cancer Res. 1985 Jun;45(6):2732–2740. [PubMed] [Google Scholar]
- Kobayashi M., Kanfer J. N. Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J Neurochem. 1987 May;48(5):1597–1603. doi: 10.1111/j.1471-4159.1987.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N., Paley A. E. 1,2-Diacylglycerols and phorbol esters stimulate phosphatidylcholine metabolism in GH3 pituitary cells. Evidence for separate mechanisms of action. J Biol Chem. 1987 Jul 5;262(19):9204–9210. [PubMed] [Google Scholar]
- Kolesnick R. N. Thyrotropin-releasing hormone and phorbol esters induce phosphatidylcholine synthesis in GH3 pituitary cells. Evidence for stimulation via protein kinase C. J Biol Chem. 1987 Oct 25;262(30):14525–14530. [PubMed] [Google Scholar]
- Kraft A. S., William F., Pettit G. R., Lilly M. B. Varied differentiation responses of human leukemias to bryostatin 1. Cancer Res. 1989 Mar 1;49(5):1287–1293. [PubMed] [Google Scholar]
- Liscovitch M. Phosphatidylethanol biosynthesis in ethanol-exposed NG108-15 neuroblastoma X glioma hybrid cells. Evidence for activation of a phospholipase D phosphatidyl transferase activity by protein kinase C. J Biol Chem. 1989 Jan 25;264(3):1450–1456. [PubMed] [Google Scholar]
- Morand J. N., Kent C. Localization of the membrane-associated CTP:phosphocholine cytidylyltransferase in Chinese hamster ovary cells with an altered membrane composition. J Biol Chem. 1989 Aug 15;264(23):13785–13792. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Paddon H. B., Vance D. E. Tetradecanoyl-phorbol acetate stimulates phosphatidylcholine biosynthesis in HeLa cells by an increase in the rate of the reaction catalyzed by CTP:phosphocholine cytidylyltransferase. Biochim Biophys Acta. 1980 Dec 5;620(3):636–640. doi: 10.1016/0005-2760(80)90156-3. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Cook H. W., Paddon H. B., Vance D. E. Membrane-bound CTP:phosphocholine cytidylyltransferase regulates the rate of phosphatidylcholine synthesis in HeLa cells treated with unsaturated fatty acids. Biochim Biophys Acta. 1984 Oct 4;795(3):433–440. doi: 10.1016/0005-2760(84)90169-3. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Paddon H. B., Vance D. E. Phorbol esters stimulate phosphatidylcholine biosynthesis by translocation of CTP:phosphocholine cytidylyltransferase from cytosol to microsomes. Biochim Biophys Acta. 1984 Oct 4;795(3):447–451. doi: 10.1016/0005-2760(84)90171-1. [DOI] [PubMed] [Google Scholar]
- Post M., Batenburg J. J., Schuurmans E. A., Van Golde L. M. The rate-limiting step in the biosynthesis of phosphatidylcholine by alveolar type II cells from adult rat lung. Biochim Biophys Acta. 1982 Aug 18;712(2):390–394. doi: 10.1016/0005-2760(82)90357-5. [DOI] [PubMed] [Google Scholar]
- Pritchard P. H., Vance D. E. Choline metabolism and phosphatidylcholine biosynthesis in cultured rat hepatocytes. Biochem J. 1981 Apr 15;196(1):261–267. doi: 10.1042/bj1960261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radika K., Possmayer F. Inhibition of foetal pulmonary choline-phosphate cytidylyltransferase under conditions favouring protein phosphorylation. Biochem J. 1985 Dec 15;232(3):833–840. doi: 10.1042/bj2320833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg I. L., Smart D. A., Gilfillan A. M., Rooney S. A. Effect of 1-oleoyl-2-acetylglycerol and other lipids on phosphatidylcholine synthesis and cholinephosphate cytidylyltransferase activity in cultured type II pneumocytes. Biochim Biophys Acta. 1987 Oct 17;921(3):473–480. doi: 10.1016/0005-2760(87)90074-9. [DOI] [PubMed] [Google Scholar]
- Sako T., Yuspa S. H., Herald C. L., Pettit G. R., Blumberg P. M. Partial parallelism and partial blockade by bryostatin 1 of effects of phorbol ester tumor promoters on primary mouse epidermal cells. Cancer Res. 1987 Oct 15;47(20):5445–5450. [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in cultured chick embryonic muscle treated with phospholipase C. J Biol Chem. 1980 Nov 25;255(22):10644–10650. [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in mammalian cells. II. Effects of phospholipase C treatment on the activity and subcellular distribution of CTP:phosphocholine cytidylyltransferase in Chinese hamster ovary and LM cell lines. J Biol Chem. 1983 Jan 25;258(2):831–835. [PubMed] [Google Scholar]
- Terce F., Record M., Chap H., Douste-Blazy L. Different susceptibility of alkylacyl--versus diacyl--and alkenylacyl--phosphatidylcholine subclasses to stimulation of biosynthesis by phospholipase C. Biochem Biophys Res Commun. 1984 Nov 30;125(1):413–419. doi: 10.1016/s0006-291x(84)80383-6. [DOI] [PubMed] [Google Scholar]
- Tercé F., Record M., Ribbes G., Chap H., Douste-Blazy L. Intracellular processing of cytidylyltransferase in Krebs II cells during stimulation of phosphatidylcholine synthesis. Evidence that a plasma membrane modification promotes enzyme translocation specifically to the endoplasmic reticulum. J Biol Chem. 1988 Mar 5;263(7):3142–3149. [PubMed] [Google Scholar]
- Watkins J. D., Kent C. Phosphorylation of CTP:phosphocholine cytidylyltransferase in vivo. Lack of effect of phorbol ester treatment in HeLa cells. J Biol Chem. 1990 Feb 5;265(4):2190–2197. [PubMed] [Google Scholar]
- Weinhold P. A., Feldman D. A., Quade M. M., Miller J. C., Brooks R. L. Evidence for a regulatory role of CTP : choline phosphate cytidylyltransferase in the synthesis of phosphatidylcholine in fetal lung following premature birth. Biochim Biophys Acta. 1981 Jul 24;665(1):134–144. doi: 10.1016/0005-2760(81)90241-1. [DOI] [PubMed] [Google Scholar]
- Zelinski T. A., Savard J. D., Man R. Y., Choy P. C. Phosphatidylcholine biosynthesis in isolated hamster heart. J Biol Chem. 1980 Dec 10;255(23):11423–11428. [PubMed] [Google Scholar]


