Abstract
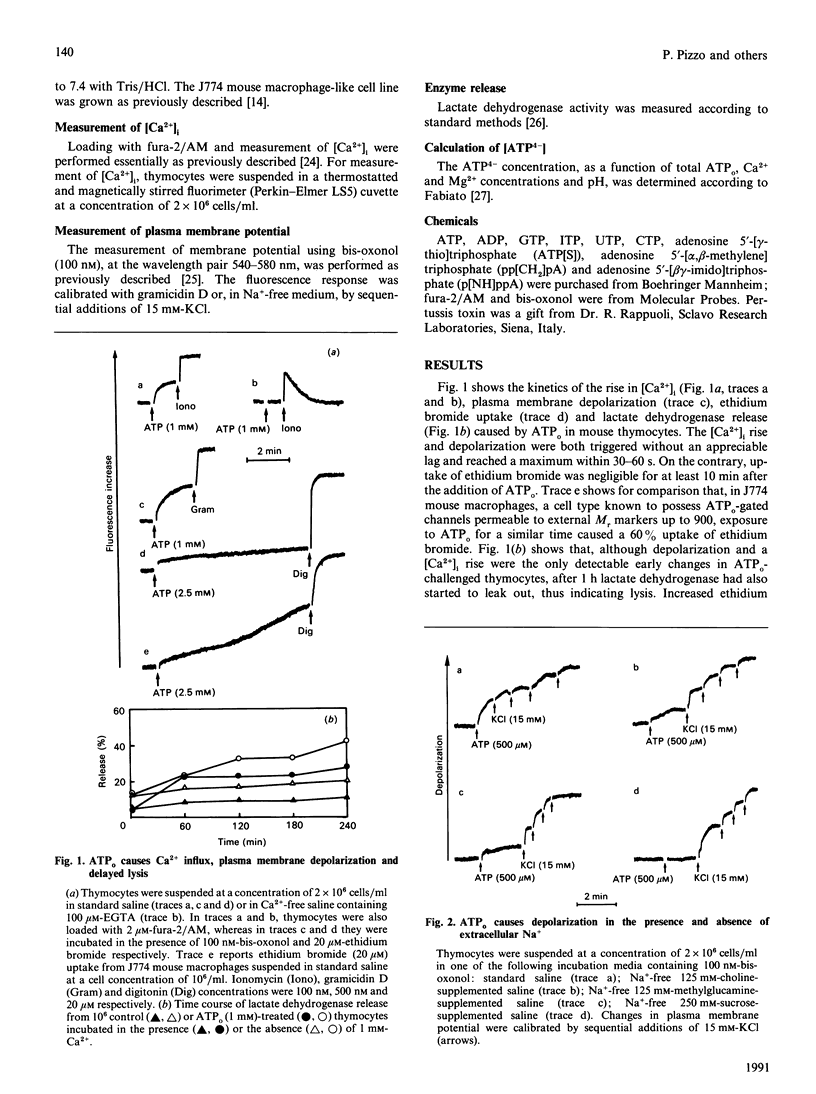
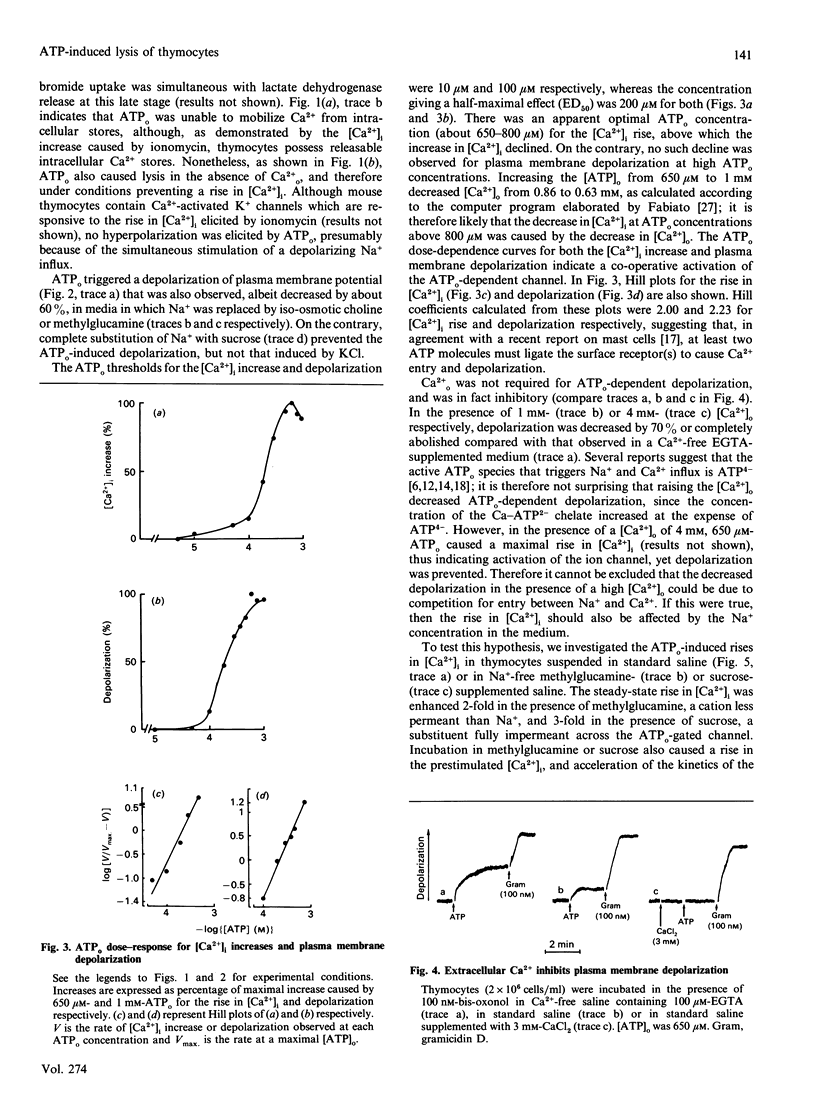
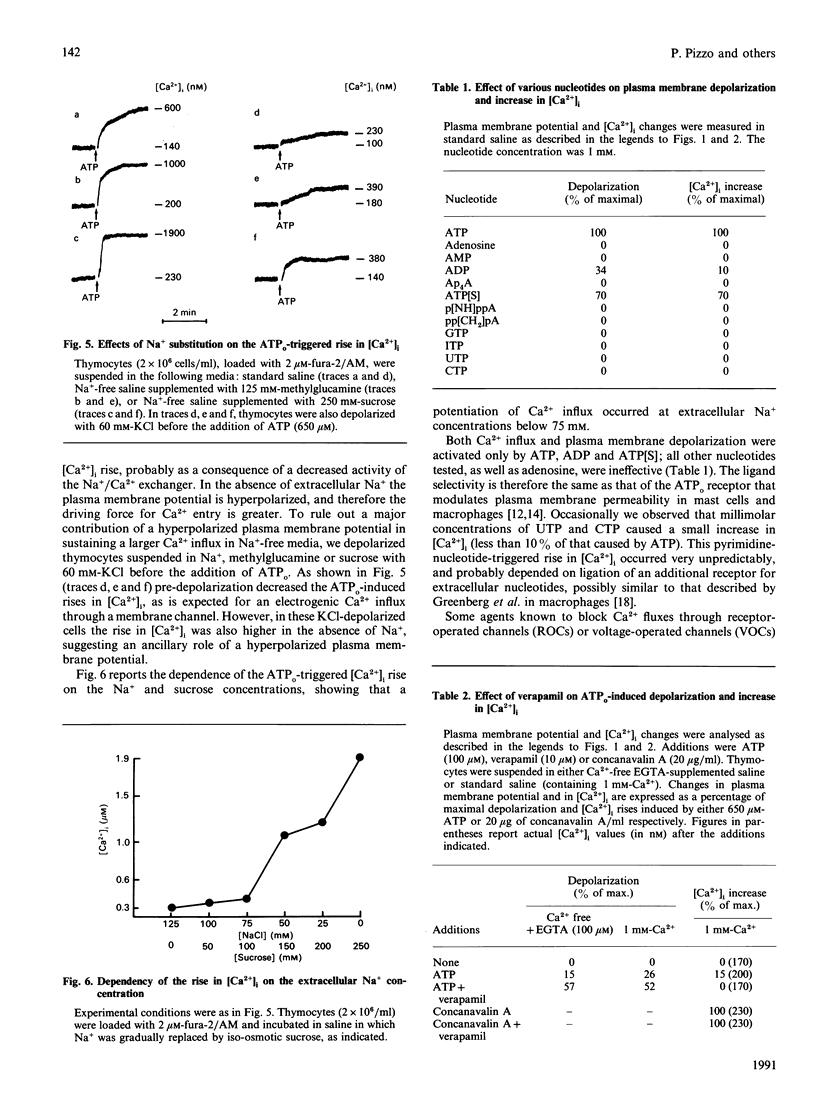
Extracellular ATP (ATPo) caused a concentration-dependent lysis of mouse thymocytes. Lysis, as judged by release of the cytosolic enzyme lactate dehydrogenase, was preceded by depolarization of the plasma membrane and by Ca2+ influx. Both Na+ uptake (which sustained plasma membrane depolarization) and Ca2+ influx showed (1) the same dependence on the ATPo concentration; (2) the same nucleotide specificity; and (3) the same Hill coefficient. However, whereas the rise in the cytosolic free Ca2+ concentration ([Ca2+]i) was fully inhibited by the known Ca2+ blocker verapamil, plasma membrane depolarization was enhanced under these conditions. Plasma membrane depolarization was greater and was shifted to lower ATPo concentrations in the absence of extracellular Ca2+ (Ca2+o), whereas the rise in [Ca2+]i was greater in Na(+)-free media. Plasma membrane depolarization also occurred in Na(+)-free choline- or methylglucamine-containing media, and was potentiated by chelation of free divalent ions with EDTA, supporting previous reports pointing to ATP4-as the active species. Among a number of purine and pyrimidine nucleotides, only adenosine 5'-[gamma-thio]triphosphate and ADP were partially effective. Furthermore, ethidium bromide (Mr 380), Lucifer Yellow (Mr 463) and Eosin Yellowish (Mr 692) did not permeate through the ATPo-activated channel. These findings suggest that lytic effects of ATPo in mouse thymocytes depend on the activation of a membrane channel with low selectivity for cations and an Mr cut-off of 200.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Buisman H. P., Steinberg T. H., Fischbarg J., Silverstein S. C., Vogelzang S. A., Ince C., Ypey D. L., Leijh P. C. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- De Togni P., Cabrini G., Di Virgilio F. Cyclic AMP inhibition of fMet-Leu-Phe-dependent metabolic responses in human neutrophils is not due to its effects on cytosolic Ca2+. Biochem J. 1984 Dec 1;224(2):629–635. doi: 10.1042/bj2240629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Bronte V., Collavo D., Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5'-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol. 1989 Sep 15;143(6):1955–1960. [PubMed] [Google Scholar]
- Di Virgilio F., Fasolato C., Steinberg T. H. Inhibitors of membrane transport system for organic anions block fura-2 excretion from PC12 and N2A cells. Biochem J. 1988 Dec 15;256(3):959–963. doi: 10.1042/bj2560959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Pizzo P., Zanovello P., Bronte V., Collavo D. Extracellular ATP as a possible mediator of cell-mediated cytotoxicity. Immunol Today. 1990 Aug;11(8):274–277. doi: 10.1016/0167-5699(90)90111-l. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Filippini A., Taffs R. E., Agui T., Sitkovsky M. V. Ecto-ATPase activity in cytolytic T-lymphocytes. Protection from the cytolytic effects of extracellular ATP. J Biol Chem. 1990 Jan 5;265(1):334–340. [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Rozengurt E., Heppel L. A. Extracellular ATP induces the release of calcium from intracellular stores without the activation of protein kinase C in Swiss 3T6 mouse fibroblasts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4530–4534. doi: 10.1073/pnas.86.12.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Kitagawa T., Amano F., Akamatsu Y. External ATP-induced passive permeability change and cell lysis of cultured transformed cells: action in serum-containing growth media. Biochim Biophys Acta. 1988 Jun 22;941(2):257–263. doi: 10.1016/0005-2736(88)90186-1. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Orrenius S., Jondal M. Cellular signalling in programmed cell death (apoptosis). Immunol Today. 1990 Apr;11(4):120–121. doi: 10.1016/0167-5699(90)90048-e. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Dogterom P., De Bont H. J., Mulder G. J. Prolonged high intracellular free calcium concentrations induced by ATP are not immediately cytotoxic in isolated rat hepatocytes. Changes in biochemical parameters implicated in cell toxicity. Biochem J. 1989 Oct 15;263(2):347–353. doi: 10.1042/bj2630347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Reciprocal control of membrane permeability of transformed cultures of mouse cell lines by external and internal ATP. J Biol Chem. 1979 Feb 10;254(3):708–714. [PubMed] [Google Scholar]
- Steinberg T. H., Silverstein S. C. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987 Mar 5;262(7):3118–3122. [PubMed] [Google Scholar]
- Su C. Extracellular functions of nucleotides in heart and blood vessels. Annu Rev Physiol. 1985;47:665–676. doi: 10.1146/annurev.ph.47.030185.003313. [DOI] [PubMed] [Google Scholar]
- Tatham P. E., Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen Physiol. 1990 Mar;95(3):459–476. doi: 10.1085/jgp.95.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol. 1980 Dec 7;87(3):609–621. doi: 10.1016/0022-5193(80)90239-8. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanovello P., Bronte V., Rosato A., Pizzo P., Di Virgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol. 1990 Sep 1;145(5):1545–1550. [PubMed] [Google Scholar]
- el-Moatassim C., Maurice T., Mani J. C., Dornand J. The [Ca2+]i increase induced in murine thymocytes by extracellular ATP does not involve ATP hydrolysis and is not related to phosphoinositide metabolism. FEBS Lett. 1989 Jan 2;242(2):391–396. doi: 10.1016/0014-5793(89)80508-3. [DOI] [PubMed] [Google Scholar]


