Abstract
The neurotoxicant trimethyltin (TMT) triggers cognitive impairment and hippocampal neurodegeneration. TMT is a useful research tool for the study of Alzheimer's disease (AD) pathogenesis and treatment. Although the antidiabetic agent metformin has shown promising neuroprotective effects, however, its precise modes of action in neurodegenerative disorders need to be further elucidated. In this study, we investigated whether metformin can mitigate TMT cognition impairment and hippocampal neurodegeneration. To induce an AD-like phenotype, TMT was injected i.p. (8 mg/kg) and metformin was administered daily p.o. for 3 weeks at 200 mg/kg. Our results showed that metformin administration to the TMT group mitigated learning and memory impairment in Barnes maze, novel object recognition (NOR) task, and Y maze, attenuated hippocampal oxidative, inflammatory, and cell death/pyroptotic factors, and also reversed neurodegeneration-related proteins such as presenilin 1 and p-Tau. Hippocampal level of AMP-activated protein kinase (AMPK) as a key regulator of energy homeostasis was also improved following metformin treatment. Additionally, metformin reduced hippocampal acetylcholinesterase (AChE) activity, glial fibrillary acidic protein (GFAP)-positive reactivity, and prevented the loss of CA1 pyramidal neurons. This study showed that metformin mitigated TMT-induced neurodegeneration and this may pave the way to develop new therapeutics to combat against cognitive deficits under neurotoxic conditions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10571-024-01502-4.
Keywords: Alzheimer’s, Metformin, Trimethyltin, AMP-activated protein kinase, Neurodegeneration, Neuroprotection
Introduction
Alzheimer’s disease (AD) is presently the leading chronic neurodegenerative disorder which affects beyond six million individuals in the US and nearly 50 million people globally (Alzheimer Association 2020). AD is gradually and irrevocably associated with cognition deterioration and eventual dementia and disability in performing regular activities (Kawas and Corrada 2006). AD is associated with significant loss of neurons in the hippocampal and cortical areas, appearance of hyperphosphorylated tau-composed neurofibrillary tangles (NFT), and amyloid beta (Aβ) deposition as senile plaques, and higher level of presenilin 1 (Anderson et al. 2024; Gholami 2023). AMP-activated protein kinase (AMPK) is a pivotal regulator of energy homeostasis with potential implication in the AD pathogenesis (Cai et al. 2012). Treatment options for AD are quite few and may be ineffective. Current drugs for this disease cannot reverse or prevent its neurodegenerative nature (Chen et al. 2021a). Trimethyltin (TMT) as an organotin and a specific neurotoxin of the hippocampus is used to induce a consistent model of neurodegeneration and cognitive impairment and is therefore a valuable tool to study AD pathogenesis (Jung et al. 2013; Rostami et al. 2022). In addition, cholinergic dysfunction which is observed in AD patients also occurs in TMT-intoxicated rats (Kang et al. 2016).
Metformin as a biguanide is one of the most commonly prescribed hypoglycemic agents (Hundal et al. 2000; Patel et al. 2023). Clinical studies have shown that metformin administration is associated with lower risk of AD and ensuing dementia in diabetic patients in addition to its improvement of cognitive performance (Campbell et al. 2018). Antidiabetic metformin can exert a therapeutic effect in intracerebroventricular streptozotocin sporadic model of AD, as shown by lower levels of memory loss, Aβ deposition, and tau phosphorylation in addition to its attenuation of brain insulin resistance (Abosharaf et al. 2024), it is capable to prevent N-nitrosodiethylamine-induced cognitive decline via attenuation of hippocampal amyloid beta deposition and inflammatory factors like tumor necrosis factor (TNF) and interleukin-6 (IL-6) (Ponce-Lopez et al. 2023), and can also mitigate amyloid β-induced cognitive deficit through lowering oxidative and nitrosative stress as well as neuroinflammation (Khaleghi-Mehr et al. 2023; Liao et al. 2021). Furthermore, metformin can ameliorate paclitaxel neurotoxicity in neural crest cells (Khodabakhsh et al. 2024) and can alleviate sevoflurane-induced cognitive decline and neurogenesis deficit via nuclear factor erythroid 2-related factor 2 (Nrf2)/ Glucose-6-phosphate dehydrogenase (G6PD) pathway (Fan et al. 2023). Moreover, metformin due to its anti-inflammatory, antiapoptotic, and antioxidant potential can attenuate ethanol neurotoxicity (Sabzali et al. 2022) in addition to its protection against methamphetamine-induced cognition deficit, anxiety, depression, and neurodegeneration through cAMP response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF)/protein kinase B (Akt)/glycogen synthase kinase 3 (GSK3) signaling (Keshavarzi et al. 2019). Accordingly, this study was designed to evaluate the potential effect of metformin in reversing cognitive deficits subsequent to TMT neurotoxin and to unravel some involved modes of action.
Materials and Methods
Animals
Thirty-two male Wistar rats at 11–12 weeks of age and weighing 195–230 g were purchased from Laboratory Animal House of Tehran University (Tehran) and were housed (4 rats/cage) at controlled room conditions for temperature (21–23 °C) and humidity (45–50%) and with free access to rat food and tap water.
Experimental Protocol
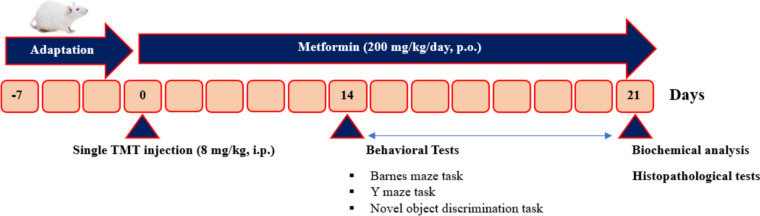
Rats were divided into 4 groups using random number protocol (n = 8/group), i.e., Control, Control+Metformin200, TMT, and TMT+Metformin200. In addition, a blinding strategy was uniformly applied for the experimenter. Number of used animals was obtained from our relevant previous study (Rostami et al. 2022). TMT groups received a single intraperitoneal injection of TMT-chloride (Cat # sc-301942, Santa Cruz Biotechnology, Inc., USA; dissolved in normal saline) at 8 mg/kg (Rostami et al. 2022). Metformin hydrochloride (Cat # PHR1084, Merck, Germany) was solubilized in Cremophor as the vehicle and administered daily via the gavage one h after TMT for 3 weeks. Dose of metformin was chosen from our earlier study on its neuroprotective effect in amyloid β phenotype of AD (Khaleghi-Mehr et al. 2023). Study experimental protocol is outline in Fig. 1.
Fig. 1.
Design of the study. Trimethyltin (TMT) was intraperitoneally given to generate model of cognitive dysfunction and neuronal degeneration. Metformin was orally given at 200 mg/kg for 3 weeks
Y Maze
The Y maze was made of black Plexiglas and composed of three arms and a central area. Each rat was tested for 8 min. The Y maze allows the animals to freely explore the three arms of the maze, with a natural tendency to explore an arm that was not visited immediately before. A high percentage of alternation between the three arms is indicative of good spatial memory. The maze was wiped with diluted ethanol between sessions and the percentage of alternation was calculated as described in a past study (Rostami et al. 2022).
Novel Object Discrimination
This test was done in 1 day in two 5-min sessions, with an interval of 4 h. In the first session, the rats were exposed to two identical objects. Four hours later, one of the objects was randomly replaced with another one. In both stages, exploration of each object was recorded. Discrimination index was defined as the time spent exploring the novel object relative to the total time, as reported before (Rostami et al. 2022).
Barnes Maze
This maze is a spatial memory task. Maze had a flat circular field with a distance of 100 cm above the floor and a diameter of 120 cm, and had 20 holes at a diameter of 10 cm in its periphery. In addition, a white light bulb was placed in the center to create an aversive stimulus. This experiment had two sessions: training and probe. Each rat had 2-min exploration in each session. Escape response from the lit arena and learning the position of escape box was trained. In the probe test, latency to find the escape box and the number of errors (number of explorations of holes different from the escape box) were recorded, with its protocol reported before (Rostami et al. 2022).
Tissue Homogenate Preparation
Rats were anesthetized and euthanized with ketamine (100 mg/kg) (Cat # 36408/3000, Alfasan, Netherlands) and xylazine (15 mg/kg) (Cat # 36408/3007, Alfasan, Netherlands). The brain was separated for homogenate preparation (right side) and histochemical assessment (left side in 10% formalin solution). Hippocampal tissue was homogenized in cold 150 mM Tris buffer (pH 7.4). The obtained homogenate was centrifuged at 7826 × g for 15 min (4 °C) to obtain supernatant. All biochemical tests and histological analyses were conducted in duplicate and their average was taken for each sample. For biochemical tests, inter-assay CV was less than 12% and intra-assay CV was less than 10%. For standard curves and formulae, if any, we used Microsoft Excel 2016.
Total protein concentration was determined using the bicinchoninic acid kit, Kiazist, Iran (Cat # KBCA96), in which proteins reduce bivalent Cu into monovalent Cu at 55 °C for 30 min and absorbance was taken at 560 nm and with albumin as the standard.
Quantification of Presenilin 1, p-Tau, Nrf2, TNF, Il-10, and AMPK
ELISA method was applied for the measurement of TNF (Cat # sc-52746; RRID # AB_630341), presenilin 1 (Cat # sc-365495; RRID # AB_10844473), p-Tau (Cat # sc-32275; RRID # AB_628325), interleukin-10 (IL-10) (Cat # sc-365858; RRID # AB_10859554), and AMP-activated protein kinase (AMPK) (Cat # sc-398861) and detection horseradish peroxidase (HRP)-conjugated (Cat # sc-516102; RRID # AB_2687626) antibodies, all from Santa Cruz Biotechnology, Inc. (USA) and results were brought in pg/mg of protein. For each parameter, ODs were determined at 450 nm and these were converted into actual concentration using relevant standard curves. Specificity of the used antibodies in this study had been determined by our supplier, i.e., Santa Cruz Biotechnology, Inc. (USA).
Measurement of Oxidative Stress and Cell Death-Related Factors
Malondialdehyde (MDA) level was measured using a commercial kit from KiaZist, Iran (Cat # KMDA96) using thiobarbituric acid in a diluted solution of glacial acetic acid and heating at 95 °C for 20 min and with tetraethoxypropane as the standard. Absorption was read at 535 nm and data were converted into nmol/mg of protein.
For measuring superoxide dismutase (SOD) activity, a commercial kit (Cat # 706002, Cayman Chemical, USA) was used utilizing a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. In this test, sample and radical detector solution were reacted with xanthine oxidase and with standard as erythrocyte SOD. After 30 min, absorbance was taken at 445 nm and SOD activity was calculated from an equation obtained from the linear regression of the standard curve. One unit of SOD activity was defined as the amount of enzyme needed for exhibiting 50% dismutation of superoxide radicals. Final data were reported as SOD activity/mg of protein.
For measuring catalase activity, a commercial kit (Cat # KCAT96, KiaZist, Iran) was used using methanol, hydrogen peroxide, potassium hydroxide solution, purpald, and potassium periodate and absorbance was read at 550 nm. Final data were presented as catalase activity/mg of protein and with formaldehyde as the standard.
To determine caspase 1 as a pyroptosis index, a commercial assay kit from Abcam, USA (Cat # ab273268) was used with YVAD-p-NA as the substrate and final production of chromophore p-nitroanilide and detection at 405 nm and data were presented as ODs of samples.
For determining caspase 3 as an indicator of cell death, DEVD-p-NA (Abcam, USA; Cat # ab39401) was used as the substrate and final production of chromophore p-nitroaniline and absorbance was detected at 405 nm and data were presented as ODs of samples.
Acetylcholinesterase (AChE) Activity Determination
For this test, AChE kit from Abcam, USA (Cat # ab138871) was used with its reagent containing DTNB and acetylthiocholine and after 30 min, absorbance was taken at 408 nm. To exclude butyrylcholinesterase, we also used in parallel donepezil as a specific inhibitor of AChE. Final data were presented in nmol/min/mg of protein.
Histological Studies
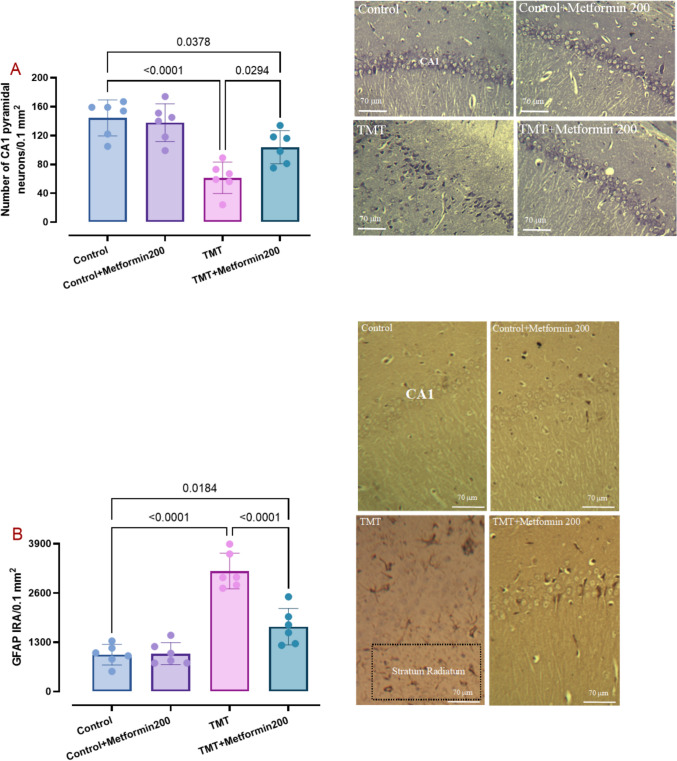
Hippocampal blocks were processed and embedded in paraffin and sectioned on a rotary microtome (DidSabz, Urmia, Iran) and 5-μm coronal sections were taken. Alternate sections were stained with Cresyl violet acetate (Cat # C5042, SigmaAldrich, USA). CA1 pyramidal neurons irrespective of their size were counted at planes 3.3–3.8 mm behind the bregma and at 1.2–2.4 mm from the midline in an area of 0.1 mm2 in accordance to the rat stereotaxic atlas. For counting, at least five sections and 50 μm apart for each sample were assessed twice and their average was obtained. Cells with visible boundary and nucleolus were counted. ImageJ 1.49 (NIH) was used for this analysis.
For glial fibrillary acidic protein (GFAP) immunohistochemistry (IHC), sections were exposed to primary monoclonal antibody against GFAP (Cat # sc-166481, RRID # AB_2294569; 1:65), then with secondary horseradish peroxidase (HRP)-conjugated antibody (Cat # sc-51s6102, RRID # AB_2687626; 1:80), both from Santa Cruz Biotechnology, USA, and final reaction with 3,3′ -diaminobenzidine (Cat # sc-209686, Santa Cruz Biotechnology, USA) and hydrogen peroxide. Sections were counterstained with Hematoxylin (15 s) and IRA for GFAP-reacted astrocytes was determined at planes 3.3–3.8 mm behind the bregma and at 1.2–2.4 mm from the midline in an area of 0.1 mm2 in accordance to the rat stereotaxic atlas. For assessment of GFAP immunoreactivity (IRA), at least four sections from each animal and 50 μm apart, were evaluated twice and their average was obtained. GFAP IRA was assessed in the stratum radiatum area which is rich in GFAP-immunoreactive astrocytes. The GFAP-positive area, marked in brown, was calculated with the exclusion of background staining from the measurement. All histologic assessments were done employing OBN 141 microscope (KERN & SOHN GmbH, Germany) and using ImageJ 1.49, NIH.
Statistical Tests
Statistical tests were done in GraphPad Prism 9.5 and data were presented as means ± SD. After ascertaining normal distribution of data for each factor in Shapiro–Wilk test and testing for outliers by Grubbs’ method, the statistical tests two-way ANOVA and Tukey post-test were used with p less than 0.05 as significant. To assess variance homogeneity, we used Bartlett test. Results for data normality using Shapiro–Wilk test and variance homogeneity using Bartlett test have been brought in the Supplement 1. For evaluation of relationship between the designated factors, Pearson linear correlation analysis was used.
Results
This research work was conducted to show the effect of metformin in reversing cognitive deficits following TMT neurotoxic effect with employment of different behavioral tests besides a multitude of biochemical and histochemical analyses.
Metformin Mitigated TMT Behavioral Deficits
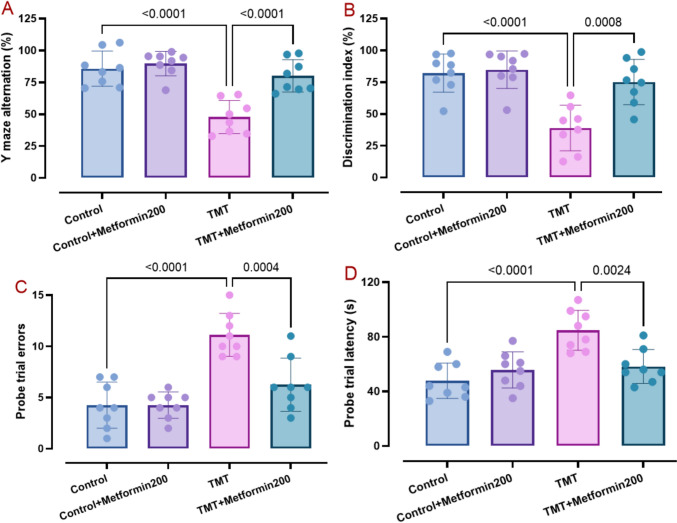
To analyze short-term spatial and working memory, the Y-maze test was used (Kraeuter et al. 2019). TMT reduced the percentage of Y maze alterations when administered alone (p < 0.001), but this reduction was significantly less when administered together with metformin. Metformin alone had no effect (Fig. 2A, two-way ANOVA, interaction TMT x metformin: F1, 28 = 10.52, p = 0.003).
Fig. 2.
Data of behavioral tests comprising Y maze (pane A), novel object discrimination (pane B), and Barnes maze (panes C and D). Metformin mitigated TMT behavioral deficits in these tasks, as analyzed by two-way ANOVA and Tukey tests. (means ± SD, n = 8/group)
Novel object discrimination test was used to assess the animals’ capacity to memorize previously-visited objects and to explore novel objects (Hawiset et al. 2023), as shown by the discrimination index (Fig. 2B). TMT group demonstrated a significantly lower discrimination score (p < 0.001) as compared with the control data and this deficit was less in metformin-treated TMT group. Metformin alone did not have a significant effect (Fig. 2B, two-way ANOVA, interaction TMT x metformin: F1, 28 = 8.33, p = 0.007).
Figure 2C and D present the impact of treatments on the Barnes maze performance to evaluate in more detail spatial learning and memory (Tancheva et al. 2023). Our data analysis showed that TMT group had significantly higher errors (p < 0.001) and greater latency (p < 0.001) and metformin treatment of the TMT group significantly reduced errors (p < 0.001) (Fig. 2C, two-way ANOVA, interaction TMT x metformin F1, 28 = 10.62, p = 0.002) and latency (p = 0.002) (Fig. 2D, two-way ANOVA, interaction TMT x metformin: F1, 28 = 13.35, p = 0.001). In addition, metformin alone did not have a significant effect on these factors.
Metformin Attenuated Cell Death, Inflammation, Pyroptosis, and Oxidative Stress, and Markers of AD Pathology
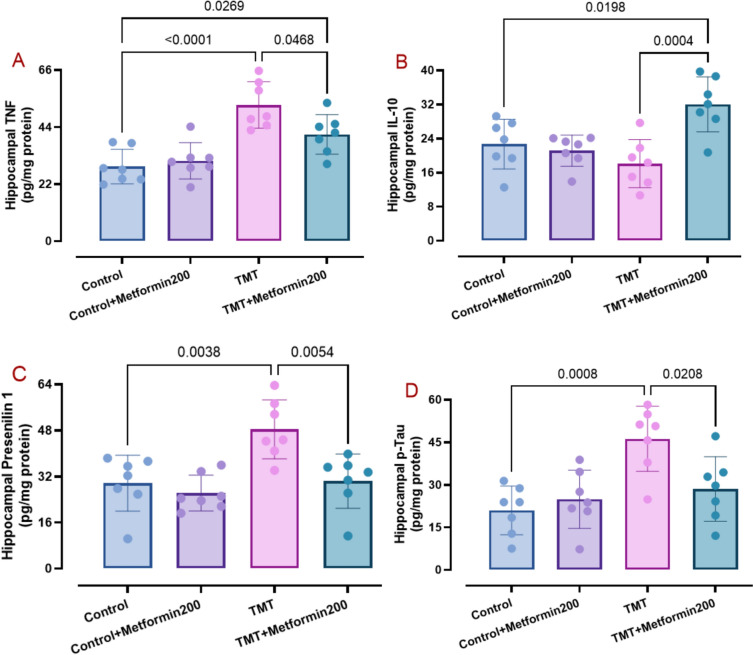
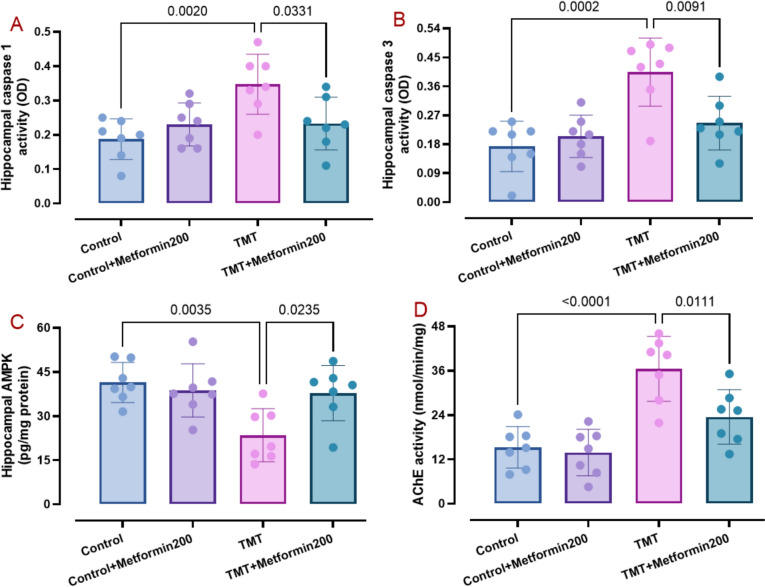
AD phenotype is typified with elevated brain levels of oxidative, apoptotic, and inflammatory burden (Zhang et al. 2023) as well as enhancement of presenilin 1 (Martinez-Feduchi et al. 2024) and p-Tau (Pradeepkiran et al. 2024). Accordingly, we measured hippocampal levels of relevant factors. Data analysis showed elevated levels of MDA (Fig. 3A, two-way ANOVA, p < 0.001; interaction TMT x metformin: F1, 24 = 5.90, p = 0.02), TNF (Fig. 4A, two-way ANOVA, p < 0.001, interaction TMT × metformin: F1, 24 = 5.54, p = 0.02), presenilin 1 (Fig. 4C, two-way ANOVA, p = 0.003, interaction TMT × metformin: F1, 24 = 4.55, p = 0.04), p-Tau (Fig. 4D, two-way ANOVA, p < 0.001, interaction TMT x metformin: F1, 24 = 7.45, p = 0.01), caspase 1 (Fig. 5A, two-way ANOVA, p = 0.002, interaction TMT × metformin: F1, 24 = 8.21, p = 0.008), and caspase 3 (Fig. 5B, two-way ANOVA, p < 0.001, interaction TMT × metformin: F1, 24 = 8.83, p = 0.006) and lower levels of catalase activity (Fig. 3B, two-way ANOVA, p < 0.001, interaction TMT × metformin: F1, 24 = 7.10, p = 0.01) and SOD activity (Fig. 3C, two-way ANOVA, p < 0.001, interaction TMT × metformin: F1, 24 = 5.36, p = 0.02) in the TMT group. On the other hand, metformin significantly reversed MDA (p = 0.007), SOD (p = 0.007), catalase (p = 0.006), TNF (p = 0.04), presenilin 1 (p = 0.005), p-Tau (p = 0.02), caspase 1 (p = 0.03), and caspase 3 (p = 0.01) besides significant elevation of IL-10 (Fig. 4B, two-way ANOVA, p < 0.001, interaction TMT x metformin: F1, 24 = 13.87, p = 0.001). In addition, metformin alone did not have a significant effect on these parameters.
Fig. 3.
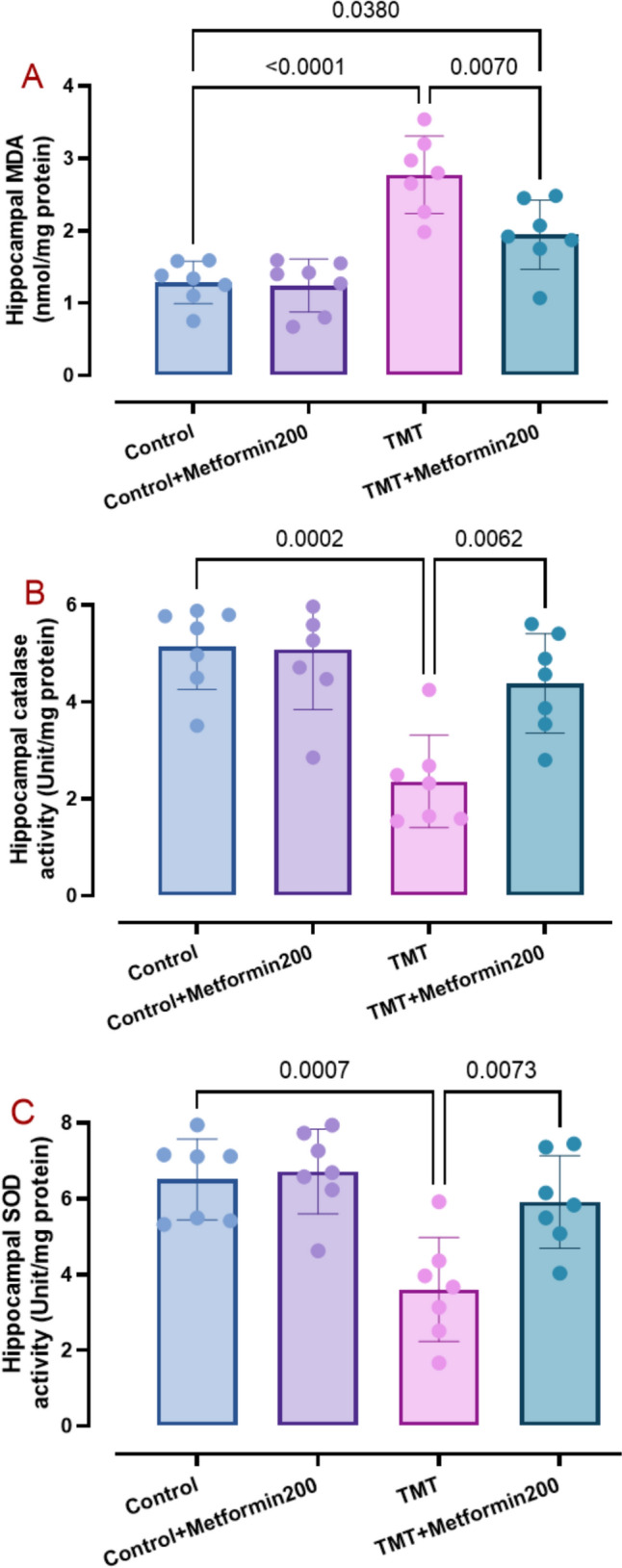
Data of oxidative stress factors comprising MDA (pane A), catalase activity (pane B), and SOD activity (pane C). Metformin properly attenuated TMT oxidative burden and improved antioxidants, as analyzed by two-way ANOVA and Tukey tests. (means ± SD, n = 7/group)
Fig. 4.
Data of inflammation-associated factors including TNF (pane A) and IL-10 (pane B) in addition to AD pathology markers consisting of presenilin 1 (pane C) and p-Tau (pane D), as analyzed by two-way ANOVA and Tukey tests. Metformin reversed TMT-induced changes regarding these factors. (means ± SD, n = 7/group)
Fig. 5.
Data for hippocampal level of caspase 1 (pane A), caspase 3 (pane B), AMPK (pane C), and AChE activity (pane D), as analyzed by two-way ANOVA and Tukey tests. Metformin was capable to reverse TMT-induced changes regarding these factors. (means ± SD, n = 7/group)
Metformin Improved the Energy Homeostasis Marker AMPK
The energy homeostasis marker AMPK in association with mitochondrial biogenesis play important roles in AD-related pathogenesis (Cai et al. 2012). Thus, we investigated how metformin affects its hippocampal levels following TMT injury. Statistical analysis of data for AMPK (Fig. 5C, two-way ANOVA, interaction TMT x metformin: F1, 24 = 6.82, p = 0.01) showed that hippocampal level of AMPK is lower in the TMT group (p = 0.003) when compared to the control data and metformin at 200 mg/kg caused its significant improvement (p = 0.02) in relation to the TMT group. Additionally, metformin alone did not have a significant impact on this factor.
Metformin Decreased Hippocampal Activity of AChE
AD-related pathology is associated with cholinergic dysfunction (Tripathi et al. 2024). Thus, the impact of TMT injection and oral metformin on the hippocampal activity of AChE was assessed in different groups. Our data analysis (Fig. 5D, two-way ANOVA, interaction TMT x metformin: F1, 24 = 4.67, p = 0.04) showed that TMT group had pronounced enhancement of AChE (p < 0.001, an increase by 136.2%) in relation to the control data. Conversely, metformin at 200 mg/kg was capable to reduce AChE activity in the TMT group (p = 0.01). However, metformin alone did not cause a significant change of AChE activity.
Metformin Prevented Hippocampal CA1 Astrogliosis and Neurodegeneration
Robust neurodegenerative changes (Tripathi et al. 2024) and reactive astrogliosis (Fontana et al. 2023) are notably observed in brain of AD patients. Upon Nissl staining (Fig. 6A), we noted karyorrhexis and pyknosis, necrotic changes, and even cell membrane disintegrity in the TMT-injected rats in the hippocampal CA1 subfield and these improper changes were less frequent in the TMT group treated with metformin at 200 mg/kg. Our data analysis (two-way ANOVA, interaction TMT x metformin: F1, 20 = 6.20, p = 0.02) showed that TMT injection notably reduced cell density (p < 0.001) and metformin at 200 mg/kg significantly attenuated this decline (p = 0.02). Additionally, metformin treatment alone did not significantly change number of Nissl-stained neurons.
Fig. 6.
Density of CA1 Nissl-stained neurons (pane A) and immunoreactivity for GFAP as a marker of astrocytes (pane B) and related photomicrograph. Metformin at 200 mg/kg was able to attenuate CA1 neurodegeneration and astrogliosis. Dotted-border rectangle shows stratum radiatum area for assessment of GFAP immunoreactivity. Two-way ANOVA and Tukey tests. (means ± SD, n = 6/group)
Measurement of GFAP immunoreactivity (IRA) (Fig. 6B) around the CA1 subfield (stratum radiatum) as an index of astrocytic response to toxic insult (two-way ANOVA, interaction TMT x metformin: F1, 20 = 21.88, p < 0.001) showed higher IRA in the TMT-injected rats (p < 0.001) (an increase of 227.3%) and GFAP IRA was less noted in the TMT group treated with metformin at 200 mg/kg (p < 0.001). Moreover, metformin treatment alone did not produce significant change of GFAP IRA.
Correlation Analysis for Oxidative and Neurodegenerative Changes
To explore the relationship between oxidative and neurodegenerative changes for TMT and metformin-treated groups, we conducted Pearson correlation analysis for MDA as a specific oxidative factor and the number of CA1 pyramidal neurons as a relevant factor for neurodegeneration. Such analysis showed a significantly negative relationship for the TMT group (r = − 0.92, p = 0.007) and the metformin-treated TMT group (r = − 0.90, p = 0.01).
Discussion
In the current work, protective impact of metformin against TMT-instigated neurotoxicity and neurodegeneration was investigated. We observed beneficial effects of antidiabetic metformin in the mitigation of hippocampal CA1 neurodegeneration alongside its reduction of deficits in various behavioral tests. Spatial memory deficits correlate well with hippocampal neuronal injury (Geloso et al. 2011; Thong-asa et al. 2020). TMT neurotoxin causes profound damage to the hippocampal CA1 subfield (Thong-asa et al. 2020), as observed in our study. This high vulnerability of CA1 area to TMT is related to its lower level of calcium-binding protein calretinin as a key protein to combat TMT-provoked calcium overload (Geloso et al. 1998; Thong-asa et al. 2020).
Our histochemical findings indicated that most of CA1 pyramidal neurons in the TMT group are degenerated and exhibiting robust pyknosis and shrinkage as signs of cell death besides their disorganized appearance, as reported before (Thong-Asa et al. 2020). Hippocampal CA1 subfield plays pivotal role in spatial cognition (O'Keefe 1993). Significant injury of CA1 area coexisted with deficits of spatial learning and memory in the TMT-intoxicated group in this study. In contrast, oral metformin at 200 mg/kg was associated with better cognition as well as preservation of CA1 neuronal organization, indicating its neuroprotective effect. In support of these data, metformin can prevent cognitive dysfunction and histopathological changes in a model of sporadic AD (Rabieipoor et al. 2023) and can ameliorate sevoflurane-instigated neurogenesis damage and cognitive decline linked to its enhancement of Nrf2 and glucose-6-phosphate dehydrogenase expression and its attenuation of cell death (Fan et al. 2023), which was also shown in this study for cell death and pyroptotic factors including caspases 1 and 3.
TMT neuronal injury is attributed to various pathologic mechanisms including oxidativestress, loss of mitochondrial integrity, apoptotic events, calcium overload, and neuroinflammation (Geloso et al. 2011; Go et al. 2023). In this study, higher hippocampal levels of oxidative factors such as MDA, the pro-inflammatory factor TNF, cell death factors consisting of caspases 1 and 3 and concomitant lower activity of catalase and SOD were shown in the rats from the TMT group. Conversely, metformin treatment at 200 mg/kg was successful to significantly reverse these abnormal alterations and accordingly protects hippocampus against TMT toxicity. In support of these data, metformin can exert neuroprotective effect via activating AMPK pathway in 3-acetylpyridine model of cerebellar ataxia which was associated with lower levels of pro-inflammatory cytokines (Atella et al. 2024), it is capable to protect hippocampal slices against methylglyoxal glutamatergic toxicity and to lower neuroinflammation (Vizuete et al. 2023), and can also exert neuroprotective effect and lower oxidative stress linked to GSK3β cascade and enhancement of antioxidants following glutamate neurotoxicity (Oruc et al. 2024).
There is convincing evidence that NFT tau phosphorylation and Aβ plaques as typical pathological markers of AD increase in the brain of AD patients (Xia et al. 2021; Yu and Wu 2021) and also in TMT-induced AD phenotype (Park et al. 2019). Higher level of hyperphosphorylated tau is associated with cognition impairment through prompting neuronal damage and apoptosis and with final appearance of neurofibrillary tangles (NFTs) (Park et al. 2019). Higher hippocampal levels of presenilin 1 and p-tau correlated with TMT-induced neurotoxicity, neuroinflammation, and culminant apoptosis and cell death (Brown et al. 2019; Nurmasitoh et al. 2023), as was similarly shown in the present work. Conversely, oral metformin was able to prevent such alterations regarding p-tau and presenilin 1. Validating this finding, antidiabetic metformin could attenuate hippocampal and cortical tau pathology and amyloid-β level through enhancement of microglial autophagy, increasing number of microglial cells around Aβ plaques, and promotion of phagocytosis of tau in APP/PS1 model of AD (Chen et al. 2021b). In our study, metformin administration at a dose of 200 mg/kg to the TMT-injured rats was associated with significantly lower hippocampal level of presenilin 1. However, Picone et al. (2015) have shown elevated level of presenilin 1 in the brain of normal mouse following metformin administration for 7 days via drinking water (2 mg/ml) which was comparable to a dose of 300 mg/kg/day of metformin (Picone et al. 2015). Interestingly, we did not find significant change of presenilin 1 in the control group treated with metformin at a dose of 200 mg/kg. A substantial volume of recent researches supports the beneficial impact of metformin on reducing the risk of AD through reducing its pathogenic factors (Ale Mahmoud Mehraban et al. 2024; Khaleghi-Mehr et al. 2023; Ou et al. 2018; Pilipenko et al. 2020). These reports clearly show that further research works are still required to evaluate the exact effect of the antidiabetic drug metformin on the AD-related factors.
Cholinergic dysfunction is observed in parallel to the cognitive impairment following TMT administration (Rostami et al. 2022; Tu et al. 2017). In this respect, a similar condition may also occur in animal models of AD with enhanced activity and/or production of AChE (Elseweidy et al. 2023; Gu et al. 2023; Khaleghi-Mehr et al. 2023). For this reason, AChE inhibitors have beneficial effects in clinical attenuation of AD symptoms (Moss and Perez 2021). AChE activity increases in some regions of the brain in association with amyloid beta plaques and even in blood samples of AD patients (Carvajal and Inestrosa 2011). It has been reported that increased AChE is associated with acceleration of Aβ peptides deposition in AD and it could increase Aβ neurotoxicity (Carvajal and Inestrosa 2011). Degeneration of cholinergic system and cognitive deficits are enhanced in neurodegenerative disorders like AD (Chen et al. 2022). The brains from AD patients exhibit marked neurodegeneration in parallel to reduction of cholinergic neurons and a severe deficiency of the neurotransmitter ACh (Bowen et al. 1976). AChE directly binds to presenilin 1 as an important enzyme in the processing of amyloid beta and also increases its expression and in this way increases the level of amyloid beta which aggravates cognitive functions (Campanari et al. 2014; Ramos-Rodriguez et al. 2013). In addition, abnormal changes of brain cholinergic system can provoke tau phosphorylation, neuroinflammation, and apoptosis (Chen et al. 2022). Therefore, higher hippocampal activity of AChE following TMT challenge in this study may be attributed to its interaction with presenilin 1, amyloid beta, and tau protein. In this regard, it has been indicated that both amyloid beta and hyperphosphorylated tau can influence AChE expression (García-Ayllón et al. 2011). Meanwhile, TMT-induced neurodegeneration is associated with higher AChE activity (Loullis et al. 1985). In addition, it is also possible that prevailing AChE molecules released from degenerated cholinergic neurons may be another explanation for enhanced activity of AChE. All of these suggestions besides controversies regarding changes of AChE under cognitive decline conditions have merit for further research studies (García-Ayllón et al. 2011). Conversely, metformin administration at 200 mg/kg was associated with lower hippocampal activity of AChE. Corroborating this evidence, it has been shown that metformin can lessen amyloid β-instigated cognitive impairment via mitigation of hippocampal oxidative/nitrosative stress and inflammation which is associated with lower activity of AChE (Khaleghi-Mehr et al. 2023) and can inhibit cardiometabolic-associated cognitive impairment in rats under high fat diet, partly linked to its downregulation AChE activity and monoamine oxidase (Chellammal et al. 2022).
It has been reported that Aβ overproduction by sequential cleavage of amyloid precursor protein (APP) is linked to neuronal dysfunction and demise (Assefa et al. 2020; Selkoe and Hardy 2016). AMPK activation affects Aβ metabolism and is accordingly involved in AD pathogenesis (Assefa et al. 2020). However, there exists controversial reports in this field. For instance, contrary to our findings, Liu et al. (2021) have shown higher hippocampal expression of p-AMPK after 1–6 days in mice injected with TMT (Liu et al. 2021). This discrepancy may be due to time of assessment of AMPK, which has been after 21 days in our work. It has been even claimed that AMPK activation may not have neuroprotective effect and may even have detrimental outcomes such as Aβ production and tau phosphorylation (Cai et al. 2012). Hence, it is still unknown whether AMPK could be postulated as a promising therapeutic target for AD and further researches will be needed to elucidate the role of AMPK in AD pathology. In contrast, metformin at 200 mg/kg improved p-AMPK in the TMT-intoxicated group. In agreement with this finding, neuroprotective effects of metformin may be principally ascribed to its activation of AMPK signaling (Jinpiao et al. 2020) which causes upregulation of brain-derived neurotrophic factor (BDNF) expression which has pivotal roles in synaptic neurotransmission and memory consolidation processes (Miranda et al. 2019). Additionally, metformin can alleviate cognitive impairments in D-galactose model of aging through enhancement of AMPK/BDNF/phosphoinositide 3-kinase (PI3K) pathway (Ameen et al. 2022).
Although considerable development has been gained to unravel the involved pathogenic mechanisms for AD in addition to finding novel therapeutics, it still remains an incurable disorder. Widely used animal models for AD are not able to satisfactorily represent its pathological events (Chen and Zhang 2022). One of the established animal models for testing efficacy of promising agents against AD-like neurodegeneration and cognitive deficit is through intraperitoneal injection of TMT (Chvojkova et al. 2021). However, TMT like other neurotoxicants could not mimic all pathophysiological aspects of AD and it might be considered more a useful research tool for evaluation of hippocampus-specific neurodegeneration (Lee et al. 2016). In other words, TMT is not an AD-specific neurotoxin to precisely simulate AD-associated cognitive, pathological, and biochemical alterations and it induces other behavioral changes including seizure-like phenotype, irritability, body weight loss, hypothermia, tremor, and even tail mutilation, as reviewed before (More et al. 2016).
Lack of a priori sample size calculation, Western blotting experiments for the analyzed factors, and verification of the specificity of the used antibodies employing relevant scientific methods such as Knockout Validation protocol was some limitations of the present study. In addition, since we did not perform experiments on the involvement of AMPK pathway in the beneficial effect of metformin after its inhibition and/or blockade, this limitation should also be taken into account in the future pertinent works.
Conclusions
To conclude, this study provided essential insight into how metformin exerts its positive preclinical effects in TMT-induced neurodegeneration. This may pave the way to develop new therapeutics to combat against cognitive deficits in AD-like pathologies and type 2 diabetes. However, exact mechanism of metformin effect to counteract TMT neurotoxicity remains to be explored.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- AMPK
AMP-activated protein kinase
- Aβ
Amyloid beta
- BDNF
Brain-derived neurotrophic factor
- CREB
CAMP response element-binding protein
- GFAP
Glial fibrillary acidic protein
- G6PD
Glucose-6-phosphate dehydrogenase
- GSH
Reduced glutathione
- GSK3β
Glycogen synthase kinase 3
- IL-6
Interleukin 6
- MDA
Malondialdehyde
- NFT
Neurofibrillary tangles
- NOR
Novel object recognition
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PI3K
Phosphoinositide 3-kinase
- p-Tau
Hyperphosphorylated Tau
- SOD
Superoxide dismutase
- TMT
Trimethyltin
- TNF
Tumor necrosis factor
Author Contributions
MT performed experiments and helped in manuscript writing, MR designed the study and protocol of experiments, supervised conductance of experiments and wrote the manuscript, and RS helped in study design, data analysis, and writing the manuscript.
Funding
This research project was partly supported in 2022 by Shahed University (# 503285).
Data Availability
Data generated and analyzed during the present study will be available from the corresponding author on reasonable request.
Declarations
Conflict of interest
There is no conflict of interest to express.
Ethical Approval
All experimental procedures of this study were conducted under ethics committee supervision of Shahed University (# IR.SHAHED.REC.1401.131) that was in accordance to NIH guidelines for the care and use of laboratory animals. All efforts were made to minimize number of animals and to minimize their sufferings.
Informed Consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abosharaf HA, Elsonbaty Y, Tousson E, Mohamed TM (2024) Alzheimer’s disease-related brain insulin resistance and the prospective therapeutic impact of metformin. J Neuroendocrinol 36(1):e13356 [DOI] [PubMed] [Google Scholar]
- Ale Mahmoud Mehraban R, Babaei P, Rohampour K, Jafari A, Golipoor Z (2024) Metformin improves memory via AMPK/mTOR-dependent route in a rat model of Alzheimer’s disease. Iran J Basic Med Sci 27(3):360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen O, Samaka RM, Abo-Elsoud RAA (2022) Metformin alleviates neurocognitive impairment in aging via activation of AMPK/BDNF/PI3K pathway. Sci Rep 12(1):17084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T, Sharma S, Kelberman MA, Ware C, Guo N, Qin Z, Weinshenker D, Parent MB (2024) Obesity during preclinical Alzheimer’s disease development exacerbates brain metabolic decline. J Neurochem 168(5):801–821 [DOI] [PubMed] [Google Scholar]
- Assefa BT, Tafere GG, Wondafrash DZ, Gidey MT (2020) The bewildering effect of AMPK activators in Alzheimer’s disease: review of the current evidence. Biomed Res Int 2020:9895121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A (2020) 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- Atella TC, Medina JM, Atella GC, Allodi S, Kluck GEG (2024) Neuroprotective effects of metformin through AMPK activation in a neurotoxin-based model of cerebellar ataxia. Mol Neurobiol 61(8):5102–5116 [DOI] [PubMed] [Google Scholar]
- Bowen DM, Smith CB, White P, Davison AN (1976) Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99(3):459–496 [DOI] [PubMed] [Google Scholar]
- Brown BM, Peiffer J, Rainey-Smith SR (2019) Exploring the relationship between physical activity, beta-amyloid and tau: a narrative review. Ageing Res Rev 50:9–18 [DOI] [PubMed] [Google Scholar]
- Cai Z, Yan LJ, Li K, Quazi SH, Zhao B (2012) Roles of AMP-activated protein kinase in Alzheimer’s disease. Neuromolecular Med 14(1):1–14 [DOI] [PubMed] [Google Scholar]
- Campanari ML, García-Ayllón MS, Belbin O, Galcerán J, Lleó A, Sáez-Valero J (2014) Acetylcholinesterase modulates presenilin-1 levels and γ-secretase activity. J Alzheimers Dis 41(3):911–924 [DOI] [PubMed] [Google Scholar]
- Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E (2018) Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis 65(4):1225–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal FJ, Inestrosa NC (2011) Interactions of AChE with Aβ aggregates in Alzheimer’s brain: therapeutic relevance of IDN 5706. Front Mol Neurosci 4:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellammal HSJ, Hasan MH, Kshirsagar RP, Musukula VKR, Ramachandran D, Diwan PV (2022) Metformin inhibits cardiometabolic syndrome associated cognitive deficits in high fat diet rats. J Diabetes Metab Disord 21(2):1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Zhang Y (2022) Animal models of Alzheimer’s disease: applications, evaluation, and perspectives. Zool Res 43(6):1026–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Drew J, Berney W, Lei W (2021a) Neuroprotective natural products for Alzheimer’s disease. Cells 10(6):1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao S, Fan Z, Li Z, Zhu Y, Shen T, Li K, Yan Y, Tian J, Liu Z, Zhang B (2021b) Metformin attenuates plaque-associated tau pathology and reduces amyloid-β burden in APP/PS1 mice. Alzheimers Res Ther 13(1):40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-R, Huang J-B, Yang S-L, Hong F-F (2022) Role of cholinergic signaling in Alzheimer’s disease. Molecules 27(6):1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvojkova M, Kubova H, Vales K (2021) Effects of dizocilpine, midazolam and their co-application on the trimethyltin (TMT)-induced rat model of cognitive deficit. Brain Sci 11(3):400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseweidy MM, Mahrous M, Ali SI, Shaheen MA, Younis NN (2023) Pentoxifylline as add-on treatment to donepezil in copper sulphate-induced Alzheimer’s disease-like neurodegeneration in rats. Neurotox Res 41(6):546–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Lu Y, Wei H, Wang K, Jia P, Zhang Y, Zhang Y, Wang T, Yang L, Zhao J, Zhang S, Lu H, Chen X, Liu Y, Zhang P (2023) Metformin attenuates sevoflurane-induced neurogenesis damage and cognitive impairment: involvement of the Nrf2/G6PD pathway. Metab Brain Dis 38(6):2037–2053 [DOI] [PubMed] [Google Scholar]
- Fontana IC, Scarpa M, Malarte ML, Rocha FM, Ausellé-Bosch S, Bluma M, Bucci M, Chiotis K, Kumar A, Nordberg A (2023) Astrocyte signature in Alzheimer’s disease continuum through a multi-PET tracer imaging perspective. Cells 12(11):1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ayllón M-S, Small DH, Avila J, Saez-Valero J (2011) Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-amyloid. Front Mol Neurosci 4:22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geloso MC, Vinesi P, Michetti F (1998) Neuronal subpopulations of developing rat hippocampus containing different calcium-binding proteins behave distinctively in trimethyltin-induced neurodegeneration. Exp Neurol 154(2):645–653 [DOI] [PubMed] [Google Scholar]
- Geloso MC, Corvino V, Michetti F (2011) Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem Int 58(7):729–738 [DOI] [PubMed] [Google Scholar]
- Gholami A (2023) Alzheimer’s disease: the role of proteins in formation, mechanisms, and new therapeutic approaches. Neurosci Lett 817:137532 [DOI] [PubMed] [Google Scholar]
- Go MJ, Kim JM, Lee HL, Kim TY, Joo SG, Kim JH, Lee HS, Kim D-O, Heo HJ (2023) Anti-amnesia-like effect of pinus densiflora extract by improving apoptosis and neuroinflammation on trimethyltin-induced ICR mice. Int J Mol Sci 24(18):14084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Lv X, Guo Y, Qi M, Ge B (2023) Total flavonoids of Cynomorium songaricum attenuates cognitive defects in an Aβ 1–42 -induced Alzheimer’s disease rat model by activating BDNF/TrkB signaling transduction. NeuroReport 34(17):825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiset T, Sriraksa N, Kamsrijai U, Praman S, Inkaew P (2023) Neuroprotective effect of Tiliacora triandra (Colebr.) diels leaf extract on scopolamine-induced memory impairment in rats. Heliyon 9(12):22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49(12):2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinpiao Z, Zongze Z, Qiuyue Y, Peng F, Qi Z, Yanlin W, Chang C (2020) Metformin attenuates sevoflurane-induced neurocognitive impairment through AMPK-ULK1-dependent autophagy in aged mice. Brain Res Bull 157:18–25 [DOI] [PubMed] [Google Scholar]
- Jung EY, Lee MS, Ahn CJ, Cho SH, Bae H, Shim I (2013) The neuroprotective effect of gugijihwang-tang on trimethyltin-induced memory dysfunction in the rat. Evid Based Complem Alternat Med 2013:542081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Park SK, Guo TJ, Ha JS, Lee DS, Kim JM, Lee U, Kim DO, Heo HJ (2016) Reversal of trimethyltin-induced learning and memory deficits by 3,5-dicaffeoylquinic acid. Oxid Med Cell Longev 2016:6981595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM (2006) Alzheimer’s and dementia in the oldest-old: a century of challenges. Curr Alzheimer Res 3(5):411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi S, Kermanshahi S, Karami L, Motaghinejad M, Motevalian M, Sadr S (2019) Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology 72:74–84 [DOI] [PubMed] [Google Scholar]
- Khaleghi-Mehr M, Delshad AA, Shafie-Damavandi S, Roghani M (2023) Metformin mitigates amyloid β(1–40)-induced cognitive decline via attenuation of oxidative/nitrosative stress and neuroinflammation. Metab Brain Dis 38(4):1127–1142 [DOI] [PubMed] [Google Scholar]
- Khodabakhsh P, Asgari Taei A, Shafaroodi H, Pournajaf S, Dargahi L (2024) Effect of metformin on epidermal neural crest stem cells and their potential application in ameliorating paclitaxel-induced neurotoxicity phenotype. Stem Cell Rev Rep 20(1):394–412 [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 1916:105–111 [DOI] [PubMed] [Google Scholar]
- Lee S, Yang M, Kim J, Kang S, Kim J, Kim J-C, Jung C, Shin T, Kim S-H, Moon C (2016) Trimethyltin-induced hippocampal neurodegeneration: a mechanism-based review. Brain Res Bull 125:187–199 [DOI] [PubMed] [Google Scholar]
- Liao W, Xu J, Li B, Ruan Y, Li T, Liu J (2021) Deciphering the roles of metformin in alzheimer’s disease: a snapshot. Front Pharmacol 12:728315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lv J, Zhang Z, Wang B, Duan L, Li C, Xie H, Li T, Zhou X, Xu R, Chen N, Liu W, Ming H (2021) The main mechanisms of trimethyltin chloride-induced neurotoxicity: Energy metabolism disorder and peroxidation damage. Toxicol Lett 345:67–76 [DOI] [PubMed] [Google Scholar]
- Loullis CC, Dean RL, Lippa AS, Clody DE, Coupet J (1985) Hippocampal muscarinic receptor loss following trimethyl tin administration. Pharmacol Biochem Behav 22(1):147–151 [DOI] [PubMed] [Google Scholar]
- Martinez-Feduchi P, Jin P, Yao B (2024) Epigenetic modifications of DNA and RNA in Alzheimer’s disease. Front Mol Neurosci 17:1398026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13:363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SV, Kumar H, Cho D-Y, Yun Y-S, Choi D-K (2016) Toxin-induced experimental models of learning and memory impairment. Int J Mol Sci 17(9):1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DE, Perez RG (2021) Anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: nexus of cholinergic and nerve growth factor dysfunction. Curr Alzheimer Res 18(13):1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmasitoh T, Sari DCR, Susilowati R (2023) Moderate-intensity intermittent exercise prevents memory deficit, hippocampal neuron loss, and elevated level of Alzheimer’s dementia markers in the hippocampus of trimethyltin-induced rats. Ann Anat 249:152103 [DOI] [PubMed] [Google Scholar]
- O’Keefe J (1993) Hippocampus, theta, and spatial memory. Curr Opin Neurobiol 3(6):917–924 [DOI] [PubMed] [Google Scholar]
- Oruc A, Oruc KY, Yanar K, Mengi M, Caglar A, Kurt BO, Altan M, Sonmez OF, Cakatay U, Uzun H, Simsek G (2024) The role of glycogen synthase kinase-3β in the zinc-mediated neuroprotective effect of metformin in rats with glutamate neurotoxicity. Biol Trace Elem Res 202(1):233–245 [DOI] [PubMed] [Google Scholar]
- Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, Huang J, Xu B, Long D, Li J, Li Q, Xu L, Xuan A (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69:351–363 [DOI] [PubMed] [Google Scholar]
- Park SK, Kang JY, Kim JM, Yoo SK, Han HJ, Chung DH, Kim DO, Kim GH, Heo HJ (2019) Fucoidan-rich substances from ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid β production/tau hyperphosphorylation. Mar Drugs 17(10):591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Ayesha IE, Monson NR, Klair N, Patel U, Saxena A, Hamid P (2023) The effectiveness of metformin in diabetes prevention: a systematic review and meta-analysis. Cureus 15(9):e46108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone P, Nuzzo D, Caruana L, Messina E, Barera A, Vasto S, Carlo M (2015) Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-κB activation: use of insulin to attenuate metformin’s effect. Biochim Biophys Acta 1853(5):1046–1059 [DOI] [PubMed] [Google Scholar]
- Pilipenko V, Narbute K, Pupure J, Langrate IK, Muceniece R, Kluša V (2020) Neuroprotective potential of antihyperglycemic drug metformin in streptozocin-induced rat model of sporadic Alzheimer’s disease. Eur J Pharmacol 881:173290 [DOI] [PubMed] [Google Scholar]
- Ponce-Lopez T, González Álvarez Tostado JA, Dias F, Montiel Maltez KH (2023) Metformin prevents NDEA-induced memory impairments associated with attenuating beta-amyloid, tumor necrosis factor-alpha, and interleukin-6 levels in the hippocampus of rats. Biomolecules 13(9):1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeepkiran JA, Baig J, Islam MA, Kshirsagar S, Reddy PH (2024) Amyloid-β and phosphorylated Tau are the key biomarkers and predictors of Alzheimer’s disease. Aging Dis 10:123. 10.14336/AD.2024.0286 [DOI] [PubMed] [Google Scholar]
- Rabieipoor S, Zare M, Ettcheto M, Camins A, Javan M (2023) Metformin restores cognitive dysfunction and histopathological deficits in an animal model of sporadic Alzheimer’s disease. Heliyon 9(7):e17873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Rodriguez JJ, Pacheco-Herrero M, Thyssen D, Murillo-Carretero MI, Berrocoso E, Spires-Jones TL, Bacskai BJ, Garcia-Alloza M (2013) Rapid β-amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J Neuropathol Exp Neurol 72(4):272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A, Taleahmad F, Haddadzadeh-Niri N, Joneidi E, Afshin-Majd S, Baluchnejadmojarad T, Roghani M (2022) Sinomenine Attenuates trimethyltin-induced cognitive decline via targeting hippocampal oxidative stress and neuroinflammation. J Mol Neurosci 72(8):1609–1621 [DOI] [PubMed] [Google Scholar]
- Sabzali M, Eidi A, Khaksari M, Khastar H (2022) Anti-inflammatory, antioxidant, and antiapoptotic action of metformin attenuates ethanol neurotoxicity in the animal model of fetal alcohol spectrum disorders. Neurotox Res 40(2):605–613 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8(6):595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancheva L, Kalfin R, Minchev B, Uzunova D, Tasheva K, Tsvetanova E, Georgieva A, Alexandrova A, Stefanova M, Solak A, Lazarova M, Hodzhev Y, Grigorova V, Yarkov D, Petkova-Kirova P (2023) Memory recovery effect of a new bioactive innovative combination in rats with experimental dementia. Antioxidants 12(12):2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong-asa W, Prasartsri S, Klomkleaw N, Thongwan N (2020) The neuroprotective effect of betanin in trimethyltin-induced neurodegeneration in mice. Metab Brain Dis 35(8):1395–1405 [DOI] [PubMed] [Google Scholar]
- Tripathi PN, Lodhi A, Rai SN, Nandi NK, Dumoga S, Yadav P, Tiwari AK, Singh SK, El-Shorbagi AA, Chaudhary S (2024) Review of pharmacotherapeutic targets in Alzheimer’s disease and its management using traditional medicinal plants. Degener Neurol Neuromuscul Dis 14:47–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu TT, Sharma N, Shin EJ, Tran HQ, Lee YJ, Nah SY, Tran HP, Jeong JH, Jeong JH, Ko SK, Byun JK, Kim HC (2017) Treatment with mountain-cultivated ginseng alleviates trimethyltin-induced cognitive impairments in mice via IL-6-dependent JAK2/STAT3/ERK signaling. Planta Med 83(17):1342–1350 [DOI] [PubMed] [Google Scholar]
- Vizuete AFK, Fróes F, Seady M, Hansen F, Ligabue-Braun R, Gonçalves CA, Souza DO (2023) A mechanism of action of metformin in the brain: prevention of methylglyoxal-induced glutamatergic impairment in acute hippocampal slices. Mol Neurobiol 61(6):3223–3239 [DOI] [PubMed] [Google Scholar]
- Xia Y, Prokop S, Giasson BI (2021) “Don’t Phos Over Tau”: recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol Neurodegener 16(1):37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu J (2021) Amyloid-β: a double agent in Alzheimer’s disease? Biomed Pharmacother 139:111575 [DOI] [PubMed] [Google Scholar]
- Zhang R, Zeng M, Zhang X, Zheng Y, Lv N, Wang L, Gan J, Li Y, Jiang X, Yang L (2023) Therapeutic candidates for Alzheimer’s disease: saponins. Int J Mol Sci 24(13):10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated and analyzed during the present study will be available from the corresponding author on reasonable request.