Abstract
Abstract
Blood oxygen in the fetus is substantially lower than in the newborn infant. In the minutes after birth, arterial oxygen saturation rises from around 50–60% to 90–95%. Initial respiratory efforts generate negative trans-thoracic pressures that drive liquid from the airways into the lung interstitium facilitating lung aeration, blood oxygenation, and pulmonary artery vasodilatation. Consequently, intra- (foramen ovale) and extra-cardiac (ductus arteriosus) shunting changes and the sequential circulation switches to a parallel pulmonary and systemic circulation. Delaying cord clamping preserves blood flow through the ascending vena cava, thus increasing right and left ventricular preload. Recently published reference ranges have suggested that delayed cord clamping positively influenced the fetal-to-neonatal transition. Oxygen saturation in babies with delayed cord clamping plateaus significantly earlier to values of 85–90% than in babies with immediate cord clamping. Delayed cord clamping may also contribute to fewer episodes of brady-or-tachycardia in the first minutes after birth, but data from randomized trials are awaited.
Impact
Delaying cord clamping during fetal to neonatal transition contributes to a significantly earlier plateauing of oxygen saturation and fewer episodes of brady-and/or-tachycardia in the first minutes after birth.
We provide updated information regarding the changes in SpO2 and HR during postnatal adaptation of term and late preterm infants receiving delayed compared with immediate cord clamping.
Nomograms in newborn infants with delayed cord clamping will provide valuable reference ranges to establish target SpO2 and HR in the first minutes after birth.
Introduction
To objectively guide resuscitation, monitoring of arterial oxygen saturation (SpO2) and heart rate (HR) using pulse oximetry has become standard of care.1–3 In 2010, Dawson et al.4,5, merging three databases from the Royal Women’s Hospital (Melbourne) and the University and Polytechnic Hospital La Fe (Valencia), established a reference range for SpO2 and HR. In a prospective observational study, a pulse oximeter sensor was placed on the right hand or wrist (preductal), and minute-by-minute SpO2 and HR were measured in 308 term and 160 preterm infants during the first 10 min after birth. Percentile charts for SpO2 and HR in term and preterm infants were constructed using these data.4,5 In 2015, the American Heart Association (AHA) recommended the following target ranges for SpO2: at 1 min 60–65%, at 2 min 65–70%, at 3 min 70–75%, at 4 min 75–80%, at 5 min 80–85%, and at 10 min 85–95%, keeping HR > 100 beats per minute (bpm) during postnatal stabilization, and these target ranges have been recommended to date.6
Recently, international guidelines have strongly recommended delaying cord clamping for 30–60 s.7 Delayed cord clamping (DCC) as opposed to immediate cord clamping (ICC) preserves a steady blood flow from the placenta to the newly born circulation enhancing ventricular preload and will contribute to a more stable hemodynamic transition when lung aeration occurs in before cord clamping.8
The aim of this review article is to provide readers with updated information regarding the changes in SpO2 and HR during postnatal adaptation of term and late preterm infants receiving DCC.
Intrauterine oxygenation
The partial pressure of oxygen gradient between maternal, placental, and fetal blood is the driving force that regulates fetal oxygen supply. Both placenta and embryo grow and differentiate in a relatively low oxygen environment during the first trimester. The blood flow to the intervillous space (IVS) becomes fully established at the 11–12th weeks of gestation. Coincidentally, oxygen tension within the IVS abruptly rises from 18 mmHg in the eighth week to 60 mmHg at 12 weeks.9 In the second half of pregnancy, IVS partial pressure of oxygen slowly decreases from around 60 mmHg in the 12th week of gestation to 45 mmHg at term assuring a steady-state oxygenation of the fetus.10 The limited supply of oxygen to the fetus requires adaptive changes in the placental structure together with changes in fetal metabolism. The relatively low metabolic rate of the fetus combined with thermoregulation provided by the mother leads to an important reduction in fetal oxygen consumption relative to the newborn infant.11 Another important factor contributing to adequate fetal oxygenation is the sustained increase in fetal hemoglobin concentration throughout gestation. Fetal hemoglobin has high affinity for oxygen at the placental level. Moreover, due to fetal hemoglobin and a low level of 2,3 diphosphoglycerate, the oxygen dissociation curve shifts to the left, which results in a greater release in oxygen at a lower arterial PO2 compared to adult hemoglobin, facilitating the liberation of oxygen into peripheral tissues.12 However, the most important mechanism that ensures that fetal oxygenation is kept within physiologic limits, comparable to those of the newborn infant, is the high perfusion of the fetal organs. The high fetal cardiac output of 250–300 mL/kg/min is achieved due to the fast HR and the central shunting that allows the fetal ventricles to work in parallel, rather than in series as in the adult-type circulation. Central shunting redirects oxygenated blood to the left ventricle for preferential distribution to the brain and myocardium.13
Oxygenation during fetal-to-neonatal transition
Oxygenation plays an important role in the physiological changes needed for a successful fetal-to-neonatal transition. In uncompromised and adequately breathing infants, SpO2 increases from 50–60% in the fetus to 90–95% in the first minutes after birth.4 This increase is needed to match the rise in metabolic rate that comes with the physiological adaptation to extra-uterine life (thermoregulation, breathing effort).14 The activation of non-shivering thermogenesis attained through the oxidation of brown fat requires increased oxygen supply. It has been demonstrated that hypoxia acts as a suppressor of this non-shivering thermogenesis and infants struggle to maintain thermoregulation in a cold environment.14
Fetal breathing movements are inhibited by low oxygen levels. It has been shown that hypoxia directly inhibits neural input to the respiratory center from a region located in the upper lateral pons, at the level of the middle cerebral peduncle in the region of the principle sensory and motor nuclei of the trigeminal nerve.15 During apnea in the fetus, glottis adduction results in a restriction of loss of airway liquid and is therefore vital for lung development.16 However, this adduction of the glottis during apnea also persists after birth, thereby restricting the aeration of the lung using non-invasive techniques.17,18 A temporal change in O2 sensitivity takes place after birth,19 and while most preterm infants breathe,20 it is not known when the switch from respiratory suppression to stimulation occurs in response to hypoxia. Large inspiratory efforts are needed immediately at birth to establish lung liquid clearance and lung aeration. Hypoxia may interfere with these processes as it leads to depressed or absent breathing efforts, particularly in preterm infants.20,21 On the other hand, hyperoxia also seems to inhibit the chemoreceptors as the onset of breathing was delayed in asphyxiated animals and infants receiving 100% oxygen.22
Oxygen is known as a potent vasodilator, and it is commonly assumed that oxygenation of the lungs is predominantly responsible for the physiological fall in pulmonary vascular resistance at birth. However, it has recently been demonstrated in a lamb model that the lung liquid clearance and aeration established with ventilation is the primary trigger for a prompt decrease in pulmonary vascular resistance.23,24 Although the onset of ventilation seems to be “the master switch”, oxygen seems to be additive to this as a “mediator”.
Introduction of pulse oximetry monitoring in the delivery room
For many years, evaluation of the condition of the newborn infant in the first minutes of life was based on clinical signs including HR and color. HR was considered the most objective measure of a newborn infant’s condition and thresholds (60 and 100 bpm) became widely accepted indications for the commencement of interventions including respiratory support and cardiac compressions. Studies of HR assessment by auscultation and umbilical cord palpation revealed that these methods were imprecise, and systematically underestimated HR.25 Similarly, when asked to assess color, clinicians of all levels of experience could not agree when an infant became pink.26 In 2003, John Kattwinkel27 suggested in a commentary accompanying an air vs 100% oxygen trial that rather than choosing a fixed oxygen concentration to use throughout a resuscitation, “perhaps we should be aiming to restore normoxia quickly and to achieve normal levels of blood oxygen throughout and beyond the resuscitation process. More aggressive use of the pulse oximeter in the delivery setting may facilitate achieving this goal”. Pulse oximetry offers the additional benefit of being able to display HR in real time without clinicians having to shift their focus from the provision of resuscitation.
Kattwinkel’s paradigm-shifting suggestion led to several questions. Although used for many years in the neonatal intensive care unit, pulse oximetry was untested in the delivery room (DR). Questions at the time were whether it would provide reliable readings when applied to a wet, often poorly perfused newborn infant in need of resuscitation, and if it did, would it provide oxygen saturation and HR values early enough in the infant’s course to be clinically useful?
O’Donnell et al.28 showed that pulse oximetry provided data in over 90% of babies in the DR, and within 90 s of life in 90%. They also demonstrated that applying the pulse oximeter probe to the infant before connecting to the oximeter resulted in quickest acquisition of accurate HR.29 In the first minutes of life, right-to-left ductal shunting predominates and therefore SpO2 is higher in the preductal circulation than in the postductal circulation.30 Therefore, it is recommended that the probe should be applied to the right hand or wrist to ensure that preductal oxygen saturation levels which reflect cerebral values are displayed. Kamlin et al.31 showed that the pulse oximeter had reasonable sensitivity (89%) and excellent specificity (99%) in detecting HRs less than 100 bpm.31
For clinicians used to targeting oxygen therapy to achieve saturation levels in the 90 s for babies in the neonatal intensive care unit, it quickly became apparent that the concept of “normoxia” suggested by Kattwinkel needed a new set of target values to be clinically useful.
The Dawson nomogram
In 2010, Dawson et al.4[,5 published two seminal studies aimed at providing reference ranges for SpO2 and HR in transitioning infants.4,5 Although this work was preceded by other attempts to describe normal ranges,32–34 Dawson’s smoothed percentile charts that included data collected every 2 s rapidly became the most widely used. The SpO2 nomograms provided a reference against which an infant’s oxygenation status could be objectively compared.
Dawson’s nomograms were derived from a prospective cohort of 468 infants (n = 39 born at <32 weeks PMA, n = 121 at 32–36 weeks PMA, n = 308 at ≥37 weeks PMA) at two tertiary centers. As was usual care at the time, infants received ICC. Infants who received supplemental oxygen or ventilation were excluded.4,5 Informed by the results, resuscitation guidelines have generally used approximations of the 25th percentile of Dawson’s nomogram as the lower acceptable SpO2 level, broadly recommending preductal SpO2 levels of >65% by 2 min and >80% by 5 min.6,35,36 Of note, Dawson et al.4 found that preterm infants on average had median SpO2 levels 3–8% lower than that of term infants. Infants born by cesarean section had median SpO2 levels approximately 10% lower than those of vaginally born infants in the first 5 min.
The nomograms quickly evolved from descriptive reference ranges to recommended SpO2 targets. Clinicians caring for an infant at the lower end of the distribution for oxygen saturation were provided with a trigger SpO2 level at which to commence oxygen therapy, and targets to titrate that therapy over the first 10 min after birth. There are limited data on the consequences of the broad strategy of titrating oxygen to mimic SpO2 levels of infants who transition without intervention.37 The practice has likely reduced the incidence of untreated hypoxia in the delivery room and associated mortality in preterm infants.38 However, an infant who starts at and progresses along the 10th centile is likely to receive oxygen therapy, and it is not known whether this is the optimal strategy.
The HR nomograms derived from infants in the same cohort have attracted less attention.5 DR practice has continued to focus on a dichotomous threshold of <100 bpm as a trigger for intervention. Dawson’s nomograms suggested that 50% of infants have HRs below this threshold at 1 min after birth and cautioned against intervention if the infant appeared clinically well. This observation has contributed to an ongoing body of work aimed at establishing whether delaying cord clamping would provide greater physiological stability during fetal-to-neonatal transition.39,40 Additional comparisons of infant HR measured concurrently using 3-lead electrocardiogram and pulse oximetry have highlighted discrepancies in the first 2 min after birth.41 This discrepancy, not apparent when using the Nelcor oximeter,42 raises the possibility of artifact contributing to measurements of low HR using the Masimo pulse oximeter.
Postnatal oxygenation with DCC
When considering oxygenation at birth, it seems logical to defer cord clamping, which removes the placenta as a gas exchange organ, until the lungs have been aerated and are able to provide gas exchange. Deferring cord clamping until after ventilation (physiological based cord clamping, PBCC) sustains preload and cardiac output and avoids the large disturbances in systemic and cerebral circulation associated with ICC.37,43 These studies, conducted in preterm lambs, demonstrated that a substantial drop in SpO2 occurred after ICC, with a subsequent tachycardia in the 3 min after ventilation onset, whereas when PBCC was applied, these values remained stable.37,43 This was later confirmed in a clinical setting in preterm infants.44
Early published clinical studies have focused on data of term infants. The first prospective cohort included 109 term infants receiving DCC of ≥1 min and immediate skin-to-skin care after low-risk births. Pulse oximetry was used to record SpO2 and HR.45 In comparison with the ICC reference ranges, these infants had significantly higher SpO2 values in the first 3 min of life, and lower values (but with median SpO2 90–95%) between 5 and 10 min. The DCC infants had lower HR than the ICC reference ranges from 2 to 10 min of age: the largest difference in median HR was at 2 min (85 vs 160 bpm) with a difference of 18 bpm or less thereafter. The DCC infants were less likely to have tachycardia of >180 bpm (2.6% vs 19.3%) and more likely to have bradycardia of <80 bpm (6.5% vs 4.8%) during the first 10 min. The authors noted that the high rate of immediate skin-to-skin care and breastfeeding in the DCC cohort may have contributed.45
Two RCTs have included term infants born by elective cesarean section, allocating them to either ICC, or DCC of at least 60 s. Cavallin et al.46 included 80 infants and did not identify a significant difference in either SpO2 or HR in the first 10 min after birth. Their sample size was not powered for these outcomes, and data analysis excluded the first 3 min after birth, where differences may have been more pronounced. Both groups had median SpO2 > 80% and median HR > 160 at 3 min. De Bernardo et al.47 included 132 infants born at 37–42 weeks, finding similar SpO2 and HR values, but again the times of assessment (5 and 10 min) excluded the first minutes after birth.
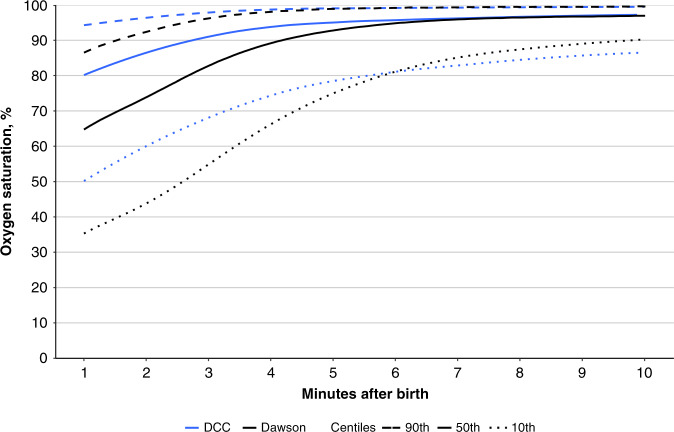
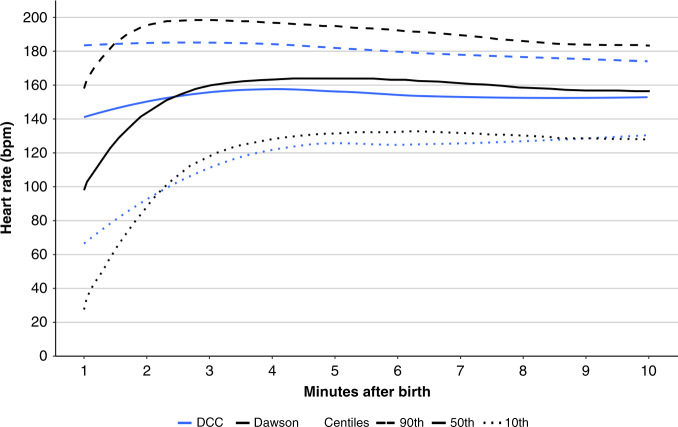
Padilla-Sanchez et al.48 published oximetry data from a cohort of 282 infants of ≥37 weeks’ gestation born vaginally, who did not receive resuscitation and underwent DCC for ≥1 min (mean 110 s). They compared their findings with the Dawson nomograms for vaginally born infants, noting that the DCC infants had significantly higher SpO2 values in the minutes after birth, particularly the first 3 min, where the median SpO2 values were ≥10% higher (Fig. 1). The HR values in DCC infants were stable (median 148–158 throughout the first 10 min), whereas the prior cohort of ICC infants displayed more fluctuation, by starting significantly lower, reaching a higher peak, and then stabilizing (median HR 99 at 1 min, 164 at 5 min, and 157 at 10 min)48 (Fig. 2). However, in the Dawson nomogram for vaginally born infants, a proportion of infants born <37 weeks of gestation was included (160/466 infants), which could have resulted in a slightly larger difference between the compared groups.4
Fig. 1. Comparison of Dawson’s with delayed cord clamping (DCC) nomogram for arterial oxygen saturation (SpO2).
Comparison of the 10th, 50th, and 90th percentiles for SpO2 measured with preductal pulse oximetry between the reference range in Dawson’s nomogram for oxygen saturation (ref. 4) and babies with delayed cord clamping (DCC) for >60 s in the study by Padilla-Sánchez et al.49 reproduced with permission of The Journal of Pediatrics.
Fig. 2. Comparison of Dawson’s with delayed cord clamping (DCC) nomogram for heart rate (HH).
Comparison of the 10th, 50th, and 90th percentiles for heart rate (HR) measured with preductal pulse oximetry between the reference range in Dawson’s nomogram for heart rate (ref. 5) and babies with delayed cord clamping (DCC) for >60 s in the study by Padilla-Sánchez et al.49 reproduced with permission of The Journal of Pediatrics.
Björland et al.49, recruited a further cohort of 898 term infants born vaginally with DCC of at least 1 min, incorporating the use of a dry electrode ECG device to allow early assessment of HR. More than 50% of infants had HR data available within 15 s after birth. Their findings suggested a rapid increase in HR from median 122 bpm at 5 s to 174 bpm by 60 s, gradually reducing thereafter. The authors suggested that this pattern might have differed from those of previous studies due to underestimation of HR when measured by pulse oximetry.
Newborn infants born at 34–36 completed gestational weeks are defined as late preterm (LPT) and represent approximately 7–8% of all deliveries in high-income countries. The proportion of infants delivered LPT has steadily increased in recent decades.50 Compared to term (≥37 completed weeks of gestation) infants, LPT are physiologically immature and have a higher risk of death and/or metabolic, infectious, and respiratory morbidities. Moreover, LPT infants exhibit a tendency towards poorer postnatal adaptation with lower Apgar scores.51–53 Consequently, LPT infants frequently need resuscitation, including positive pressure ventilation and oxygen supplementation in the first minutes after birth.54
Data from the “healthy” moderate-late preterm population are currently sparse, in part because published work in this group has focused on infants receiving resuscitation, rather than the group that do not require intervention.55 One feasibility trial included 44 infants ≥32 weeks’ gestation, of whom 32 did not receive resuscitation during at least 2 min of DCC.56 HR was derived from ECG, oximetry, or ultrasound. Outcomes were reported for the entire group of 44 infants, with only two infants with HR < 100 bpm at any point in the first 5 min after birth, and median HR > 160 throughout this time. This trial was the precursor to a recently completed trial that will generate HR and SpO2 data from a larger cohort of infants ≥32 weeks’ gestation (ACTRN12618000621213).
One large RCT conducted by Ashish et al.57 has reported outcomes for >000 infants of ≥33 weeks born by uncomplicated vaginal birth, who breathed spontaneously. The majority were born at term (mean 39.4 weeks). Infants were allocated to cord clamping at either <60 s or DCC at ≥180 s, with HR and SpO2 data recorded by a ultrasound transducer and oximetry, respectively. The infants receiving DCC had higher SpO2 values throughout the first 10 min (80% vs 61%, 91% vs 78%, and 98% vs 88% at 1, 5, and 10 min respectively). The HR of the DCC group was lower at 1 and 5 min after birth (107 vs 116 and 132 vs 134 bpm, respectively), but similar thereafter. The mean HR remained between 130 and 140 bpm from 5 to 10 min in both groups of this study, lower than that observed in previous studies such as Smit et al.45, where the median HR with DCC was between 146 and 152 in this time. This may reflect differences in setting, method of HR ascertainment, or gestation (term or late preterm), and further data in the late preterm group will be of value.
Conclusions
In summary, the fetus develops in a lower oxygen environment compared to the newborn infant. However, oxygen provided to fetal tissues is equivalent to that supplying the newborn infant due to metabolic and cardiocirculatory adjustments. Healthy term babies after an uncomplicated birth have a low initial SpO2 that rapidly increases, plateauing at values of 90–95% around 3–5 min. Currently available data support the concept that expected ranges for HR and SpO2 differ, depending in the timing of cord clamping. Larger cohorts of vaginally born infants indicate that infants receiving DCC experienced a more rapid rise in SpO2 than those receiving ICC. HR findings have been less consistent between studies, but it appears that DCC infants may achieve HR stability at an earlier time point after birth than ICC infants. The mode of delivery (cesarean section vs vaginal birth), the mode of HR assessment (ECG vs pulse oximeter), and other factors such as immediate skin-to-skin care may have some influence on measured values. Information regarding SpO2 and HR in late preterm infants born after vaginal delivery, and not needing resuscitation, is lacking. Adequately powered prospective studies will provide interesting information on how SpO2 and HR evolve during postnatal adaptation in this important cohort of preterm infants.
Acknowledgements
Figures 1 and 2 are authorized reproductions from the article published in The Journal of Pediatrics, 227, Celia Padilla-Sánchez, Susana Baixauli-Alacreu, Antonio José Cañada-Martinez, Alvaro Solaz-García, María José Alemany-Anchel, Maximo Vento. Delayed vs immediate cord clamping changes oxygen saturation and heart rate patterns in the first minutes after birth. 149–156.e1, Copyright Elsevier 2020.
Author contributions
M.V. acted as the corresponding author, designed the structure of the article, assigned tasks to the rest of the co-authors, wrote the draft, and approved the final version of the manuscript. I.L.-C., S.B., J.D., P.D., C.R., and A.B.tP. contributed to writing and reviewing the draft and approved the final version of the manuscript.
Funding information
M.V. acknowledges RETICS funded by the PN 2018-2021 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD16/0022/0001 and grant PI20/0964 from the Instituto Carlos III (Spanish Ministry of Science and Innovation). I.L.-C. acknowledges CM20/0187 grant from the Instituto Carlos III (Spanish Ministry of Science and Innovation). C.R. and P.D. acknowledge Investigator Grants 1175634 (to C.R.) and 1157782 (to P.D.) from NHMRC, respectively. S.B. is supported by an Australian Government Research Training Scholarship.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Finer, N. & Leone, T. Oxygen saturation monitoring for the preterm infant: the evidence basis for current practice. Pediatr. Res.65, 375–380 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Kamlin, C. O. F., O’Donnell, C. P. F., Davis, P. G. & Morley, C. J. Oxygen saturation in healthy infants immediately after birth. J. Pediatr.148, 585–589 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Rabi, Y., Yee, W., Chen, S. Y. & Singhal, N. Oxygen saturation trends immediately after birth. J. Pediatr.148, 590–594 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Dawson, J. A. et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics125, e1340–e1347 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Dawson, J. A. et al. Changes in heart rate in the first minutes after birth. Arch. Dis. Child Fetal Neonatal Ed.95, F177–F181 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Wyckoff, M. H. et al. Part 13. Neonatal resuscitation; 2015. American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation132(Suppl 2), S543.60 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Aziz, K. et al. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation142(Suppl 2), S524–S550 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Katheria, A., Hosono, S. & El-Naggar, W. A new wrinkle: umbilical cord management (how, when, who). Semin. Fetal Neonatal Med.23, 321–326 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Pringle, K. G., Kind, K. L., Sferruzzi-Perri, A. N., Thompson, J. G. & Roberts, C. R. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum. Reprod.16, 415–431 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider, H. Oxygenation of the placental-fetal unit in humans. Respir. Physiol. Neurobiol.178, 51–58 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Singer, D. Metabolic adaptation to hypoxia: a cost and benefit of being small. Respir. Physiol. Neurobiol.141, 215–228 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Vali, P. & Lakshminrusimha, S. The fetus can teach us: oxygen and the pulmonary vasculature. Children4, 67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, C. B. Normal fetal physiology and behavior, and adaptive responses with hypoxemia. Semin. Perinatol.32, 239–242 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Singer, D. & Muhlfeld, C. Perinatal adaptation in mammals: the impact of metabolic rate. Comp. Biochem. Physiol. A Mol. Integr. Physiol.148, 780–784 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Gluckman, P. D. & Johnston, B. M. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J. Physiol.382, 373–383 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding, R. & Hooper, S. B. Regulation of lung expansion and lung growth before birth. J. Appl. Physiol.81, 209–224 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Crawshaw, J. R. et al. Laryngeal closure impedes non-invasive ventilation at birth. Arch. Dis. Child Fetal Neonatal Ed.103, F112–F119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thuot, F., Lemaire, D., Dorion, D., Létourneau, P. & Praud, J. P. Active glottal closure during anoxic gasping in lambs. Respir. Physiol.128, 205–218 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Huberts, T. J. P. et al. The breathing effort of very preterm infants at birth. J. Pediatr.194, 54–59 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Dekker, J. et al. Low FiO2 on breathing effort in preterm infants at birth: a randomized controlled trial. Front. Pediatr.7, 504 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekker, J. et al. Increasing respiratory effort with 100% oxygen during resuscitation of preterm rabbits at birth. Front. Pediatr.7, 427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookatz, G. B. et al. Effect of supplemental oxygen on reinitiation of breathing after neonatal resuscitation in rat pups. Pediatr. Res. 61, 608–702 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Lang, J. A. et al. Increase in pulmonary blood flow at birth; role of oxygen and lung aeration. J. Physiol.594, 1389–98 (2016). [DOI] [PMC free article] [PubMed]
- 24.Lang, J. A. R. et al. Vagal denervation inhibits the increase in pulmonary blood flow during partial lung aeration at birth. J. Physiol.595, 1593–1606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamlin, C. O., O’Donnell, C. P., Everest, N. J., Davis, P. G. & Morley, C. J. Accuracy of clinical assessment of infant heart rate in the delivery room. Resuscitation71, 319–321 (2006). [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell, C. P., Kamlin, C. O., Davis, P. G., Carlin, J. B. & Morley, C. J. Clinical assessment of infant colour at delivery. Arch. Dis. Child Fetal Neonatal Ed.92, F465–F467 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kattwinkel, J. Evaluating resuscitation practices on the basis of evidence: the findings at first glance may seem illogical. J. Pediatr.142, 221–222 (2003). [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell, C. P., Kamlin, C. O., Davis, P. G. & Morley, C. J. Feasibility of and delay in obtaining pulse oximetry during neonatal resuscitation. J. Pediatr.147, 698–699 (2005). [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell, C. P., Kamlin, C. O., Davis, P. G. & Morley, C. J. Obtaining pulse oximetry data in neonates: a randomised crossover study of sensor application techniques. Arch. Dis. Child Fetal Neonatal Ed.90, F84–F85 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariani, G. et al. Pre-ductal and post-ductal O2 saturation in healthy term neonates after birth. J. Pediatr.150, 418–421 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Kamlin, C. O. et al. Accuracy of pulse oximetry measurement of heart rate of newborn infants in the delivery room. J. Pediatr.152, 756–760 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Altuncu, E., Özek, E., Bilgen, H., Topuzoglu, A. & Kavuncuoglu, S. Percentiles of oxygen saturations in healthy term newborns in the first minutes of life. Eur. J. Pediatr.167, 687–688 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Dimich, I. et al. Evaluation of oxygen saturation monitoring by pulse oximetry in neonates in the delivery system. Can. J. Anaesth.38, 985–988 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Gonzales, G. F. & Salirrosas, A. Arterial oxygen saturation in healthy newborns delivered at term in Cerro de Pasco (4340 m) and Lima (150 m). Reprod. Biol. Endrocrinol. 3, 46 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ANZCOR Guideline 13.4—Airway management and mask ventilation of the newborn infant. https://www.nzrc.org.nz/assets/Guidelines/Neonatal-Resus/ANZCOR-Guideline-13.4-April-2021.pdf (2016). [DOI] [PubMed]
- 36.Madar, J. et al. European Resuscitation Council Guidelines 2021: newborn resuscitation and support of transition of infants at birth. Resuscitation161, 291–326 (2021). [DOI] [PubMed] [Google Scholar]
- 37.White, L. N. et al. Achievement of saturation targets in preterm infants <32 weeks’ gestational age in the delivery room. Arch. Dis. Child Fetal Neonatal Ed.102, F423–F427 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Oei, J. L. & Vento, M. Is there a ‘right’ amount of oxygen for preterm infant stabilization at birth? Front. Pediatr.7, 354 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatt, S. et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J. Physiol.591, 2113–2126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blank, D. A. et al. Baby-directed umbilical cord clamping: a feasibility study. Resuscitation131, 1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Van Vonderen, J. J. et al. Pulse oximetry measures a lower heart rate at birth compared with electrocardiography. J. Pediatr.166, 49–53 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Murphy, M. C., De Angelis, L., Mccarthy, L. K. & O’Donnell, C. P. F. Randomised study comparing heart rate measurement in newly born infants using a monitor incorporating electrocardiogram and pulse oximeter versus pulse oximeter alone. Arch. Dis. Child Fetal Neonatal Ed.104, F547–F550 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Polglase, G. R. et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PLoS ONE10, e0117504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouwer, E. et al. Physiological-based cord clamping in preterm infants using a new purpose-built resuscitation table: a feasibility study. Arch. Dis. Child Fetal Neonatal Ed.104, F396–F402 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smit, M. et al. Pulse oximetry in newborns with delayed cord clamping and immediate skin-to-skin contact. Arch. Dis. Child Fetal Neonatal Ed.99, F309–F314 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Cavallin, F. et al. Delayed cord clamping versus early cord clamping in elective cesarean section: a randomized controlled trial. Neonatology116, 252–259 (2019). [DOI] [PubMed] [Google Scholar]
- 47.De Bernardo, G. et al. A randomized controlled study of immediate versus delayed umbilical cord clamping in infants born by elective caesarean section. Ital. J. Pediatr.46, 71 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padilla-Sánchez, C. et al. Delayed vs immediate cord clamping changes oxygen saturation and heart rate patterns in the first minutes after birth. J. Pediatr.227, 149–156,e1 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Bjorland, P. A. et al. Changes in heart rate from 5 s to 5 min after birth in vaginally delivered term newborns with delayed cord clamping. Arch. Dis. Child Fetal Neonatal Ed.106, 311–315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapiro-Mendoza, C. K. & Lackritz, E. M. Epidemiology of late and moderate preterm birth. Semin. Fetal Neonatal Med.17, 120–125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer, M. S. The contribution of mild and moderate preterm birth to infant mortality. JAMA284, 843 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Williams, J. E. & Pugh, Y. The late preterm: a population at risk. Crit. Care Nurs. Clin. North Am.30, 431–443 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Delnord, M. & Zeitlin, J. Epidemiology of late preterm and early term births- an international perspective. Semin. Fetal Neonatal Med.24, 3–10 (2019). [DOI] [PubMed] [Google Scholar]
- 54.De Almeida, M. F. B. et al. Resuscitative procedures at birth in late preterm infants. J. Perinatol.27, 761–765 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Spillane, N. T., Chivily, C. & Andrews, T. Short term outcomes in term and late preterm neonates admitted to the well-baby nursery after resuscitation in the delivery room. J. Perinatol.39, 983.989 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Andersson, O. et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 min of birth (Nepcord III) – a randomized clinical trial. Matern Health Neonatol. Perinatol.5, 15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashish, K. C., Singhal, N., Gautam, J., Rana, N. & Andersson, O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 min of birth—randomized clinical trial. Matern Health Neonatol. Perinatol.5, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]