Abstract
The chondroitin sulphate chains of proteoglycans are not uniformly sulphated. Commonly, regions of under- and over-sulphation are found. It is probable that variability in chondroitin sulphation has physiological significance, although such structure-function relationships largely remain unexplored. Chondroitin sulphate from rat chondrosarcoma proteoglycan has been found to possess no oversulphated residues. It is primarily chondroitin 4-sulphate, although a significant proportion of unsulphated disaccharides (14%) are also present. It appears that some unsulphated disaccharides are concentrated only at the point of attachment to the linkage region (i.e. it is the major unsaturated disaccharide remaining attached to chondrosarcoma proteoglycan core produced by chondroitinase ABC digestion). This proteoglycan core binds monoclonal antibody (MAb) 3B3. Although 3B3 principally binds to 6-sulphated 'stubs' of proteoglycan cores [Couchman, Caterson, Christner & Baker (1984) Nature (London) 307, 650-652], given a high concentration of unsulphated 'stubs', it can alternatively bind to these residues. It is also evident that caution must be exercised in using MAb 3B3 to identify chondroitin 6-sulphated proteoglycans.
Full text
PDF

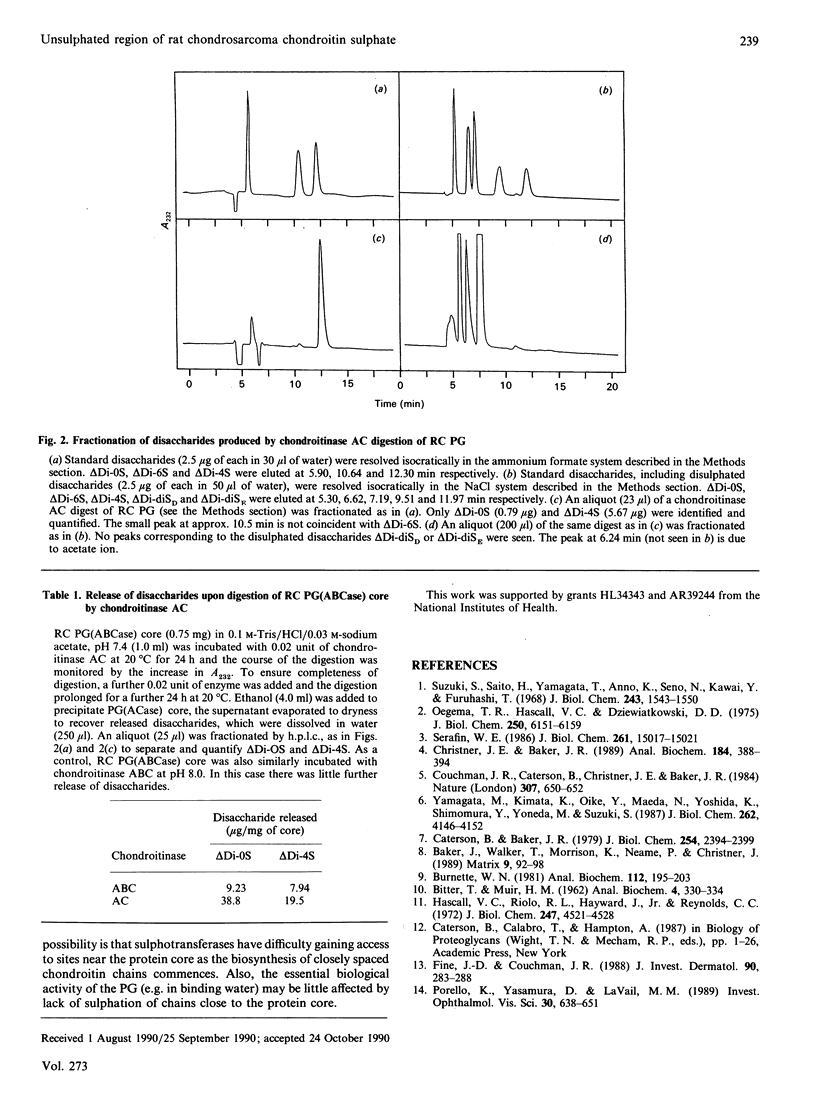
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baker J., Walker T., Morrison K., Neame P., Christner J. The specificity of a mouse monoclonal antibody to human aorta proteoglycans. Matrix. 1989 Mar;9(2):92–98. doi: 10.1016/s0934-8832(89)80026-5. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Caterson B., Baker J. R. The link proteins as specific components of cartilage proteoglycan aggregates in vivo. Associative extraction of proteoglycan aggregate from swarm rat chondrosarcoma. J Biol Chem. 1979 Apr 10;254(7):2394–2399. [PubMed] [Google Scholar]
- Christner J. E., Baker J. R. A competitive assay of lipoprotein: proteoglycan interaction using a 96-well microtitration plate. Anal Biochem. 1990 Feb 1;184(2):388–394. doi: 10.1016/0003-2697(90)90698-9. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Caterson B., Christner J. E., Baker J. R. Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature. 1984 Feb 16;307(5952):650–652. doi: 10.1038/307650a0. [DOI] [PubMed] [Google Scholar]
- Fine J. D., Couchman J. R. Chondroitin-6-sulfate-containing proteoglycan: a new component of human skin dermoepidermal junction. J Invest Dermatol. 1988 Mar;90(3):283–288. doi: 10.1111/1523-1747.ep12456049. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Porrello K., Yasumura D., LaVail M. M. Immunogold localization of chondroitin 6-sulfate in the interphotoreceptor matrix of normal and RCS rats. Invest Ophthalmol Vis Sci. 1989 Apr;30(4):638–651. [PubMed] [Google Scholar]
- Serafin W. E., Katz H. R., Austen K. F., Stevens R. L. Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J Biol Chem. 1986 Nov 15;261(32):15017–15021. [PubMed] [Google Scholar]
- Suzuki S., Saito H., Yamagata T., Anno K., Seno N., Kawai Y., Furuhashi T. Formation of three types of disulfated disaccharides from chondroitin sulfates by chondroitinase digestion. J Biol Chem. 1968 Apr 10;243(7):1543–1550. [PubMed] [Google Scholar]
- Yamagata M., Kimata K., Oike Y., Tani K., Maeda N., Yoshida K., Shimomura Y., Yoneda M., Suzuki S. A monoclonal antibody that specifically recognizes a glucuronic acid 2-sulfate-containing determinant in intact chondroitin sulfate chain. J Biol Chem. 1987 Mar 25;262(9):4146–4152. [PubMed] [Google Scholar]



