Abstract
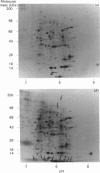
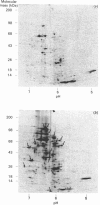
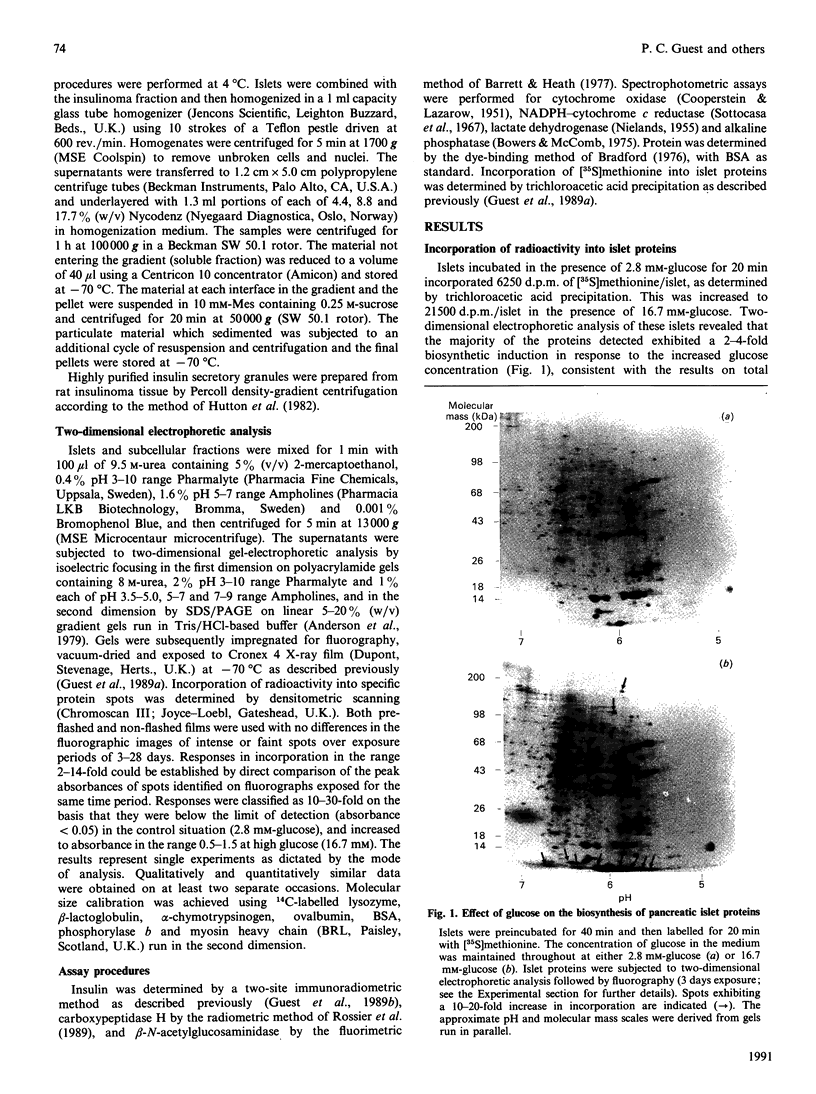
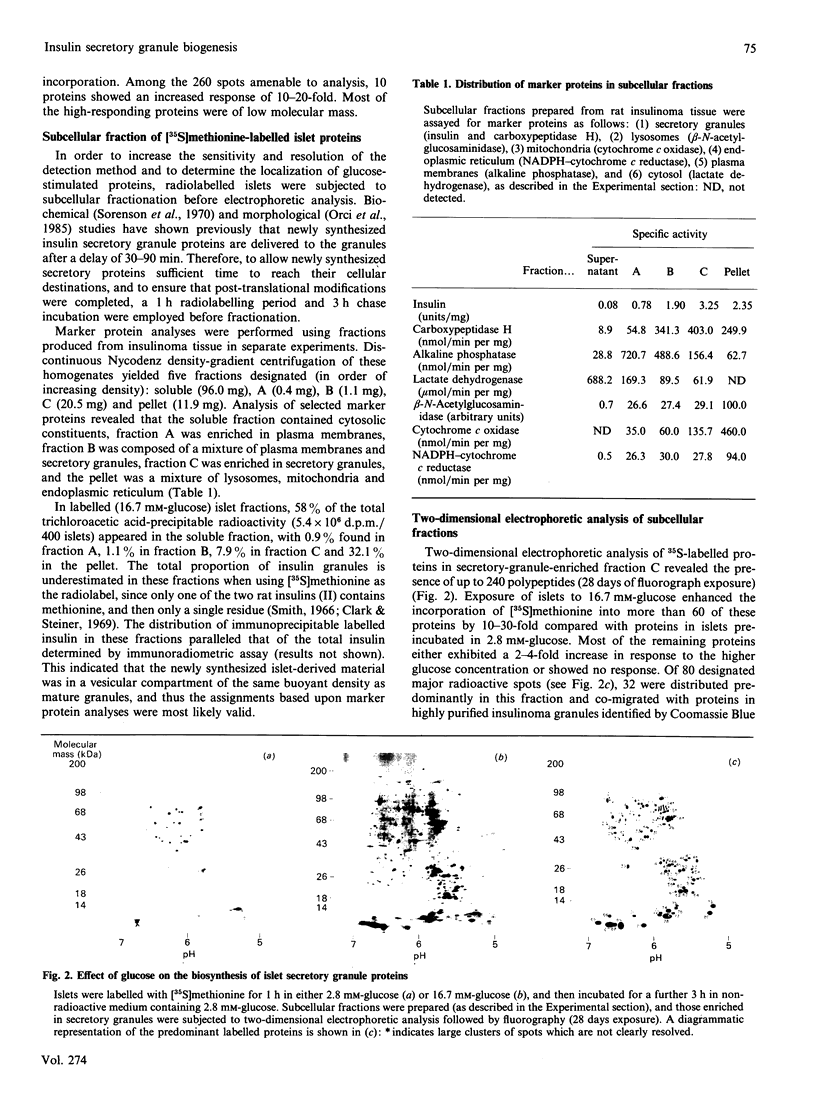
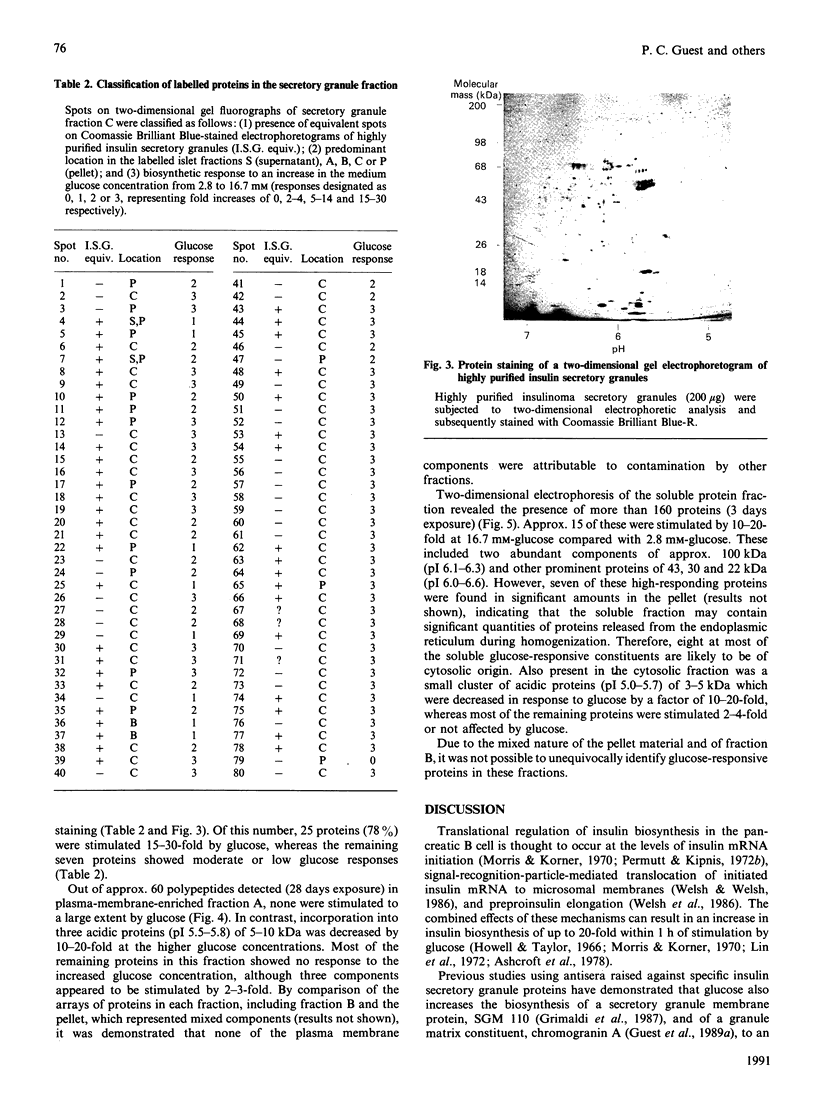
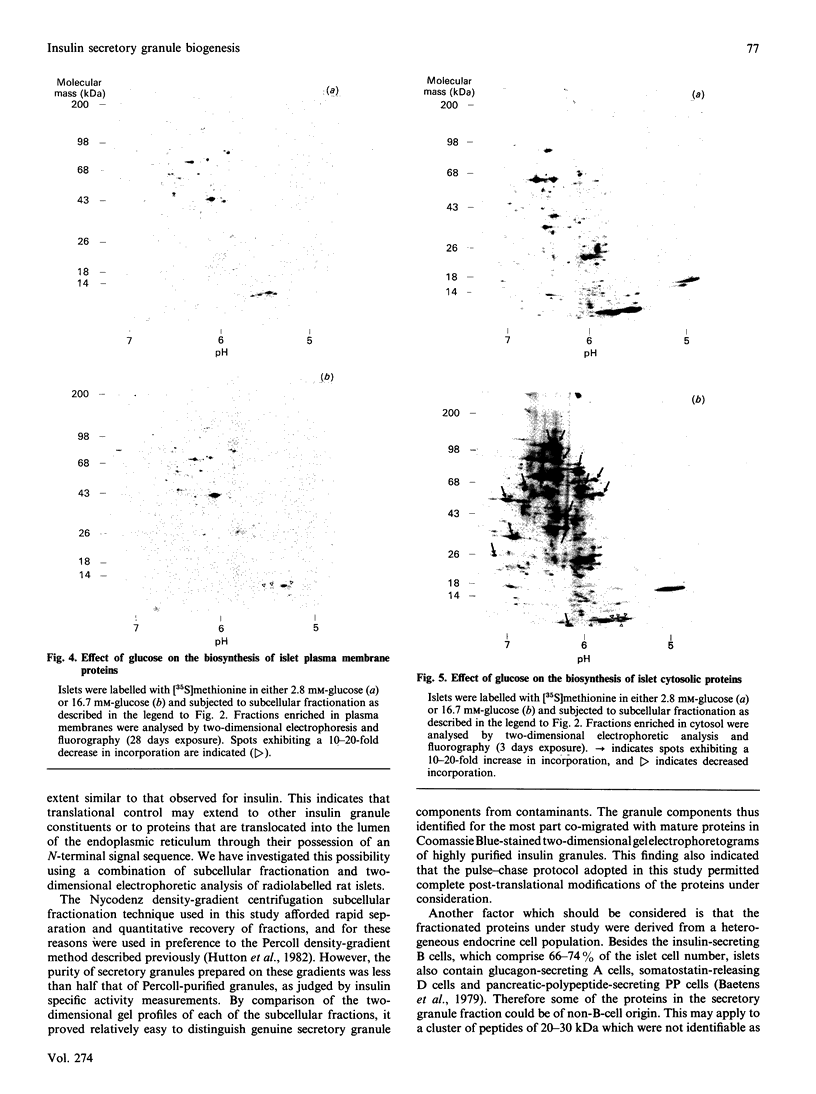
Two-dimensional gel-electrophoretic analysis combined with fluorography and densitometric quantification was used to examine the effects of glucose on the biosynthesis of rat pancreatic islet proteins. An increase in the medium glucose concentration from 2.8 to 16.7 mM produced a 10-20 fold stimulation in the synthesis of 10 out of 260 detected islet proteins, as judged by incorporation of [35S]methionine during a 20 min incubation. The synthetic rates of the majority of the remaining proteins were stimulated by 2-4-fold. Greater resolution achieved by pulse-chase labelling and subcellular fractionation showed that, of 32 major proteins localized to insulin secretory granules, the biosynthesis of 25 were stimulated 15-30-fold by glucose. By contrast, only eight of 160 proteins in the soluble fraction showed a response of similar magnitude. It is concluded that there is a major and co-ordinated activation of the biosyntheses of proteins destined for secretory granules, which most likely occurs at the level of translational initiation and signal-recognition-particle-mediated translocation into the endoplasmic reticulum lumen. However, it is clear that not all granule proteins, or the majority of proteins translocated across the endoplasmic reticulum membrane, are affected in an equivalent manner. In addition, the synthesis of a small number of cytosolic proteins may be increased markedly by insulinotropic stimuli.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Bunce J., Lowry M., Hansen S. E., Hedeskov C. J. The effect of sugars on (pro)insulin biosynthesis. Biochem J. 1978 Aug 15;174(2):517–526. doi: 10.1042/bj1740517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetens D., Malaisse-Lagae F., Perrelet A., Orci L. Endocrine pancreas: three-dimensional reconstruction shows two types of islets of langerhans. Science. 1979 Dec 14;206(4424):1323–1325. doi: 10.1126/science.390711. [DOI] [PubMed] [Google Scholar]
- Bowers G. N., Jr, McComb R. B. Measurement of total alkaline phosphatase activity in human serum. Clin Chem. 1975 Dec;21(13):1988–1995. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Grimaldi K. A., Siddle K., Hutton J. C. Biosynthesis of insulin secretory granule membrane proteins. Control by glucose. Biochem J. 1987 Jul 15;245(2):567–573. doi: 10.1042/bj2450567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest P. C., Lowing C., Arden S. D., Gray I. P., Hutton J. C. A rapid, sensitive and versatile two-site immunoradiometric assay for insulin. Mol Cell Endocrinol. 1989 Dec;67(2-3):173–178. doi: 10.1016/0303-7207(89)90207-4. [DOI] [PubMed] [Google Scholar]
- Guest P. C., Rhodes C. J., Hutton J. C. Regulation of the biosynthesis of insulin-secretory-granule proteins. Co-ordinate translational control is exerted on some, but not all, granule matrix constituents. Biochem J. 1989 Jan 15;257(2):431–437. doi: 10.1042/bj2570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton D. A., Millward B. A., Gray P., Tun Y., Hales C. N., Pyke D. A., Leslie R. D. Evidence of beta cell dysfunction which does not lead on to diabetes: a study of identical twins of insulin dependent diabetics. Br Med J (Clin Res Ed) 1987 Jan 17;294(6565):145–146. doi: 10.1136/bmj.294.6565.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of glucose concentration on incorporation of [3H]leucine into insulin using isolated mammalian islets of Langerhans. Biochim Biophys Acta. 1966 Dec 28;130(2):519–521. doi: 10.1016/0304-4165(66)90250-9. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Bailyes E. M., Rhodes C. J., Rutherford N. G., Arden S. D., Guest P. C. Biosynthesis and storage of insulin. Biochem Soc Trans. 1990 Feb;18(1):122–124. doi: 10.1042/bst0180122. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Isolation and characterisation of insulin secretory granules from a rat islet cell tumour. Diabetologia. 1982 Oct;23(4):365–373. doi: 10.1007/BF00253746. [DOI] [PubMed] [Google Scholar]
- Lin B. J., Nagy B. R., Haist R. E. Effect of various concentrations of glucose on insulin biosynthesis. Endocrinology. 1972 Jul;91(1):309–311. doi: 10.1210/endo-91-1-309. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Korner A. RNA synthesis and the stimulation of insulin biosynthesis by glucose. FEBS Lett. 1970 Oct 5;10(3):165–168. doi: 10.1016/0014-5793(70)80444-6. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Vassalli J. D., Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985 Sep;42(2):671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972 Feb 25;247(4):1194–1199. [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis: studies of Islet polyribosomes (nascent peptides-sucrose gradient analysis-gel filtration). Proc Natl Acad Sci U S A. 1972 Feb;69(2):505–509. doi: 10.1073/pnas.69.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier J., Barrès E., Hutton J. C., Bicknell R. J. Radiometric assay for carboxypeptidase H (EC 3.4.17.10) and other carboxypeptidase B-like enzymes. Anal Biochem. 1989 Apr;178(1):27–31. doi: 10.1016/0003-2697(89)90350-3. [DOI] [PubMed] [Google Scholar]
- Smith L. F. Species variation in the amino acid sequence of insulin. Am J Med. 1966 May;40(5):662–666. doi: 10.1016/0002-9343(66)90145-8. [DOI] [PubMed] [Google Scholar]
- Sorenson R. L., Steffes M. W., Lindall A. W. Subcellular localization of proinsulin to insulin conversion in isolated rat islets. Endocrinology. 1970 Jan;86(1):88–96. doi: 10.1210/endo-86-1-88. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. K., LaCava E. C., Paquette T. L., Beard J. C., Wallum B. J., Porte D., Jr Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987 Sep;30(9):698–702. doi: 10.1007/BF00296991. [DOI] [PubMed] [Google Scholar]
- Welsh M., Scherberg N., Gilmore R., Steiner D. F. Translational control of insulin biosynthesis. Evidence for regulation of elongation, initiation and signal-recognition-particle-mediated translational arrest by glucose. Biochem J. 1986 Apr 15;235(2):459–467. doi: 10.1042/bj2350459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986 Nov 14;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]