Abstract
Coronary CT angiography (CTA) derived fractional flow reserve (FFRCT) is recommended for physiological assessment in intermediate coronary stenosis for guiding referral to invasive coronary angiography (ICA). In this study, we report real-world data on the feasibility of implementing a CTA/FFRCT test algorithm as a gatekeeper to ICA at referral hospitals. Retrospective all-comer study of patients with new onset stable symptoms and suspected coronary stenosis (30–89%) by CTA. Evaluation of CTA datasets, interpretation of FFRCT analysis, and decisions on downstream testing were performed by skilled CT-cardiologists. CTA was performed in 3974 patients, of whom 381 (10%) were referred directly to ICA, whereas 463 (12%) to non-invasive functional testing: FFRCT 375 (81%) and perfusion imaging 88 (19%). FFRCT analysis was rejected in 8 (2%) due to inadequate CTA image quality. Number of patients deferred from ICA after FFRCT was 267 (71%), while 100 (27%) were referred to ICA. Obstructive coronary artery disease (CAD) was confirmed in 62 (62%) patients and revascularization performed in 53 (53%). Revascularization rates, n (%), were higher in patients undergoing FFRCT-guided versus CTA-guided referral to ICA: 30–69% stenosis, 28 (44%) versus 8 (21%); 70–89% stenosis, 39 (69%) versus 25 (46%), respectively, both p < 0.05. Implementation of FFRCT at referral hospitals was feasible, reduced the number of invasive procedures, and increased the revascularization rate.
Keywords: Angina pectoris, Myocardial fractional flow reserve, Computed tomography, Xray, Coronary angiography, Myocardial revascularization
Introduction
In Denmark, as stated in international guidelines, coronary computed tomography angiography (CTA) has for years been used as a first-line test for evaluation of patients presenting with symptoms suggestive of new onset stable angina pectoris (SAP) [1]. CTA has proved superior to traditional non-invasive testing algorithms in reducing long-term incidence of myocardial infarction [2]. However, as CTA is a strict anatomic test and the correlation between stenosis severity and impact on coronary flow as measured by fractional flow reserve (FFR) is only moderate [3], additional non-invasive functional testing is recommended prior to referral to invasive assessment in stable patients with suspected coronary stenosis, unless stenosis severity and patient symptoms calls for direct invasive assessment [4, 5].
FFR derived from CTA (FFRCT) is a contemporary modality that allows physiological estimation of the impact on blood flow by coronary artery disease (CAD) detected by coronary CTA [6, 7]. This novel non-invasive modality has been validated for functional assessment of intermediate coronary stenosis [3, 8]. FFRCT has demonstrated high and superior diagnostic performance compared to CTA alone [3], improved diagnostic sensitivity as compared to commonly applied stress perfusion imaging modalities [9–11], a high per-patient and -vessel agreement with invasive FFR [3, 8], and favourable prognostic outcomes in case of a normal FFRCT test result [12–15]. Consequently, FFRCT has recently been recommended for guiding referral to invasive coronary angiography (ICA) in patients with SAP and intermediate coronary stenosis by CTA [5, 16, 17]. However, only few hospitals have reported data on the applicability of FFRCT consecutively in an all-comer setting. [14, 18, 19]. In this study of patients undergoing CTA at referral hospitals as a first-line test for suspected SAP, we evaluated the gate-keeping potential of using selective FFRCT as the preferred second-line test in terms of feasibility, use of downstream procedures, and revascularization practice.
Methods
Study design and patient population
Two-center, retrospective all-comer study. Data represents the initial clinical results following implementation of FFRCT as the preferred test for functional assessment of intermediate stenosis by CTA in patients with new onset suspected SAP, Fig. 1. Data were collected in 2018 or 2019 at two departments of cardiology at University Hospital of Southern Denmark (Vejle and Esbjerg), Region of Southern Denmark. Only patients with sinus rhythm, a body mass index ≤ 40 kg/m2, an estimated glomerular filtration rate ≥ 45 ml/min, and no previous revascularization were eligible for CTA. Patients with left main disease, multivessel disease or severe proximal disease by CTA were referred directly to ICA according to best practice guidelines [5, 20]. Clinical data were obtained from electronic patient journals. This study was approved by regional authorities (journal nr.: 21/10587 and 18/44285).
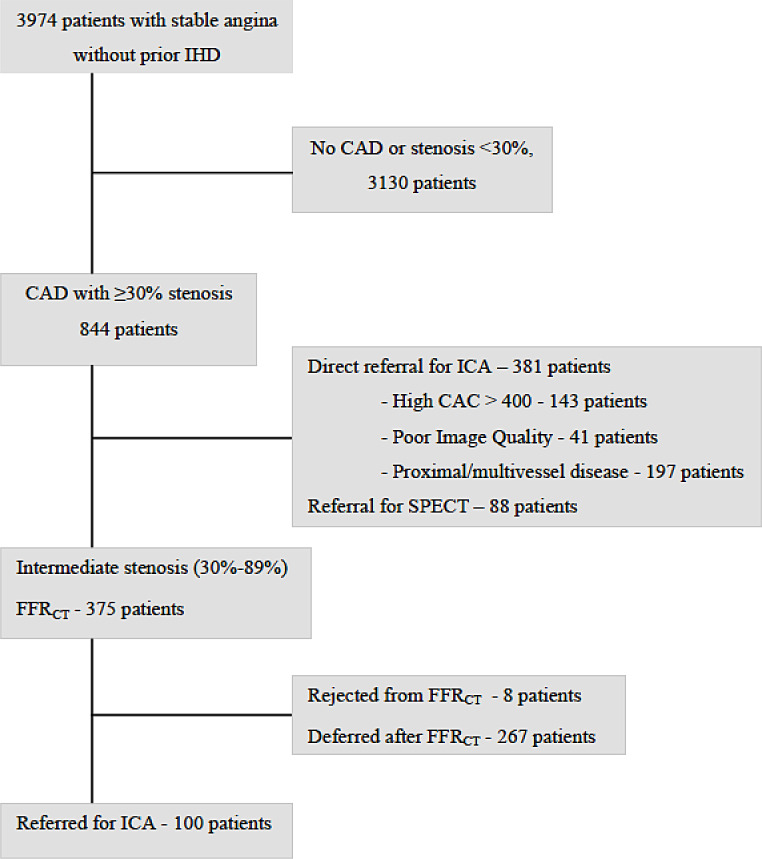
Fig. 1.
Flow chart. Schematic representation of flowchart for patients with new onset SAP and suspected coronary stenosis, who are eligible for coronary CTA and referral for FFRCT. Abnormal FFRCT test: An FFRCT value ≤ 0.80, registered 10–20 mm distal to the stenosis (2 cm-FFRCT) was the primary criterium for abnormality. Distal in vessel FFRCT, ∆FFRCT (difference of FFRCT-values immediately proximal and 10 mm distal to stenosis), high risk plaque features, plaque burden, stenosis location and number of stenosis (21) represented alternative criteria for abnormality. Abnormal MPI-SPECT: Diagnosis of an abnormal test result based on traditional criteria, including a summed difference score ≥ 2/an ungated stress-and-rest volume ratio of > 1.19/a significant decrease in left ventricular ejection fraction from rest to stress. Abbreviations: SAP: stable angina pectoris CTA: computed tomography angiography CAD: coronary artery disease OMT: optimal medical treatment FFRC: coronary computed tomography angiography derived fractional flow reserve ICA: invasive coronary angiography MPI-SPECT: myocardial perfusion imaging by single-photon emission computerized tomography SDS: summed difference score
Coronary CTA
CTA was performed using either a SOMATOM Definition Flash or a FORCE CT scanner (both from Siemens Healthineers, Forchheim, Germany). Oral beta-blockers or ivabradine were administered if necessary, targeting a heart rate ≤ 60 beats/min. All patients received sublingual nitroglycerin. An initial non-enhanced scan for calcium scoring was performed. CTA was assessed by skilled CT cardiologists. Vessels ≥ 2 mm in diameter were evaluated and graded visually by the interpreters. Stenosis severity by CTA was classified as; non-obstructive, 1–29%; suspected obstructive 30–89%; obstructive ≥ 90%. Suspected obstructive stenosis was divided into categories 30–69% and 70–89% stenosis. Information regarding stenosis severity was obtained by reviewing CTA interpretation reports in the electronic patient journal.
FFRCT-analysis and interpretation
Standard acquired coronary CTA datasets were transmitted for central analysis (HeartFlow Inc., Redwood City, California) as previously described. A generated individualized 3D-model of the FFRCT-analysis served as the platform for registration and interpretation of the FFRCT-data. Interpretation of FFRCT-data and decisions on referral to ICA were performed by skilled CT cardiologists and were guided by current recommendations for interpretation of FFRCT-data [21]. Briefly, an FFRCT value ≤ 0.80, registered 10–20 mm distal to a stenosis (2 cm-FFRCT) was the primary criterion for classifying a stenosis as hemodynamic significant and the patient as potential candidate for ICA. Alternative criteria for referral to invasive procedures included severity of decrease of distal in vessel FFRCT, magnitude of ∆FFRCT (difference of FFRCT-values immediately proximal and 10 mm distal to stenosis), high risk plaque features (positive remodeling, spotty calcification or low-attenuation plaque), plaque burden, stenosis location, and number of stenoses [21].
Invasive procedures and revascularization
Diagnostic ICA was performed at the two CTA hospitals. A multidisciplinary heart team conference and/or the treating physician made decisions on revascularization strategy. Patients were classified as having obstructive CAD, if ≥ 1 coronary vessel had ≥ 50% stenosis (visual assessment), or if ≥ 1 coronary stenosis had an FFR-value ≤ 0.80 distal to stenosis. Percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) were performed at one tertiary hospital and in accordance with international guidelines [4, 22].
Statistical methods
Baseline characteristics are presented as mean (SD) or medians (interquartile range [IQR]) as appropriate for continuous variables and proportions for categorical variables. Logistic regression was used to compare coronary CTA versus FFRCT with respect to incidences of obstructive CAD by ICA and revascularization rates, and to assess differences in revascularization rate according to the applied FFRCT-interpretation algorithm (2 cm distal to stenosis criterium versus alternative criteria) for stenosis categories 30–69% and 70–89%.
Diagnostic performance of baseline risk variables (diabetes, hypertension, dyslipidaemia, and smoking), coronary stenosis at CTA, symptoms, and coronary CTA–derived FFR were assessed using receiver operating characteristic curves, and differences between areas under the receiver operating characteristic curve were evaluated using the DeLong method.
A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using Stata version 16.1 software (Stata Corp, College Station, Texas).
Results
Coronary CTA was performed in 3974 patients: 3130 (79%) had no CAD or stenosis < 30%, 381 (10%) were referred directly to ICA, and 463 (12%) were referred for non-invasive functional assessment, Fig. 1. In patients undergoing non-invasive functional testing, single photon emission computerized tomography (SPECT) myocardial perfusion imaging was performed in 88 (19%) patients and FFRCT in 375 (81%). FFRCT analysis was successful in 367 (98%) patients and 8 (2%) were rejected based on poor image quality due to coronary calcification, misalignment and/or motion artifacts. Turn-around time for FFRCT was < 48 h in all patients. Baseline characteristics of patients referred to ICA, directly after CTA or after selective FFRCT are shown in Table 1.
Table 1.
Baseline characteristics of patients referred to invasive coronary angiography according to non-invasive testing strategy
| CTA-guided n = 381 |
FFRCT-guided n = 367 |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age | 66 ± 10 | 64 ± 10 | 0.007 |
| Gender, male | 244 (64) | 228 (61) | 0.621 |
| Risk factors | |||
| Diabetes | 53 (14) | 40 (11) | 0.213 |
| Hypertension | 262 (69) | 218 (58) | 0.008 |
| Dyslipidemia | 259 (68) | 236 (63) | 0.289 |
| Current smoker | 268 (70) | 209 (56) | < 0.001 |
| Coronary CTA | |||
| Agatston score, U | 574 (198–1234) [0-6067] | 211 (81–456) [0-3168] | < 0.001 |
| Stenosis severity | |||
| 30–69% | 38 (10) | 319 (87) | < 0.001 |
| 70–89% | 84 (22) | 48 (13) | |
| ≥90% | 75 (20) | 0 (0) | |
| Non evaluable* | 184 (48) | 0 (0) | |
Values given as n (%), mean ± SD or median (interquartile range) [range]
*Due to high calcium (n = 143) or poor image quality (n = 41)
Patients classified according to most severe stenosis
Abbreviations: CTA = computerized tomography angiography; FFRCT=coronary CTA derived fractional flow reserve; ICA = invasive coronary angiography
The clinical consequences of using selective FFRCT testing for decision making are shown in Table 2, case examples in Fig. 2. FFRCT had a positive predictive value of 62%, and 71% of patients referred for FFRCT were deferred from ICA or other downstream tests, Table 2. The proportion of patients who were deferred from ICA based on the FFRCT test result was 80% (n = 255) in those with a 30–69% stenosis and 25% (n = 12) in those with a 70–89% stenosis.
Table 2.
Downstream testing and treatment after selective FFRCT testing in stable angina pectoris
| No need for additional downstream testing | 267 (71) | |
| Referred for ICA | 100 (27) | |
| Rejected FFRCT analysis | 8 (2) | |
| Findings by ICA and treatment | ||
| Obstructive CAD | 62 (62) | |
| Revascularization | 53 (53) | |
| 1-vessel PCI | 43 (81) | |
| 2-vessel PCI | 6 (11) | |
| 3-vessel PCI | 0 (0) | |
| CABG | 4 (8) | |
| OMT | 9 (9) | |
| Non-obstructive CAD, OMT | 38 (38) | |
| Turn-around time < 48 h | 367 (98) | |
Values given as n (%) Obstructive CAD, n (%) was defined by eye-balling by the interventionist, 49 (79) or by a measured fractional flow reserve ≤ 0.80, 13 (21). Non-obstructive CAD was defined by eye-balling in 34 (89%) or by invasive FFR ≤ 0.80 in 4 (11%). Abbreviations CABG = coronary arterial bypass grafting; CAD = coronary artery disease; CTA = computerized tomography angiography; FFRCT=coronary CTA derived fractional flow reserve; ICA = invasive coronary angiography; OMT = optimal medical therapy; PCI = percutaneous coronary intervention
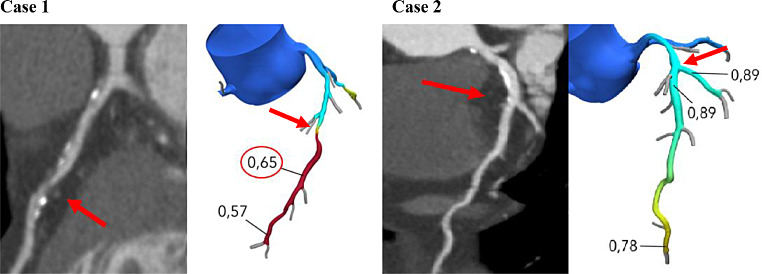
Fig. 2.
Case examples. Representation of two patient cases with proximal (70–89%) LAD stenosis. Case 1 was referred to ICA and was revascularized and treated with OMT. Case 2 was deferred from further testing and treated with OMT Arrows indicate location of stenoses. Markers illustrate the point in the coronary tree 2 cm distal to stenoses, where FFRCT values were registered Abbreviations LAD = left anterior descending artery; ICA = invasive coronary angiography; OMT = optimal medical therapy; FFRCT=coronary computed tomography angiography derived fractional flow reserve
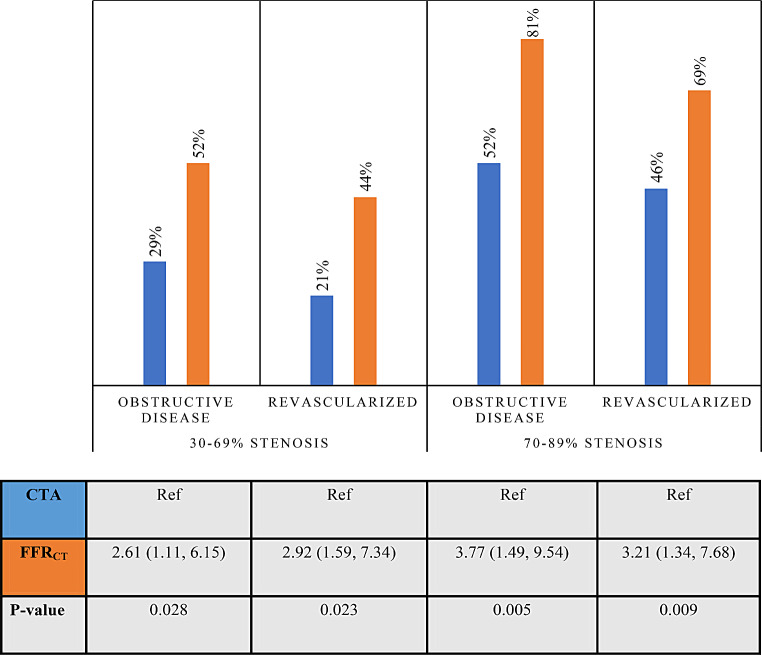
The finding that FFRCT superiorly detected obstructive disease and carried a higher revascularization rate compared with CTA alone was observed both in patients with a 30–69% stenosis and in those with a 70–89% stenosis, Fig. 3. Patients referred to ICA based on CTA without FFRCT were older, had more risk factors for cardiovascular disease, a higher coronary artery calcium score, and a more severe degree of CTA-assessed stenosis when compared to patients referred to ICA after FFRCT, Table 1. Invasive FFR was performed in 11% of patients in the CTA group and in 21% of patients in the FFRCT group.
Fig. 3.
FFRCT-guided versus coronary CTA-guided referral to ICA in suspected coronary stenosis. Invasive findings and treatment. The diagram illustrates findings by ICA and treatment depending on coronary CTA-guided or FFRCT-guided referral to ICA. The number of patients, n, referred directly to ICA according to stenosis severity by coronary CTA were: 30–69%, 38; 70–89%, 84. The corresponding numbers for patients referred based on FFRCT-testing were: 30–69%, 64; 70–89%, 36. Differences in findings by ICA/revascularization rates between referral practices are given as odds ratios (95% confidence intervals). Abbreviations ICA = invasive coronary angiography; coronary CTA = coronary computerized tomography angiography; FFRCT=coronary CTA derived fractional flow reserve
Of the 100 patients referred to ICA after FFRCT, 85 patients met the primary criterium of a 2 cm-FFRCT value ≤ 0.80, while 15 patients had the alternative criteria for referral to ICA. Revascularization rates were: primary criterium, 51 (60%), versus alternative criteria, 2 (13%), OR (95% CI) 9.75 (2.07, 45.97) p-value < 0.005. In 100 patients referred to ICA by FFRCT, revascularization was guided by visual assessment in 83 (83%) and by FFR in 17 (17%). There was no difference in revascularization rates between patients in whom treatment decisions were based on visual assessment, 49 (59%), compared to FFR, 13 (76%), p = 0.185.
Amongst the 15 patients referred to ICA based on the alternative criteria, the main drivers were a high delta FFRCT (n = 13), and/or a distal FFRCT <0.80 (n = 12), and/or a proximal stenosis (n = 11), and/or multivessel stenoses (n = 6).
In total, 184 patients were referred directly to ICA from CTA without using FFRCT as second line test. When this was due to high content of coronary calcification (n = 143, 78%), obstructive CAD by ICA was present in 69 patients (48%) and revascularization was performed in 54 (38%). When FFRCT was not performed due to image quality, obstructive CAD by ICA was present in 10 (24%) and revascularization was performed in 7 (17%).
Receiver operating characteristics curve analysis showed that the addition of FFRCT to baseline risk variables (diabetes, hypertension, dyslipidemia, and smoking), symptoms and degree of stenosis improved overall discrimination to the prediction of revascularization AUC 0.95[0.91–0.98] vs. AUC 0.72 [0.64–0.79] (P < 0.001), Fig. 4.
Fig. 4.
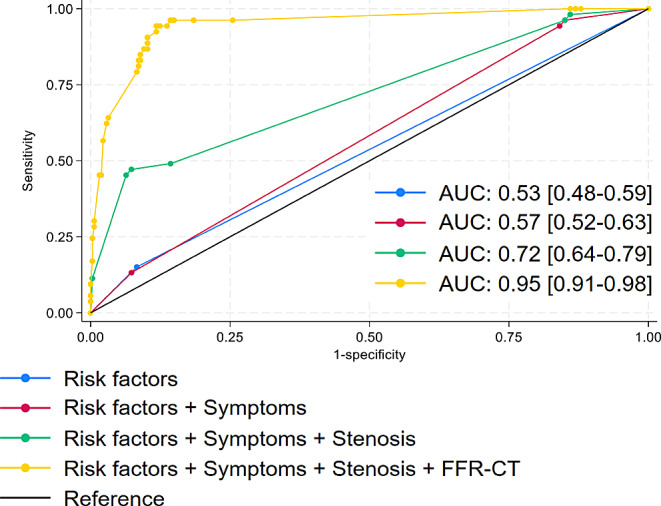
Graphs show performance evaluation of models created using combinations of baseline participants characteristics for discriminating the prediction of revascularization. Receiver operating characteristics performed best for discriminating when adding FFRCT to risk factors (diabetes, hypertension, dyslipidemia, and smoking), symptoms (dyspnoea and chest pain) and stenosis (+/-70%), AUC 0.95 [0.91–0.98], risk factors alone with AUC 0.53 [0.48–0.59], Risk factors and symptoms, AUC 0.72 [0.64–0.79] and risk factors, symptoms and stenosis AUC 0.72[0.64–0.79], p < 0.001
Amongst the 53 patients that were revascularized in the FFRCT group, FFRCT correctly detected the culprit vessel in 51 cases (93%), whereas another vessel was revascularized in 2 cases (7%).
Discussion
In this study of stable angina patients examined with coronary CTA at referral hospitals without PCI facilities, we found FFRCT to be a feasible second line test that reduced the need for invasive procedures and increased the revascularization rate as compared with coronary CTA alone.
The high proportion of patients that were deferred from downstream diagnostic testing following a normal FFRCT analysis in the present study is in line with previous studies, in which the safety of using an FFRCT based approach for guiding deferral from ICA was documented [12–14, 18, 23, 24]. In particular, our results are in line with the ADVANCED multicentre registry [18], in which 2/3 of all patients referred for FFRCT were deferred from invasive procedures and further tests. The observed higher revascularization rates associated with an FFRCT-guided approach as compared to decision-making based on visual assessment by experienced CT-cardiologists corresponds with previous head-to-head comparisons, showing a better diagnostic performance when adding FFRCT to conventional CTA for prediction of the hemodynamic significance of intermediate stenosis [3, 8, 9]. In our study, the benefit of FFRCT was observed despite the fact that patients referred to ICA without FFRCT had more risk factors, more central lesions, and a higher degree of stenosis by CTA than patients in the FFRCT group. The ROC analysis supports that FFRCT improved the ability to predict obstructive coronary disease in our study population.
Our post hoc analysis provides an opportunity to evaluate some aspects of the initial clinical experiences of implementing FFRCT in an all-comer population. We observed a higher revascularization rate if referral to ICA was based on the recommendations [20] for interpretation of FFRCT, applying lesion-specific criteria, as compared to referral driven by alternative criteria for FFRCT test abnormality. However, some studies have shown alternative criteria like the delta FFRCT value to be an important predictor of revascularization. [25]. We also report that revascularization in some cases was deferred by PCI operators despite a positive FFRCT. This was not only due to false positive FFRCT results as compared to invasive measurements, but also based on an overall clinical judgment including comorbidity, burden of symptoms, and localization and extent of calcification of stenosis. Possibly, such aspects are more likely to be observed in a real world setting than in protocolled trials.
In our study, 19% of patients were referred for myocardial perfusion imaging (MPI) mainly because the quality of the CT-scan was not suitable for FFRCT, a strategy that is recommended according to contemporary guidelines [4, 5]. The results of downstream testing in this group of patients were not registered in this study. However, we have previously reported that SPECT as a second-line test strategy following CTA had a sensitivity of 41% and a specificity of 86% as compared to invasive FFR [10], which is similar to the results obtained in the Dan-NICAD trial [26]. Thus, FFRCT has been associated with a better over all diagnostic performance and a higher sensitivity than perfusion imaging with SPECT and magnetic resonance [10, 11].
Overall, FFRCT testing seems well-suited for implementation in the diagnostic algorithm for patients with suspected SAP. First, patients do not need to physically attend additional examinations, as the FFRCT analysis is generated from available coronary CTA data sets, thus minimizing patient discomfort. Second, overall exposure to radiation and contrast is reduced. Third, the FFRCT test result is available within 24 h after CTA, whereas referring patients to other second-line tests after CTA would generally carry a greater delay of the diagnostic process. Fourth, FFRCT has demonstrated excellent diagnostic performance, also in patients with a high CAC [15, 27, 28] or aortic stenosis [29, 30], and a normal test result is associated with a good prognosis [12–15]. Fifth, recent studies [31, 32] have indicated that implementation of FFRCT is cost neutral or cost effective as compared to traditional testing strategies. One study indicated, that CTA/FFRCT may be the most cost-effective strategy in patients with stenoses > 50% [33]. In addition, FFRCT has proven useful in guiding individual antianginal therapy, whether the treatment is optimal medical treatment or PCI [34, 35].
The present study comprises real-world data obtained from two referral hospitals without PCI-facilities. We found FFRCT to be an effective tool that minimized the number of invasive procedures and increased the revascularization rate.
Limitations
It should be emphasized that this was a retrospective study of patients included at 2 hospitals. As the study is based on thousands of consecutive patients undergoing CTA, it might be claimed that the number of patients with an intermediate stenosis suitable for FFRCT analysis was rather low.
Slightly different patient approaches in terms of utilization of MPI-SPECT/FFRCT and referral practice to ICA following CTA and the fact that management and revascularization decisions were at the discretion of the treating physicians make the results subject to potential selection bias. In the real world setting of our study, we found that invasive FFR was performed in a relatively small proportion of patients and it may be speculated that a positive FFRCT result influenced operators not to perform FFR before revascularization. A few patients were referred directly from CTA to ICA as the CT-image quality was considered inadequate for FFRCT-analysis. The low revascularization rate (17%) amongst these patients indicates that repeat CTA to optimize image quality should probably have been performed instead of direct referral to ICA. At the time of this study, patients with CAC > 400 were sometimes referred directly to ICA and sometimes to further functional testing, which constitutes a limitation.
It should also be mentioned that only one CT-vendor was applied for performing CTA and only one company providing FFRCT was used.
Conclusion
Implementation of FFRCT at referral hospitals without PCI-facilities is feasible, implies minimal need for alternative downstream non-invasive testing, leads to a substantial reduction of invasive procedures and increases the revascularization rate in patients with recent onset stable angina pectoris and intermediate stenosis by CTA.
Author contributions
This manuscript is a collaborative effort with contributions from multiple authors. ST, NRS, KTM, and MB primarily authored the main content, while all other authors provided expertise in interpreting CT scans or invasive investigations. Additionally, all authors have critically reviewed and approved the manuscript.
Funding
The entire financial support for the study was delivered by participating departments.
Open access funding provided by Aarhus University Hospital
Data availability
The data that underlines this article is available in the manuscript.
Declarations
Disclosures
Lisette Okkels Jensen has received institutional research grants from St Jude Medical, Biosensors and Biotronik. All other authors had no disclosures to declare.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nissen L et al (2020) Implementation of coronary computed tomography angiography as nationally recommended first-line test in patients with suspected chronic coronary syndrome: impact on the use of invasive coronary angiography and revascularization. Eur Heart J Cardiovasc Imaging 21(12):1353–1362 [DOI] [PubMed] [Google Scholar]
- 2.Investigators S-H et al (2018) Coronary CT angiography and 5-Year risk of myocardial infarction. N Engl J Med 379(10):924–933 [DOI] [PubMed] [Google Scholar]
- 3.Norgaard BL et al (2014) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of Coronary Blood Flow using CT angiography: next steps). J Am Coll Cardiol 63(12):1145–1155 [DOI] [PubMed] [Google Scholar]
- 4.Knuuti J et al (2020) 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 41(3):407–477 [DOI] [PubMed] [Google Scholar]
- 5.Gulati M et al (2021) AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 144(22):e368–e454 [DOI] [PubMed]
- 6.Norgaard BL et al (2017) Coronary CT Angiography Derived Fractional Flow Reserve: the game changer in Noninvasive Testing. Curr Cardiol Rep 19(11):112 [DOI] [PubMed] [Google Scholar]
- 7.Taylor CA, Fonte TA, Min JK (2013) Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 61(22):2233–2241 [DOI] [PubMed] [Google Scholar]
- 8.Koo BK et al (2011) Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (diagnosis of ischemia-causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 58(19):1989–1997 [DOI] [PubMed] [Google Scholar]
- 9.Driessen RS et al (2019) Comparison of Coronary computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia diagnosis. J Am Coll Cardiol 73(2):161–173 [DOI] [PubMed] [Google Scholar]
- 10.Sand NPR et al (2018) Prospective comparison of FFR Derived from Coronary CT Angiography with SPECT Perfusion Imaging in stable coronary artery disease: the ReASSESS study. JACC Cardiovasc Imaging 11(11):1640–1650 [DOI] [PubMed] [Google Scholar]
- 11.Ronnow Sand NP et al (2020) Prediction of coronary revascularization in stable angina: comparison of FFR(CT) with CMR stress perfusion imaging. JACC Cardiovasc Imaging 13(4):994–1004 [DOI] [PubMed] [Google Scholar]
- 12.Patel MR et al (2020) 1-Year impact on Medical Practice and Clinical outcomes of FFR(CT): the ADVANCE Registry. JACC Cardiovasc Imaging 13(1 Pt 1):97–105 [DOI] [PubMed] [Google Scholar]
- 13.Ihdayhid AR et al (2019) Prognostic Value and Risk Continuum of Noninvasive Fractional Flow Reserve Derived from Coronary CT Angiography. Radiology 292(2):343–351 [DOI] [PubMed] [Google Scholar]
- 14.Norgaard BL et al (2018) Coronary CT angiographic and Flow Reserve-guided management of patients with stable ischemic heart disease. J Am Coll Cardiol 72(18):2123–2134 [DOI] [PubMed] [Google Scholar]
- 15.Madsen KT et al (2023) Prognostic value of coronary CT angiography-derived fractional Flow Reserve on 3-year outcomes in patients with stable angina. Radiology 308(3):e230524 [DOI] [PubMed] [Google Scholar]
- 16.NICE Clinical Guidelines (2016) Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. National Institute for Health and Care Excellence (NICE), London [PubMed]
- 17.Nakano S et al (2022) JCS 2022 Guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J 86(5):882–915 [DOI] [PubMed] [Google Scholar]
- 18.Fairbairn TA et al (2018) Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE Registry. Eur Heart J 39(41):3701–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fares A et al (2019) Real World utilization of computed Tomography Derived Fractional Flow Reserve: single Center experience in the United States. Cardiovasc Revasc Med 20(12):1043–1047 [DOI] [PubMed] [Google Scholar]
- 20.Cury RC et al (2022) CAD-RADS 2.0–2022 coronary artery Disease-Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 16(6):536–557 [DOI] [PubMed] [Google Scholar]
- 21.Norgaard BL et al (2019) Coronary CT angiography-derived fractional Flow Reserve Testing in patients with stable coronary artery disease: recommendations on interpretation and reporting. Radiol Cardiothorac Imaging 1(5):e190050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann FJ et al (2019) [2018 ESC/EACTS guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-thoracic surgery (EACTS)]. G Ital Cardiol (Rome) 20(7–8 Suppl 1):1S–61S [DOI] [PubMed] [Google Scholar]
- 23.Becker LM et al (2023) Real world impact of added FFR-CT to coronary CT angiography on clinical decision-making and patient prognosis - IMPACT FFR study. Eur Radiol 33(8):5465–5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas PS et al (2023) Comparison of an initial risk-based testing strategy vs usual testing in stable symptomatic patients with suspected coronary artery disease: the PRECISE Randomized Clinical Trial. JAMA Cardiol 8(10):904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi H et al (2022) Trans-lesional fractional flow reserve gradient as derived from coronary CT improves patient management: ADVANCE registry. J Cardiovasc Comput Tomogr 16(1):19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nissen L et al (2018) Diagnosing coronary artery disease after a positive coronary computed tomography angiography: the Dan-NICAD open label, parallel, head to head, randomized controlled diagnostic accuracy trial of cardiovascular magnetic resonance and myocardial perfusion scintigraphy. Eur Heart J Cardiovasc Imaging 19(4):369–377 [DOI] [PubMed] [Google Scholar]
- 27.Mickley H et al (2022) Diagnostic and clinical value of FFR(CT) in stable chest Pain patients with extensive coronary calcification: the FACC study. JACC Cardiovasc Imaging 15(6):1046–1058 [DOI] [PubMed] [Google Scholar]
- 28.Norgaard BL et al (2021) Clinical outcomes following real-world computed tomography angiography-derived fractional flow reserve testing in chronic coronary syndrome patients with calcification. Eur Heart J Cardiovasc Imaging 22(10):1182–1189 [DOI] [PubMed] [Google Scholar]
- 29.Wienemann H et al (2022) Feasibility and comparison of resting full-cycle ratio and Computed Tomography Fractional Flow Reserve in patients with severe aortic valve stenosis. J Cardiovasc Dev Dis, 9(4) [DOI] [PMC free article] [PubMed]
- 30.Michail M et al (2021) Feasibility and Validity of Computed Tomography-Derived Fractional Flow Reserve in patients with severe aortic stenosis: the CAST-FFR study. Circ Cardiovasc Interv 14(1):e009586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douglas PS et al (2016) 1-Year outcomes of FFRCT-Guided care in patients with suspected coronary disease: the PLATFORM Study. J Am Coll Cardiol 68(5):435–445 [DOI] [PubMed] [Google Scholar]
- 32.Curzen N et al (2021) Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur Heart J 42(37):3844–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graby J et al (2021) Real-world clinical and cost analysis of CT coronary angiography and CT coronary angiography-derived fractional flow reserve (FFR(CT))-guided care in the National Health Service. Clin Radiol, 76(11): p. 862 e19-862 e28. [DOI] [PubMed]
- 34.Madsen KT et al (2024) Coronary computed tomography angiography derived fractional flow reserve and risk of recurrent angina: a 3-year follow-up study. J Cardiovasc Comput Tomogr [DOI] [PubMed]
- 35.Sonck J et al (2022) Clinical validation of a virtual planner for coronary interventions based on coronary CT angiography. JACC Cardiovasc Imaging 15(7):1242–1255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that underlines this article is available in the manuscript.