Abstract
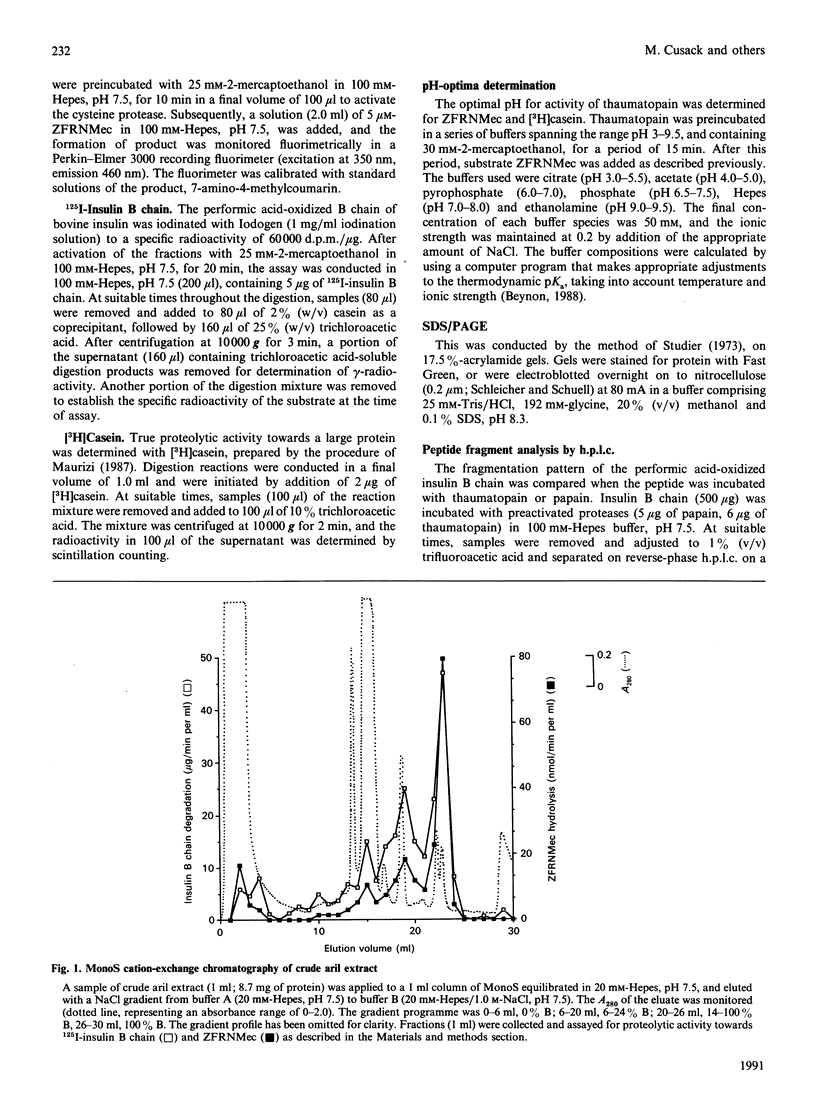
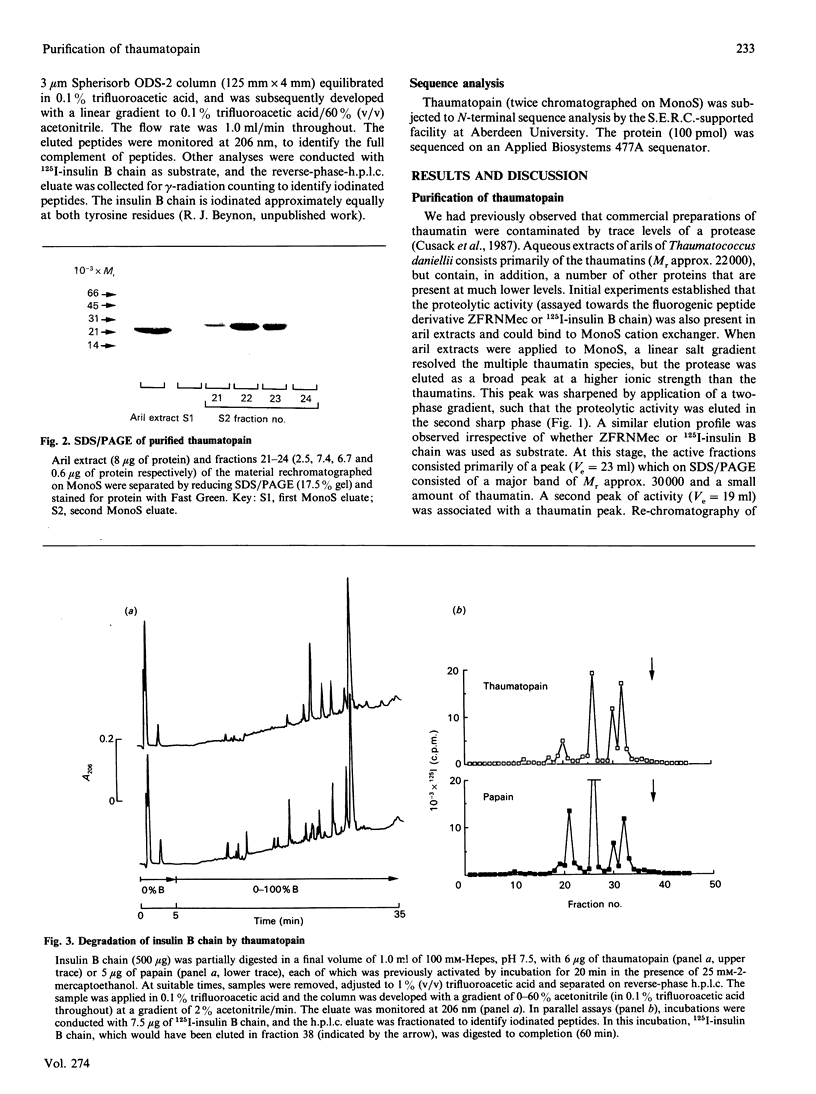
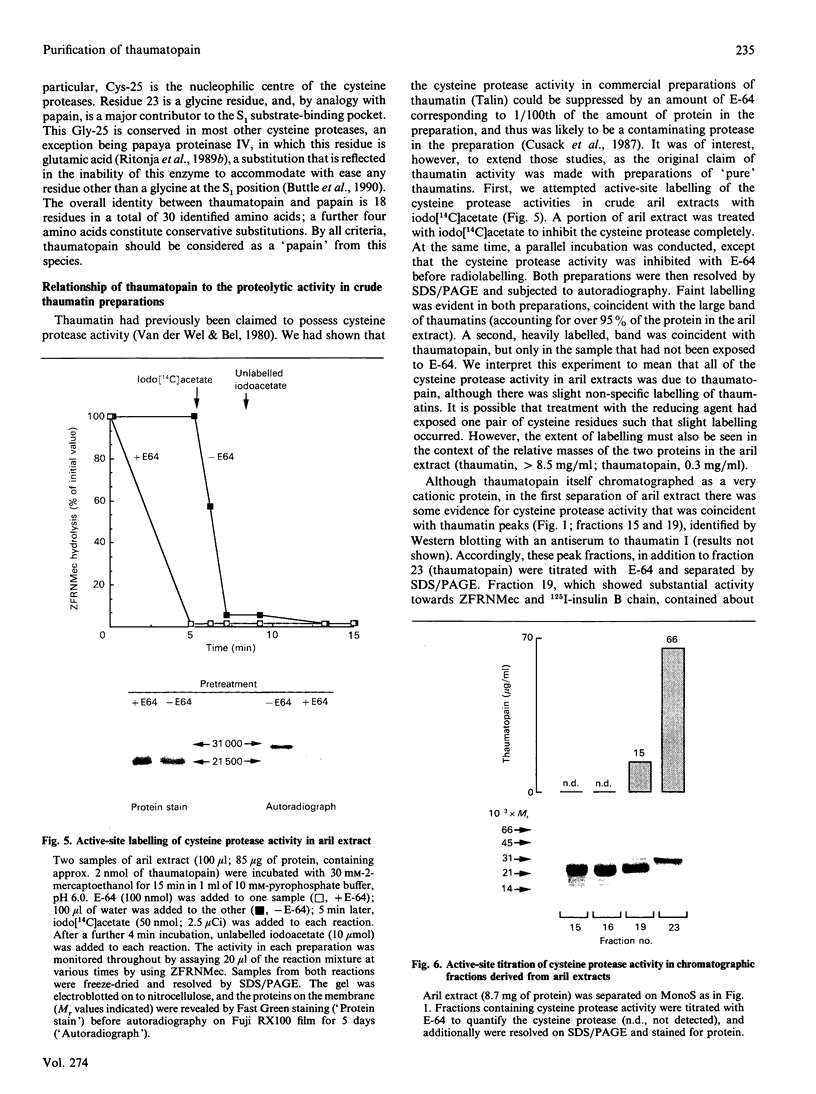
Aqueous extracts of the aril of the seed of Thaumatococcus daniellii contain, in addition to the intensely sweet protein thaumatin, a cysteine protease that we have termed thaumatopain. Thaumatopain has been purified by ion-exchange chromatography from arils, and is a monomeric protein of Mr 30,000. The protease strongly resembles papain in proteolytic activity, pH optima, susceptibility to inhibitors of cysteine proteases and in N-terminal sequence. The protease has also been identified in crude aril extracts by affinity labelling with iodo[14C]acetate. Thaumatopain is responsible for the cysteine protease activity previously attributed to thaumatin. Thaumatin is digested by thaumatopain at neutral to alkaline pH values.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasofu H., Yamauchi D., Mitsuhashi W., Minamikawa T. Nucleotide sequence of cDNA for sulfhydryl-endopeptidase (SH-EP) from cotyledons of germinating Vigna mungo seeds. Nucleic Acids Res. 1989 Aug 25;17(16):6733–6733. doi: 10.1093/nar/17.16.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin, after refinement at 1.7 A resolution. J Mol Biol. 1980 Aug 25;141(4):441–484. doi: 10.1016/0022-2836(80)90255-7. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon R. J. A Macintosh Hypercard stack for calculation of thermodynamically-corrected buffer recipes. Comput Appl Biosci. 1988 Nov;4(4):487–490. doi: 10.1093/bioinformatics/4.4.487. [DOI] [PubMed] [Google Scholar]
- Beynon R. J. A general purpose non-linear curve fitting program for the British Broadcasting Corporation Microcomputer. Comput Appl Biosci. 1985;1(2):111–115. doi: 10.1093/bioinformatics/1.2.111. [DOI] [PubMed] [Google Scholar]
- Beynon R., Cusack M. Thaumatin not proteolytic. Nature. 1990 Apr 5;344(6266):498–498. doi: 10.1038/344498a0. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Ritonja A., Pearl L. H., Turk V., Barrett A. J. Selective cleavage of glycyl bonds by papaya proteinase IV. FEBS Lett. 1990 Jan 29;260(2):195–197. doi: 10.1016/0014-5793(90)80101-n. [DOI] [PubMed] [Google Scholar]
- Carne A., Moore C. H. The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis. Biochem J. 1978 Jul 1;173(1):73–83. doi: 10.1042/bj1730073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. W., Coghlan V. M., Dihel L. C. Cloning and sequencing of papain-encoding cDNA. Gene. 1986;48(2-3):219–227. doi: 10.1016/0378-1119(86)90080-6. [DOI] [PubMed] [Google Scholar]
- Dubois T., Kleinschmidt T., Schnek A. G., Looze Y., Braunitzer G. The thiol proteinases from the latex of Carica papaya L. II. The primary structure of proteinase omega. Biol Chem Hoppe Seyler. 1988 Aug;369(8):741–754. doi: 10.1515/bchm3.1988.369.2.741. [DOI] [PubMed] [Google Scholar]
- Jacquet A., Kleinschmidt T., Schnek A. G., Looze Y., Braunitzer G. The thiol proteinases from the latex of Carica papaya L. III. The primary structure of chymopapain. Biol Chem Hoppe Seyler. 1989 May;370(5):425–434. doi: 10.1515/bchm3.1989.370.1.425. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Maurizi M. R. Degradation in vitro of bacteriophage lambda N protein by Lon protease from Escherichia coli. J Biol Chem. 1987 Feb 25;262(6):2696–2703. [PubMed] [Google Scholar]
- Ritonja A., Buttle D. J., Rawlings N. D., Turk V., Barrett A. J. Papaya proteinase IV amino acid sequence. FEBS Lett. 1989 Nov 20;258(1):109–112. doi: 10.1016/0014-5793(89)81627-8. [DOI] [PubMed] [Google Scholar]
- Ritonja A., Rowan A. D., Buttle D. J., Rawlings N. D., Turk V., Barrett A. J. Stem bromelain: amino acid sequence and implications for weak binding of cystatin. FEBS Lett. 1989 Apr 24;247(2):419–424. doi: 10.1016/0014-5793(89)81383-3. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Dean D., Heck G. R. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6512–6516. doi: 10.1073/pnas.82.19.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. D., Buttle D. J., Barrett A. J. Ananain: a novel cysteine proteinase found in pineapple stem. Arch Biochem Biophys. 1988 Nov 15;267(1):262–270. doi: 10.1016/0003-9861(88)90031-8. [DOI] [PubMed] [Google Scholar]
- Schaffer M. A., Fischer R. L. Analysis of mRNAs that Accumulate in Response to Low Temperature Identifies a Thiol Protease Gene in Tomato. Plant Physiol. 1988 Jun;87(2):431–436. doi: 10.1104/pp.87.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skern T., Zorn M., Blaas D., Kuechler E., Sommergruber W. Protease or protease inhibitor? Nature. 1990 Mar 1;344(6261):26–26. doi: 10.1038/344026a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Van Der Wel H., Bel W. J. Enzymatic properties of the sweet-tasting proteins thaumatin and monellin after partial reduction. Eur J Biochem. 1980 Mar;104(2):413–418. doi: 10.1111/j.1432-1033.1980.tb04442.x. [DOI] [PubMed] [Google Scholar]
- van der Wel H., Loeve K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur J Biochem. 1972 Dec 4;31(2):221–225. doi: 10.1111/j.1432-1033.1972.tb02522.x. [DOI] [PubMed] [Google Scholar]