Abstract
Objective
Opioid overdose survivors present to emergency departments (EDs) and many EDs have developed programs to initiate buprenorphine. The impact of the increasing use of buprenorphine in ED and by other providers is unknown while opioid mortality continues to rise. Public mortality data do not distinguish buprenorphine from other prescription opioids. Our objective was to determine when changes in overdose mortality trends occurred comparing deaths involving buprenorphine to oxycodone, hydrocodone, and methadone.
Methods
This observational study utilized the drug‐involved mortality database including US death certificates (2010‒2017) in which buprenorphine, oxycodone, hydrocodone, or methadone were contributing causes of death (determined through textual analysis). Population‐ and drug utilization‐adjusted mortality rates were examined using disjointed linear regression. Buprenorphine‐involved deaths were stratified by polysubstance involvement.
Results
The population‐adjusted mortality rates for buprenorphine‐involved deaths were lowest compared to other opioids; however, the change in rate for buprenorphine increased faster than oxycodone, hydrocodone, and methadone at 8.9% each quarter‐year (95% confidence interval [CI]: 8.0, 9.8) from 2010 to mid‐2016 when it stabilized. After adjusting for changes in dispensing over the study period, buprenorphine‐involved mortality rates were increasing at 5.3% (95% CI: 4.6, 6.1) each quarter‐year. In 2017, 94% buprenorphine‐involved deaths had at least one other drug contributing to the cause of death.
Conclusions
Given the low mortality, high proportions of polysubstance mortality, and the mixed agonist/antagonist mechanism of action, use of buprenorphine alone likely presents a lower risk for overdose than comparators. Mortality rose faster than dispensing, signaling need to ensure people understand buprenorphine risks, particularly polysubstance use, balanced against importance for treating opioid use disorders.
Keywords: buprenorphine, medication‐assisted therapies, overdose mortality, polysubstance
1. INTRODUCTION
1.1. Background
Mortality related to the opioid crisis has risen rapidly in the last two decades in the United States. Early in this crisis, overdose mortality primarily involved prescription opioids, followed by increasing heroin‐involved overdose mortality and, more recently, by a sharp increase in overdose involving synthetic opioids. 1 By 2017, 70% of all drug overdose deaths involved an opioid, and 60% of opioid deaths involved a synthetic opioid. 2 One tactic to combat the growing opioid epidemic has been increasing access to pharmacological treatments for opioid use disorders (OUDs).
Buprenorphine, which suppresses withdrawal symptoms and attenuates other opioid effects, 3 was approved by the Food and Drug Administration as a treatment for OUD in 2002. 3 Although a waiver was initially required for physicians to prescribe buprenorphine in office‐based settings and there were patient limits in place, access to buprenorphine was expanded in 2016, 4 and the waiver requirement was eliminated entirely starting in 2023. 5 Buprenorphine is a partial agonist at the μ‐opioid receptor and antagonist at the κ‐receptor, which causes it to have different pharmacological profiles than a full agonist treatment, such as methadone. 6 Buprenorphine infusion can also limit respiratory depression caused by other opioids, including fentanyl. 7
Nonetheless, as a partial μ‐opioid receptor agonist, buprenorphine may itself induce respiratory depression 8 and therefore can contribute to cause of death. 9 Therefore, it is important to understand the extent of this risk as emergency physicians will increasingly encounter those who use buprenorphine to treat OUD, may be in active withdrawal, and those who are survivors of opioid overdose as they present to the emergency department (ED). The impact of increasing use of buprenorphine in the ED and by many other providers is unknown. This study was interested in quantifying the contribution of buprenorphine in drug overdose deaths.
1.2. Importance
Buprenorphine cannot be distinguished in death records using Classification of Diseases (ICD‐10) codes. Importantly, publicly available mortality data do not distinguish buprenorphine from other synthetic prescription opioid deaths, a category that includes all fentanyl related deaths as well. 10 To understand the contribution of buprenorphine in cause of death as dictated by coroners and medical examiners, the literal text fields on death certificates must be analyzed. Furthermore, these data are restricted access. With these two features combined, important national trends in buprenorphine‐involved mortality are masked in publicly available mortality reports. Ability to access the literal text on death certificates and extract listed drugs involved in the death allows for more specific assessment of drug molecules and more detailed surveillance of individual active pharmaceutical ingredients. In addition, the co‐involvement of synthetic opioids is growing across all drug deaths (semi‐synthetic opioids, psychostimulants, cocaine, heroin, etc.). 1 Distinguishing between various synthetic opioids could identify uncommon, but relevant, trends in mortality.
1.3. Goals
The primary objective of this study was to compare change in drug overdose mortality trends among deaths involving buprenorphine versus oxycodone, hydrocodone, and methadone in the United States from health surveillance data from 2010 to 2017. This includes expansion to access that occurred in 2016. One secondary objective included describing regional differences in mortality over time. Finally, given that those who present to the ED likely are complex clinical cases, which involved polysubstance use, another secondary objective was to quantify the percentages of polysubstance deaths.
2. METHODS
2.1. Design
This surveillance study used mortality files linked to cause‐of‐death literal text from death certificates provided in the Centers for Disease Control and Prevention (CDC) restricted‐access data from the Research Data Center, the Drug Involved Mortality (DIM) database. The DIM database was essential in this study since it houses the National Vital Statistics System's repository of all deaths with drug‐related terms mentioned in the cause‐of‐death literal text field as determined by medical examiners or coroners. These deaths from 2010 to 2017 in the 50 US states and District of Columbia were included, which was the most recently available data to researchers to request access for use.
The Bottom Line
Many emergency departments have developed buprenorphine programs for treating opioid use disorders and addressing overdoses. Compared to population size in the United States, overdose rates for buprenorphine‐involved deaths were lowest compared to oxycodone, hydrocodone, and methadone (2010‒2017); however, the rates increased from 2010 to mid‐2016. Since buprenorphine dispensing has also increased, the rate per prescription was calculated, also increasing over time. In 2017, 94% of buprenorphine‐involved deaths had another drug involved. Overall buprenorphine mortality was low, but mortality rose faster than dispensing signaling need to ensure people understand buprenorphine risks, particularly polysubstance use, balanced against importance for treating opioid use disorders.
Multiple definitions for overdose deaths have been proposed including identification through ICD‐10 codes only (the standard National Center for Health Statistics [NCHS] definition), use of substance use disorder codes, and pharmaceutical adverse events. 11 For our analysis, drug overdose deaths were defined utilizing the Vermont Department of Health definition, which includes the standard NCHS definition of identifying poisonings from controlled substances 10 (ICD‐10 codes: X40‐44 unintentional/accidental poisoning, X60‐64 intentional self‐harm/suicide, X85 assault/homicide, and Y11‐14 undetermined intent) and adds additional codes to identify drug overdoses including the mental health‐related harmful use and dependence syndromes (X45, X60, F10‐F19[.0, .1], T36‐50, and T51.0). 12 Given buprenorphine was a primary drug of interest and is indicated as treatment for OUD, it was important to include these mental health‐related deaths in our definition. Among overdose cases, a drug with involvement in cause of death must have been mentioned on the death certificate based on medical examiners or coroners’ determination.
2.2. Measures/outcomes
In the United States, all deaths that involve a coroner or medical examiner are captured by use of death certificate, which is completed by the coroner or medical examiner performing the examination. The DIM dataset includes all of the drugs mentioned in the literal text fields, which the coroner or medical examiner concluded were causally involved in the death. The primary drug of interest was buprenorphine and must have been mentioned on the death certificate as a contributing cause to the poisoning. Oxycodone, hydrocodone, and methadone‐involved deaths were also examined for comparison. Individual substances involved in the death were identified through textual analysis and manual review conducted by NCHS; more details on the specifics of this process can be found elsewhere. 13 Briefly, search terms for each drug substance in the literal text fields completed by medical examiners or coroners were identified through both the listed drugs involved and contextual information. If a search term was isolated in the literal text, the drug was coded as contributing to the cause of death. This provides higher precision for counts of substances involved in deaths since it goes beyond the substances identified by individual ICD‐10 codes. If a drug mention is flagged as not being involved in the death through contextual clues in the literal text, the mention was excluded from analysis. If a death record was found to have multiple drugs involved in the cause of death, that death record was included in the analysis for each of the four drugs examined. Among buprenorphine‐involved deaths, polysubstance deaths were classified as including any additional cause of death mentions for: any additional drug, alcohol (ethanol), benzodiazepines (eg, alprazolam, diazepam), centrally active psychostimulants (eg, cocaine, amphetamine, methamphetamine), heroin, and other opioid excluding heroin (eg, fentanyl, oxycodone, morphine). Additional information (eg, demographics, geography, date of death) was used as recorded on the death certificate. Sex and race are both determined by the coroner or medical examiner on the death certificates, with known limitations to the accuracy of race and Hispanic origin. 14 Geography is based on the state where the death occurred, presented at the census region level.
Two additional data sources were utilized in this study to scale the rate adjustments by population and amount of drug dispensing which provided context to how opioid dispensing changed from 2010 to 2017. Annual population rates were calculated using population estimates from the US Census Bureau's Population Estimates Program for 1 July of each year both nationally and stratified by census region. For quarterly rates, the population estimates were interpolated via linear regression between years. Population‐adjusted rates provide a per capita understanding of drug overdose trends. Projections for amount of drug dispensing were provided by IQVIA's US‐Based Longitudinal Prescription Data and are a measure of drug availability by the number of dosage units dispensed in a geographical region. Dosage units were defined as one unit of the dispensed drug, such as a one pill. The IQVIA prescription database uses timely product and geographically specific data obtained from prescription transactions covering approximately 92% of retail pharmacy transactions in the United States to inform their projections. IQVIA uses a proprietary projection methodology to extrapolate from the observed data to the universe of all retail prescriptions in the United States. IQVIA defines the retail prescription channel to include chain pharmacies, independent pharmacies, food store pharmacies, and mass merchandizers with pharmacies. IQVIA data were only available from third quarter 2010 to 2017. Methadone is typically dispensed at opioid treatment centers and therefore not properly accounted for in IQVIA data given it is known to be underrepresented in this data source it was not included in drug utilization analyses. Dispensing adjusted rates provide understanding of drug overdose trends relative to authorized availability.
2.3. Data analyses
First, decedent demographics over the cumulative period from 2010 to 2017 were presented by drug. Second, total number of overdose deaths by drug were calculated as quarterly adjusted rates based on month of death to assess changes over time; quarter 1 included January‒March, quarter 2 included April‒June, quarter 3 included July‒September, and quarter 4 included October‒December. Number of overdose deaths with 95% confidence intervals (CIs, calculated based on the Poisson distribution) were scaled to rates per 1,000,000 population and 1,000,000 dosage units dispensed; national and regional rates were calculated. Joinpoint statistical software was utilized to fit linear disjointed regression slopes, which identified breaks in the national trend at “joinpoints.” 15 Briefly, the software utilized the log‐transformed mortality rates per quarter and searches the grid space to identify the best fit number of joinpoints, and calculates the average quarterly percent change using a Poisson model. 16 , 17 This method assumes no prior number of trend breaks, but does assume sequential linear relationships connected at joinpoints. We ensured at least two quarters must be present in between identified segments to ensure the joinpoint method was detecting changes beyond seasonality in deaths. Tests of significance used a Monte Carlo Permutation method. The advantage of this approach was that no a priori assumptions about observed trends were required. Both segmental quarterly percent change and cumulative average quarterly percent change are shown to be interpreted as the change in total counts, or absolute number, of deaths involving each of the drugs of interest without other adjustments.
To understand dispensing changes during the study period, national dispensing per capita (per 1000 population) was calculated for each drug. Buprenorphine dispensing was further stratified by census region. Finally, among 2017 deaths, the annual proportions classified as polysubstance were calculated with 95% Clopper‒Pearson CI for each additional substance mentioned for the four drugs of interest. Since reporting of all drug mentions involved in deaths has improved over time, the quantification of each drug involved in the polysubstance nature of the death was only shown for the most recently available data.
For all analyses, if death counts less than 10 occurred, the statistics for that cell were suppressed for confidentiality reasons in accordance with CDC restricted data use agreements. Given this study was conducted on only decedents, it is not considered human subject's research. All analyses were conducted in SAS (version 9.4) and Joinpoint Trend Analysis Software (version 4.9.0.0) for the trend analysis. 18
3. RESULTS
3.1. Characteristics of decedents
From 2010 to 2017 among poisoning overdose deaths, there were 3241 buprenorphine‐involved deaths, 46,244 oxycodone‐involved deaths, 26,278 hydrocodone‐involved deaths, and 31,659 methadone‐involved deaths across the 50 US states and the District of Columbia. Decedents with buprenorphine listed on their death certificate as a contributing cause were younger and slightly more likely to be white or male than those with oxycodone, hydrocodone, or methadone listed on their death certificate (Table 1). Across all drugs, accidental poisonings were the most reported underlying cause of death. The inclusion of mental health‐related codes, which was intended to yield potentially missed deaths involving buprenorphine, yielded few additional deaths (n = 169) compared to the standard NCHS definition.
TABLE 1.
Demographics characteristics of overdose deaths by involved drug (2010‒2017).
| Drug listed contributing to cause of death | |||||
|---|---|---|---|---|---|
| Characteristic | Level |
Buprenorphine (N = 3241) |
Oxycodone (N = 46,244) |
Hydrocodone (N = 26,278) |
Methadone (N = 31,659) |
| Gender | Male, N (%) | 2086 (64.4%) | 26,565 (57.4%) | 13,518 (51.4%) | 19,269 (60.9%) |
| Age | Median (IQR) | 38 (30, 49) | 45 (35, 54) | 48 (38, 56) | 43 (32, 53) |
| Race | White, N (%) | 3059 (94.4%) | 42,605 (92.1%) | 24,192 (92.1%) | 28,664 (90.5%) |
| Black, N (%) | 129 (4.0%) | 2751 (5.9%) | 1532 (5.8%) | 2365 (7.5%) | |
| Native American or Alaskan Native, N (%) | 26 (0.8%) | 560 (1.2%) | 350 (1.3%) | 448 (1.4%) | |
| Asian or Pacific Islander, N (%) | 27 (0.8%) | 328 (0.7%) | 204 (0.8%) | 182 (0.6%) | |
| Most common ICD‐10 underlying cause of death codes a | Unintentional | ||||
| X41, N (%) | 34 (1.0%) | 118 (0.3%) | 74 (0.3%) | 119 (0.4%) | |
| X42, N (%) | 1004 (31.0%) | 15,035 (32.5%) | 6940 (26.4%) | 13,558 (42.8%) | |
| X44, N (%) | 1831 (56.5%) | 21,700 (46.9%) | 12,753 (48.5%) | 13,129 (41.5%) | |
| Self‐harm/suicide | |||||
| X62, N (%) | 12 (0.4%) | 1595 (3.4%) | 835 (3.2%) | 429 (1.4%) | |
| X64, N (%) | 59 (1.8%) | 3045 (6.6%) | 2701 (10.3%) | 658 (2.1%) | |
| Undetermined intent | |||||
| Y12, N (%) | 30 (0.9%) | 1198 (2.6%) | 471 (1.8%) | 1444 (4.6%) | |
| Y14, N (%) | 89 (2.7%) | 1423 (3.1%) | 932 (3.5%) | 849 (2.7%) | |
Abbreviations: ICD‐10, Classification of Diseases; IQR, interquartile range.
Underlying cause of deaths codes contributing <1% of deaths for all drugs were not displayed.
3.2. Main results
Mortality for each of the study drugs increased over the study period except for methadone (Table 2 and Figure 1). At the end of the study period, buprenorphine had a lower population‐adjusted mortality rate of 0.67 deaths per 1,000,000 population (95% CI: 0.58, 0.76) compared to rates for hydrocodone (2.32 deaths per 1,000,000, 95% CI: 2.16, 2.48), methadone (2.60 deaths per 1,000,000, 95% CI: 2.43, 2.78), and oxycodone (4.60 deaths per 1,000,000, 95% CI: 4.37, 4.83). However, the cumulative rate of change across the study period for buprenorphine mortality outpaced oxycodone, hydrocodone, and methadone (Table 2).
TABLE 2.
Percent change over time for adjusted mortality rates by drug (2010‒2017).
| Drug | Cumulative percent change a (each quarter‐year) | Segment percent change b (each quarter‐year) | |
|---|---|---|---|
| Average % (95% CI) | Trend segment | Year quarter, % (95% CI) | |
| Population‐adjusted mortality rates | |||
| Buprenorphine | 7.8 (6.7, 9.0) *** | 2010Q1‒2016Q3 | 8.9 (8.0, 9.8) *** |
| 2016Q3‒2017Q4 | 2.4 (‒3.1, 8.2) | ||
| Oxycodone | 0.3 (‒0.3, 0.8) | 2010Q1‒2013Q3 | ‒0.9 (‒1.8, 0.0) |
| 2013Q3‒2017Q4 | 1.2 (0.5, 1.9) ** | ||
| Hydrocodone | ‒0.1 (‒0.4, 0.1) | None observed | – |
| Methadone | ‒1.4 (‒1.1, ‒1.7) *** | None observed | – |
| Dosage units dispensed‐adjusted mortality ratesc | |||
| Buprenorphine | 5.3 (4.6, 6.1) *** | None observed | – |
| Oxycodone | 0.8 (0.2, 1.4) ** | 2010Q3‒2013Q3 | ‒0.6 (‒1.7, 0.6) |
| 2013Q3‒2017Q4 | 1.8 (1.1, 2.4) *** | ||
| Hydrocodone | 2.1 (1.7, 2.4) *** | None observed | – |
Abbreviations: 20XXQY, year‐quarter indication; CI, confidence interval.
Cumulative percent change based on weighted average of segments for entire study period.
Segments based on observed joinpoints identified on log‐transformed mortality rates; when no joinpoints were observed no segment data were provided.
Dispensing data available beginning in 2010Q3; methadone dosage units dispensed mortality rates not shown due to known underestimate of methadone dispensing in data.
p < 0.01.
p < 0.001.
FIGURE 1.
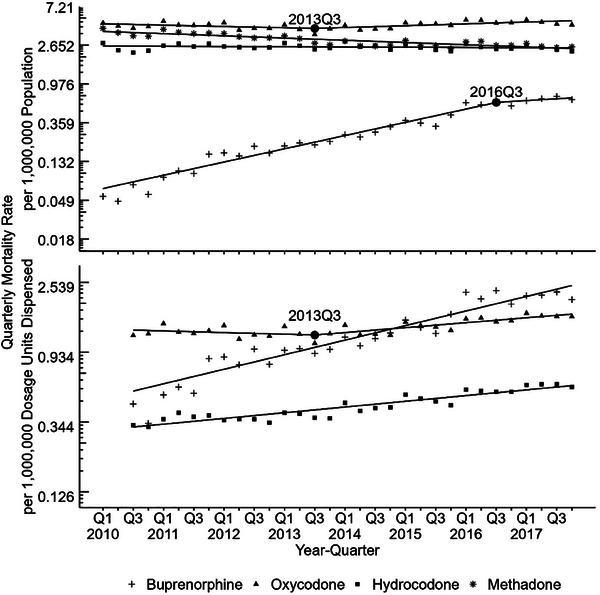
Trends over time for quarterly adjusted mortality rates by drug (2010‒2017). Overall buprenorphine involved deaths rates are lower than that of oxycodone, hydrocodone, and methadone. Buprenorphine population‐adjusted rates had an identified joinpoint at 2016Q3; whereas,) no joinpoint was identified in utilization‐adjusted rates. Trends are displayed on log‐transformed rate scale to show percent change over time. Dispensing data available beginning in 2010Q3; methadone dosage units dispensed mortality rates not shown due to known underestimate of methadone dispensing in data.
For the population‐adjusted mortality rate trends, there was one joinpoint identified in third quarter 2016 for buprenorphine (Figure 1). Before this time, crude buprenorphine mortality rates were statistically significantly increasing at 8.9% each quarter‐year (95% CI: 8.0, 9.8) and then became a non‐significant percent change of 2.4% each quarter‐year (95% CI: ‒3.1, 8.2). Oxycodone also had an identified joinpoint in third quarter 2013 where population‐adjusted rates before this time were not significantly changing and then rose to significant 1.2% change each quarter‐year (95% CI: 0.5, 1.9). Hydrocodone population‐adjusted rates were unchanged while methadone rates were significantly decreasing from 2010 to 2017 at ‒1.4% each quarter‐year (95% CI: ‒1.1, ‒1.7) with no trend changes identified.
While oxycodone and hydrocodone total dispensing per capita had decreased over the study period, buprenorphine dispensing per capita rose steadily nationally and across all US regions (Figure 2). The Northeast had the highest dosage units dispensed per capita (Figure 2). Combining this dispensing data with mortality, buprenorphine dispensing‐adjusted mortality rates had significant growth of 5.3% (95% CI: 4.6, 6.1) each quarter‐year (Figure 1) over the study period, with no identified joinpoints. Hydrocodone dispensing‐adjusted rates also had a significantly increasing percent change for each quarter‐year of 2.1% (95% CI: 1.7, 2.4) across the study period, with no identified joinpoints. For oxycodone, a joinpoint at third quarter 2013 was identified for utilization‐adjusted rates, where rates went from non‐significant change to significantly increasing by 1.8% (95% CI: 1.1, 2.4) each quarter‐year. This oxycodone joinpoint at third quarter 2013 was also found in the population‐adjusted rates.
FIGURE 2.
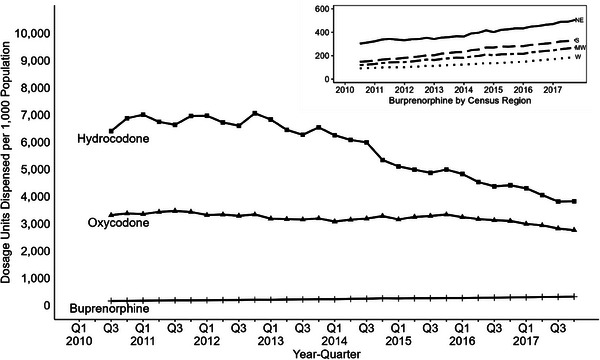
Quarterly dosage units dispensed per capita by drug (2010‒2017). Buprenorphine dispensing per capita remains lower than hydrocodone and oxycodone. However, buprenorphine dispensing per capita rose across all census regions, with dispensing per capita being greatest in the Northeast and lowest in the West. Dispensing data available beginning in 2010Q3; methadone dosage units dispensed not shown due to known underestimate of methadone dispensing in data.
There were observable regional differences in population‐adjusted mortality rates both by magnitude and shape of the trend line (Figure 3 and Table S1). In general, oxycodone had the highest population‐adjusted rate in every region, and buprenorphine had the lowest rate in every region over the study period. Buprenorphine‐involved population‐adjusted mortality increased in all census regions. The Northeast and South appear to have the steepest growth, with buprenorphine surpassing the hydrocodone morality rate in the Northeast in 2017.
FIGURE 3.
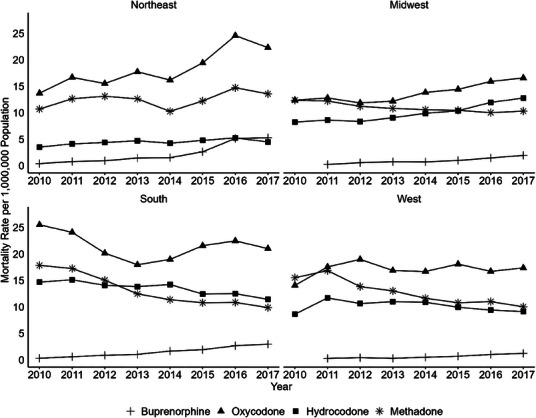
Annual regional population‐adjusted morality rates by census region and drug (2010‒2017). Morality rates per 1,000,000 population by drug differ across census region, with general increases in buprenorphine involved deaths in all regions. Buprenorphine rates from 2010 in the Midwest and West had fewer than 10 deaths and were suppressed for confidentiality reasons.
In 2017 among deaths involving buprenorphine, 93.8% also mentioned another specific drug that contributed to the cause of death. In particular, reports also mentioned other substances in the following order: benzodiazepines (46.9%), opioids other than heroin (43.5%), centrally active psychostimulants (32.0%), and alcohol or heroin (<20%). Polysubstance involvement in deaths was high for all four drugs of interest but generally speaking, buprenorphine was co‐involved with other substances more often than methadone. All polysubstance proportions and 95% CIs are shown in Table 3.
TABLE 3.
Percentages of deaths identified as polysubstance overdose deaths by drug (2017).
| Additional drug mentioned | Proportion of deaths (95% CI) | |||
|---|---|---|---|---|
| Buprenorphine | Oxycodone | Hydrocodone | Methadone | |
| Any specific drug mention | 93.8 (92.0, 95.3) | 87.7 (86.9, 88.5) | 90.2 (89.1, 91.2) | 79.9 (78.5, 81.2) |
| Benzodiazepines | 46.9 (43.6, 50.2) | 41.6 (40.4, 42.8) | 43.0 (41.3, 44.8) | 31.8 (30.3, 33.4) |
| Other opioids excluding heroin | 43.5 (40.2, 46.9) | 48.6 (47.4, 49.9) | 51.7 (49.9, 53.4) | 38.1 (36.5, 39.7) |
| Centrally active psychostimulants | 32.0 (29.0, 35.2) | 20.0 (19.0, 21.0) | 17.4 (16.1, 18.7) | 26.0 (24.6, 27.5) |
| Alcohol | 17.8 (15.3, 20.5) | 16.3 (15.4, 17.2) | 18.0 (16.7, 19.4) | 11.5 (10.4, 12.6) |
| Heroin | 17.8 (15.3, 20.5) | 10.9 (10.1, 11.7) | 9.1 (8.1, 10.1) | 16.6 (15.4, 17.9) |
Abbreviation: CI, confidence interval.
4. LIMITATIONS
The DIM database is comprised of all deaths recorded in the 50 US states and the District of Columbia. The literal text extraction, which cannot be extracted in public records, allows for more detailed surveillance of individual active pharmaceutical ingredients. However, deaths from Puerto Rico and US territories are not included, and results are likely not generalizable to these geographies. Additionally, heterogeneity exists between the state and local practices of medical examiners and coroners and toxicology practices have changed over the course of the study period. While standards for the practice of death investigation exist, these were introduced during the timeframe of this study (2014), and the diagnostic acumen of the pool of medical examiners and coroners could change over time. 19 Likely some of the increasing trend in buprenorphine‐involved deaths over the study period could be attributed to increased identification of buprenorphine. Identification of specific opioid molecules is often dependent on toxicology screens, and misclassification can occur. The proportion of drug overdose deaths in which no specific drugs are identified varies widely by state.
5. DISCUSSION
We observed an increase in dispensing‐adjusted mortality rates for buprenorphine, hydrocodone, and oxycodone. While the absolute number of deaths involving buprenorphine as a contributing cause was fewer than the comparator drugs, the mortality rates for both population‐adjusted and dispensing‐adjusted increased more rapidly than other medications. By end of 2017, the mortality rate of buprenorphine had been increasing almost 8% per year since 2010.
There are several possible explanations for the increased mortality involving buprenorphine. First, prescribing of buprenorphine has increased following extensive efforts to increase the number of health care providers that prescribe buprenorphine. 20 If increased availability resulted in the same rate of deaths per unit dispensed, then the expected result would be that the number of deaths would increase while the dispensing‐adjusted mortality rate would remain unchanged over time. Instead, the mortality rate for buprenorphine after adjustment for dosage units dispensed has increased and lacks the flattening observed after mortality rate adjustment for population. Thus, it is unlikely that increased prescribing of buprenorphine is the cause of the increasing mortality rate.
Second, most buprenorphine is prescribed to patients with OUD who are at a high risk of opioid overdose. However, expansion of prescribing may have included patients who are even higher risk of overdose such as those patient presenting after an acute opioid overdose. While it clinically appropriate for a patient to be started on buprenorphine after an overdose, this practice may shift in the cohort of patients receiving buprenorphine resulting in buprenorphine use by more individuals at high risk for drug overdose. 6 , 21 If patients treated with buprenorphine were at increased risk of death from underlying comorbidity, then the buprenorphine mortality rate might increase as more high‐risk patients received the drug. For example, if more patients with recurrent, relapsing polysubstance overdose were prescribed buprenorphine, the apparent mortality rate might increase if these patients subsequently died. We did observe larger proportions of polysubstance mortality among buprenorphine‐involved deaths (vs. comparators). Similarly, an increase in mortality might develop if efforts to expand prescribing recruited clinicians who were inexperienced in the use of buprenorphine. Of note, most clinicians that prescribe buprenorphine do so infrequently and tend to have a low caseload. 22 Of interest, the number of prescriptions written by emergency physicians is low and unlikely to affect our results, but prescriptions written by advanced practice providers have increased. However, better information is needed to address the risks associated with prescribers. The removal of the buprenorphine waiver requirement in 2023 may create similar concerns. The expanded use of buprenorphine is desirable, but monitoring for safe use in important.
Third, the nature of buprenorphine overdose is evolving. Our period of study occurred during a time when polysubstance use was increasing. Buprenorphine is a partial μ‐receptor agonist, 23 which results in a ceiling for respiratory depression when used alone. 24 Furthermore, the binding affinity of buprenorphine is much higher than full agonists. 25 These properties may contribute to its lower mortality rates relative to full agonists. Both real‐world mortality evidence and pharmacologic evidence support the relative safety of buprenorphine compared to full agonists. However, buprenorphine effects can be overcome with high doses of full agonists, 26 and does not protect against the respiratory depression caused by non‐opioids (e.g., benzodiazepines), which offers a rationale for why polysubstance involvement was frequently observed among buprenorphine involved deaths. As the serum concentration of buprenorphine decreases, the use of a highly potent opioid would be more likely to produce an overdose. Elevated, sustained concentrations of buprenorphine may be needed to protect against such high potency agonists, such as fentanyl. 27 Unfortunately, the concentration of buprenorphine is not included in mortality data.
Furthermore, substances such as xylazine, fentanyl, and fentanyl derivatives have contributed to the marked increase in polysubstance mortality. 28 The impact of increasing polysubstance misuse is still being evaluated, but the presumption is that the involvement of multiple drugs that may affect multiple nervous system or myocardial conduction pathways will increase the risk of death. The interaction of this factor with other factors such as prescribers remains to be determined.
Lastly, drug‐involved death attribution over time is subject to ascertainment bias, which is both a limitation of the study and a possible partial explanation of the observed trends. The increase may be at least partially attributed to changes and improvements in toxicology screening practices and increased identification of buprenorphine‐involved deaths. 19 Federal mortality data are comprised of medical death certificates completed by local authorities in hundreds of jurisdictions across the United States and practices could vary, not only by jurisdiction, but within a jurisdiction, as well as over time. Buprenorphine is not necessarily part of standard toxicology panels, but testing has increased since the introduction of buprenorphine treatment for OUD in 2002. Therefore, mortality data could suffer from increased reporting over time due to local preferences and budgetary concerns.
It is important to understand that being listed as a contributing drug in these data does not mean buprenorphine was the primary cause of death, but rather a medical judgement made on a local basis, not determined by national guidelines. For example, if a decedent was treated with buprenorphine but relapsed and inadvertently ingested a counterfeit oxycodone that actually contained a lethal dose of fentanyl, it is conceivable that buprenorphine might be listed as involved even though fentanyl was most likely the primary cause of death. However, the stabilization of buprenorphine‐involved deaths seen in the population rates suggest identification practices would have had lower impact on data quality in more recent years. Furthermore, a recent study found that the stabilization of buprenorphine‐involved overdose deaths was sustained in 2019‒2021. 29
Separately from the findings around the change in trends, it is important to note that in 2017 nearly all deaths involving buprenorphine included another specific substance (94%), with almost half of deaths including benzodiazepines and opioids other than heroin. This suggests the emergency treatment of opioid overdose may become more complicated. Benzodiazepines can act synergistically with buprenorphine pharmacodynamically resulting in respiratory depression 30 and patients with comorbid sedative use disorders are at increased risk of adverse events, 31 which may partially explain the large percentage of polysubstance deaths involving benzodiazepines. Among deaths involving opioids other than heroin, fentanyl is a large contributing factor based on total mortality trends caused by synthetic opioids 1 ; however, these data in our study were not delineated this way. Other authors have quantified the complex nature of polysubstance mortality more comprehensively. 32 This work further supports the general trend indicating the continued need for overdose prevention strategies, which focus on polysubstance use and diverse treatment approaches. 1 Emergency physicians, whether they utilize buprenorphine or other treatments, can guide patients who misuse opioids into treatment where long‐term care addressing these complexities can be given.
In summary, it is important to understand and interpret the data regarding mortality associated with buprenorphine use carefully. Buprenorphine‐involved mortality remains lower than mortality involving oxycodone, hydrocodone, or methadone; however, buprenorphine‐involved mortality is rising faster than dispensing would fully explain and, after adjustment for dispensing, was highest in 2017 compared to prior years of study. The sources of the observed mortality may arise from a combination of factors including prescribing to higher risk patients, the involvement of less‐experienced prescribers, and perhaps ascertainment bias. Educational efforts addressing the complicated utilization of buprenorphine and subsequent consequences, both in EDs and treatment clinics, would ensure risks and benefits are well understood by practitioners.
AUTHOR CONTRIBUTIONS
Joshua C. Black, Gabrielle E. Bau, and Karilynn Rockhill had access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Joshua C. Black, Janetta Iwanicki, Richard Dart, Angela DeVeaugh‐Geiss, and Howard Chilcoat. Data acquisition and analysis: Joshua C. Black, Gabrielle E. Bau, and Karilynn Rockhill. Drafting manuscript: Karilynn Rockhill. Critical revision of manuscript: All authors. Obtained funding: Angela DeVeaugh‐Geiss and Howard Chilcoat. Administrative and technical support: Joshua C. Black, Karilynn Rockhill, and Gabrielle E. Bau.
CONFLICT OF INTEREST STATEMENT
Karilynn Rockhill, Gabrielle E. Bau, Richard Dart, Dr. Iwanicki, and Joshua C. Black are employed by the Denver Health and Hospital Authority. The RADARS system is supported by subscriptions from pharmaceutical manufacturers, government and non‐government agencies for surveillance, and research and reporting services. RADARS system is the property of Denver Health and Hospital Authority, a political subdivision of the State of Colorado. Subscribers neither participate in data collection nor do they have access to the raw data. Angela DeVeaugh‐Geiss and Howart Chilcoat are employees of Indivior, Inc. and own stock in the company. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Research Data Center, National Center for Health Statistics (NCHS), or the US Centers for Disease Control and Prevention.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank the staff from US Census Bureau at the Rocky Mountain Research Data Center for their assistance in creating the data set and staff from National Center for Health Statistics (NCHS) for their assistance in reviewing the data. No compensation was received. Indivior, Inc. funded the design, data access fees, data preparation, and review of the manuscript. They did not have direct access to the data.
Biography
Karilynn M. Rockhill PhD is an epidemiologist and analyst in Rocky Mountain Poison & Drug Safety in Denver, Colorado, USA.

Rockhill KM, Bau GE, DeVeaugh‐Geiss A, et al. Buprenorphine, oxycodone, hydrocodone, and methadone mortality in the United States (2010‒2017). JACEP Open. 2024;5:e13338. 10.1002/emp2.13338
Supervising Editor: Brittany Punches, PhD, RN
DATA AVAILABILITY STATEMENT
Data used in this study are restricted access through the National Vital Statistics System and therefore cannot be shared; this request process can be accessed at the following URL (https://www.cdc.gov/rdc/b1datatype/dt1229.html). After approval of the project, a dataset is created that has a de‐identified row for every death in the United States including demographic, geographic, and cause‐of‐death variables. More documentation can be found here about this dataset including an example data dictionary (https://www.cdc.gov/rdc/b1datatype/datafiles/Drug‐Involved‐Mortality‐Data‐Documentation.pdf).
REFERENCES
- 1. Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013‒2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202‐207. doi: 10.15585/mmwr.mm7006a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid‐Involved Overdose Deaths—United States, 2013‒2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419‐1427. doi: 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry. 2020;87(1):82‐88. doi: 10.1016/j.biopsych.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 4. Code of Federal Regulations . Medication Assisted Treatment for Opioid Use Disorders . Substance Abuse and Mental Health Services Administration (SAMHSA), HHS. 42 CFR 8. 2016.
- 5. Milgram A. DEA Announced Important Change to Registration Requirement. US Department of Justice. October 9, 2023 [updated January 12, 2023]. https://www.deadiversion.usdoj.gov/pubs/docs/A‐23‐0020‐Dear‐Registrant‐Letter‐Signed.pdf
- 6. Chilcoat HD, Amick HR, Sherwood MR, Dunn KE. Buprenorphine in the United States: motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148‐157. doi: 10.1016/j.jsat.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 7. Olofsen E, Algera MH, Moss L, et al. Modeling buprenorphine reduction of fentanyl‐induced respiratory depression. JCI Insight. 2022;7(9):e156973. doi: 10.1172/jci.insight.156973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94(6):825‐834. doi: 10.1093/bja/aei145 [DOI] [PubMed] [Google Scholar]
- 9. Yassen A, Olofsen E, Romberg R, et al. Mechanism‐based PK/PD modeling of the respiratory depressant effect of buprenorphine and fentanyl in healthy volunteers. Clin Pharmacol Ther. 2007;81(1):50‐58. doi: 10.1038/sj.clpt.6100025 [DOI] [PubMed] [Google Scholar]
- 10. National Vital Statistics System . Instructions for classifying the underlying cause of death. NCHS Instruction Manual: Part 2a . National Center for Health Statistics.
- 11. Dasgupta NPS, Sandford C, Funk MJ, et al. Defining controlled substances overdose: should deaths from substance use disorders and pharmaceutical adverse events be included? J Clin Toxicol. 2013;03(03):1‐8. [Google Scholar]
- 12. Vermont Department of Health . Opioid‐Related Fatalities Among Vermonters . Burlington, VT: Vermont Department of Health; 2020. https://www.healthvermont.gov/sites/default/files/documents/pdf/ADAP_Data_Brief_Opioid_Related_Fatalities.pdf
- 13. Trinidad JP, Warner M, Bastian BA, Minino AM, Hedegaard H. Using literal text from the death certificate to enhance mortality statistics: characterizing drug involvement in deaths. Natl Vital Stat Rep. 2016;65(9):1‐15. [PubMed] [Google Scholar]
- 14. Arias E, Heron M, Hakes J. The validity of race and Hispanic‐origin reporting on death certificates in the United States: an update. Vital Health Stat. 2016;2(172):1‐21. [PubMed] [Google Scholar]
- 15. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335‐351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid‐sim336>3.0.co;2‐z PubMed PMID: 10649300 [DOI] [PubMed] [Google Scholar]
- 16. Kim HJ, Luo J, Chen HS, et al. Improved confidence interval for average annual percent change in trend analysis. Stat Med. 2017;36(19):3059‐3074. doi: 10.1002/sim.7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670‐3682. doi: 10.1002/sim.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joinpoint Regression Program. Statistical Research and Applications Branch. Version 4.9.0.0. National Cancer Institute; 2021.
- 19. Davis GG. Complete republication: National Association of Medical Examiners position paper: recommendations for the investigation, diagnosis, and certification of deaths related to opioid drugs. J Med Toxicol. 2014;10(1):100‐106. doi: 10.1007/s13181-013-0323-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larochelle MR, Jones CM, Zhang K. Change in opioid and buprenorphine prescribers and prescriptions by specialty, 2016‒2021. Drug Alcohol Depend. 2023;248:109933. doi: 10.1016/j.drugalcdep.2023.109933 [DOI] [PubMed] [Google Scholar]
- 21. Kenney SR, Anderson BJ, Bailey GL, Stein MD. The relationship between diversion‐related attitudes and sharing and selling buprenorphine. J Subst Abuse Treat. 2017;78:43‐47. doi: 10.1016/j.jsat.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stein BD, Saloner B, Schuler MS, Gurvey J, Sorbero M, Gordon AJ. Concentration of patient care among buprenorphine‐prescribing clinicians in the US. JAMA. 2021;325(21):2206‐2208. doi: 10.1001/jama.2021.4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long‐acting formulations. J Addict Med. 2019;13(2):93‐103. doi: 10.1097/ADM.0000000000000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55(5):569‐580. doi: 10.1038/clpt.1994.71 [DOI] [PubMed] [Google Scholar]
- 25. Volpe DA, McMahon Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59(3):385‐390. doi: 10.1016/j.yrtph.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 26. Strain EC, Walsh SL, Bigelow GE. Blockade of hydromorphone effects by buprenorphine/naloxone and buprenorphine. Psychopharmacology. 2002;159(2):161‐166. doi: 10.1007/s002130100920 [DOI] [PubMed] [Google Scholar]
- 27. Moss LM, Algera MH, Dobbins R, et al. Effect of sustained high buprenorphine plasma concentrations on fentanyl‐induced respiratory depression: a placebo‐controlled crossover study in healthy volunteers and opioid‐tolerant patients. PLoS One. 2022;17(1):e0256752. doi: 10.1371/journal.pone.0256752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones CM, Einstein EB, Compton WM. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010‒2016. JAMA. 2018;319(17):1819‐1821. doi: 10.1001/jama.2018.2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanz LJ, Jones CM, Davis NL, et al. Trends and characteristics of buprenorphine‐involved overdose deaths prior to and during the COVID‐19 pandemic. JAMA Netw Open. 2023;6(1):e2251856. doi: 10.1001/jamanetworkopen.2022.51856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44(7):661‐680. doi: 10.2165/00003088-200544070-00001 [DOI] [PubMed] [Google Scholar]
- 31. Zoorob R, Kowalchuk A, Mejia de Grubb M. Buprenorphine therapy for opioid use disorder. Am Fam Physician. 2018;97(5):313‐320. [PubMed] [Google Scholar]
- 32. Black JC, Rockhill KM, Dart RC, Iwanicki J. Clustering patterns in polysubstance mortality in the United States in 2017: a multiple correspondence analysis of death certificate data. Ann Epidemiol. 2023;77:119‐126. doi: 10.1016/j.annepidem.2022.03.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Data used in this study are restricted access through the National Vital Statistics System and therefore cannot be shared; this request process can be accessed at the following URL (https://www.cdc.gov/rdc/b1datatype/dt1229.html). After approval of the project, a dataset is created that has a de‐identified row for every death in the United States including demographic, geographic, and cause‐of‐death variables. More documentation can be found here about this dataset including an example data dictionary (https://www.cdc.gov/rdc/b1datatype/datafiles/Drug‐Involved‐Mortality‐Data‐Documentation.pdf).


