Abstract
CXCL13 is a chemokine that plays an important role in the regulation and development of secondary lymphoid organs. CXCL13 is also involved in the regulation of pathological processes, particularly inflammatory responses, of many diseases. The function of CXCL13 varies depending on the condition of the host. In a healthy condition, CXCL13 is mainly secreted by mouse stromal cells or human follicular helper T cells, whereas in diseases conditions, they are produced by human peripheral helper T cells and macrophages in non-lymphoid tissues; this is termed ectopic expression of CXCL13. Ectopic CXCL13 expression is involved in the pathogenesis of various immune-mediated inflammatory diseases as it regulates the migration of B lymphocytes, T lymphocytes, and other immune cells in inflammatory sites as well as influences the expression of inflammatory factors. Additionally, ectopic expression of CXCL13 plays a key role in ectopic lymphoid organ formation. In this review, we focused on the sources of CXCL13 in different conditions and its regulatory mechanisms in immune-mediated inflammatory diseases, providing novel ideas for further research on targeting CXCL13 for the treatment of immune-mediated inflammatory diseases.
Keywords: CXCL13, Immune-mediated inflammatory diseases, IMIDs, ELOs, Inflammatory response
Introduction
Chemokines are low molecular weight proteins of the cytokine family that shares 20–50% gene sequence and amino acid sequence homology [1]. Based on the arrangement of the cysteine residues, > 50 human chemokines can be segregated into the following subgroups: CXC, CC, XC, and CX3C [2–4]. C-X-C motif ligand 13 (CXCL13) is a homeostatic chemokine belonging to the CXC subgroup, which is the most potent chemoattractant for B cells. Thus, CXC13 also referred to as B cell attracting chemokine 1 (BCA-1) or B lymphocyte chemokine (BLC) [5–7]. CXCL13 is an important regulator of immunity that is mostly produced by stromal and follicular helper T (Tfh) cells in a healthy state [8,9]. C-X-C chemokine receptor type 5 (CXCR5), originally known as Burkitt's lymphoma receptor 1 (BLR1), is up to now the only receptor of CXCL13 [7]. The specific binding of CXCL13 to CXCR5 activates downstream signaling pathways such as phospholipase C (PLC), protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and nuclear factor-kB (NF-kB) [10–12]. Activation of these signaling pathways not only induces the migration of B lymphocytes, Tfh cells, and macrophages in secondary lymphoid organs (SLOs), but also contributes to the formation of lymphatic follicles [7,10,13]. Experiments involving mice have revealed that CXCL13 activates naïve B cells and increased membrane LTa1b2 levels, thereby promoting the maturation of follicular dendritic cells (FDCs) and enhancing CXCL13 production [14,15]. Additionally, mice without CXCL13 have defective homing of B cells to follicles in lymph nodes and the spleen, indicating the important role of CXCL13 in the development of SLOs [15].
CXCL13 is also involved in the regulation of processes in several diseases, including chronic inflammation, infectious diseases, autoimmune diseases, neurological disorders, and neoplasms [7,12,16,17]. Evidence indicates that CXCL13 expression is upregulated in non-lymphoid tissues in diseased conditions and is termed as ectopic expression of CXCL13. Interestingly, unlike CXCL13 production in a healthy state, ectopic CXCL13 expression during infections or inflammatory conditions is a result of extrafollicular secretion of peripheral helper T cell (Tph) cells and macrophages [18]. Moreover, elevated levels of ectopic CXCL13 are related to inflammatory activity in diseased states. Ectopic CXCL13 modulates the migration of B lymphocytes, T lymphocytes, and other immune cells to sites of inflammation in disease, influences the expression of inflammatory cytokines, and is also involved in the formation of ectopic lymphoid organs or third lymphoid organs (ELOs) [19–21]. ELOs, which are clusters of T cells, B cells, macrophages, and DCs, are linked to inflammatory responses, infections, tumors and autoimmune diseases [22].
CXCL13 is a potential marker for inflammatory diseases, particularly immune-mediated inflammatory diseases (IMIDs). IMIDs are caused by autoimmune system imbalance, resulting in acute and chronic inflammation that damages tissues and/or organs [23,24]. IMIDs include inflammatory bowel disease (IBD), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), psoriasis, and asthma [25,26]. The common pathogenic mechanisms of IMIDs are activation of inflammatory pathways and imbalance of inflammatory cytokines. Immune cells that aggregate at sites of inflammation activate inflammatory pathways, release inflammatory cytokines, and promote chemokine synthesis, resulting in a local inflammatory microenvironment. The inflammatory microenvironment includes the formation of ELOs that invade different tissues, causing severe damage [27,28]. The common pathogenic mechanisms may also explain the presence of concomitant IMIDs in patients [26]. Specifically targeting immune pathways in IMIDs have demonstrated that regulating inflammation can have a favorable effect on the disease [24]. Therefore, the current direction of treatment of IMIDs is to regulate inflammation through early and aggressive targeted therapies to prevent irreversible tissue damage [24,29]. It was previously noted that CXCL13 is closely related with several IMIDs, showing its potential as a therapeutic target. Hence, a deeper comprehension of the source of CXCL13 and the mechanisms by which it modulates the inflammatory response is necessary to further evaluate the therapeutic strategies for IMIDs.
Source of CXCL13
CXCL13 is produced by a variety of cells that differ not only between mice and humans but also between different diseases in humans (Fig. 1). In the healthy state, CXCL13 is produced by stromal cells in B-cell follicles of SLOs and follicular helper T (Tfh) cells in germinal centers of SLOs in mice and humans, respectively [9,30]. In contrast, Tph cells and macrophages secrete large amounts of CXCL13 during diseased or inflammatory states as it is essential for ELO formation [18]. This suggests that the source of CXCL13 is directly related to the state of the organism, and understanding the source in different conditions will help to better analyze the role of CXCL13 in disease.
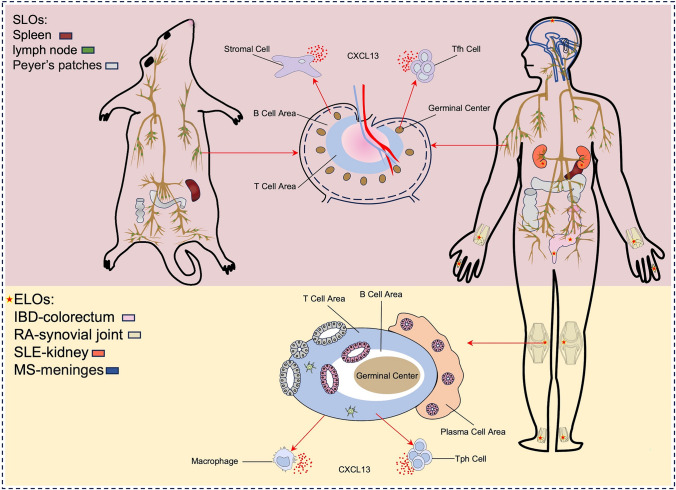
Fig. 1.
Different sources of CXCL13 In the healthy condition, CXCL13 is mainly secreted by stromal cells in mice or Tfh cells in human in SLOs. In disease, CXCL13 is mainly produced by human Tph cells and macrophages in non-lymphoid tissues, accompanied by the formation of ELOs
Stromal cells
Stromal cells are epithelial cells in immune tissues and receptors that contribute to lymphocyte development, maturation, and immune responses. Stromal cells in B-cell follicles of SLOs mainly produce CXCL13 during the healthy state mice.
In SLOs, the major subsets of stromal cells include FDCs, marginal reticular cells (MDCs) in the B-cell zone, and fibroblastic reticular cells (FRCs) in the T cell zone [8,31]. During homeostasis, stromal cells highly express CXCL13 and other chemokines, regulating the normal organization of SLOs by restricting lymphocytes to their respective regions through a gradient of secreted chemokines [8,30]; reports in subsets of stromal cells also support this theory. Notably, CXCL13 secreted by stromal cell subsets in different regions is differentially expressed. Compared with MDCs, FDCs in the B-cell zone are the dominant CXCL13-producing cells in the stromal cell subset [32,33]. Additionally, CXCL13 expression in FDCs is strongly dependent on lymphotoxin (LT) α1β2 signaling. Through the interaction between stromal cells and lymphoid tissue-inducing (LTi) cells, a positive feedback loop is established between CXCL13 expression and LTα1β2, which is important for the development of SLOs [31,34]. This indicates the important role of stromal cell-secreted CXCL13 in SLO development.
Follicular helper T cells (Tfh)
Follicular helper T (Tfh) cells are a subset of CD4+T cells that exist in the germinal center (GC) of SLOs. As an important regulatory factor of antigen specific B-cell responses, Tfh cells play an essential role in preventing infection and autoimmune diseases [35]. In human SLOs, Tfh cells are the major producers of CXCL13 in human SLOs [9]. The function of human and mouse Tfh cells is similar; however, whether CXCL13 is not produced by these cells in mice [36,37]. CXCL13 expressed by Tfh cells acts on GC B cells. In human SLOs, large amounts of CXCL13 from Tfh cells it attracts GC B cells to the light zone, wherein most Tfh cells and FDCS are located [38]. On the one hand, CXCL13 binding CXCR5 can also induce LTα1β2 expression on naïve B cells or provide signals to B cells not associated with migration [15,39]. On the other hand, TCR activation induces Tfh cells to produce CXCL13, and the production of large amounts of CXCL13 cannot be sustained without T cell activation [40]. Additionally, Tfh cells not only secrete CXCL13 but also highly express CXCR5 in response to CXCL13 migration [9]. In summary, CXCL13 expressed by Tfh cells has several effects on GC B cells, and CXCL13 production by Tfh cells depends on T cell activation to regulate the migration of Tfh cells.
Peripheral helper T cells (Tph)
Peripheral T helper (Tph) cells, which are a newly defined special CD4+ helper T cell subset, are located outside the follicle. Tph cells highly express CXCL13 but lack CXCR5 markers and are labeled as PD-1+CXCR5-CD4+ cells [18]. Unlike Tfh cells that provide support to B cells in the GC of SLOs, Tph cells assist B cells in pathologically inflamed non-lymphoid tissues [41]. Previously, in patients with breast cancer, a large number of CD4+ T cells expressing CXCL13 exhibiting PD-1+CXCR5− were found in inflammatory intestinal tissues of patients with Crohn’s disease (CD) as well as in synovial tissues of patients with RA, and these cells were consistent with the characteristics of Tph cells [18,35,42]. These findings indicate that Tph cells are major producers of ectopic CXCL13 in inflammation-associated non-lymphoid tissues. Additionally, Tph cells infiltrate inflamed tissues and secrete large amounts of CXCL13, which recruits B cells into inflamed tissues and causes them to aggregate around Tph cells, further facilitating B-cell migration and inducing them to differentiate into antibody-producing cells that eventually develop into ELOs [41]. As a receptor for CXCL13, CXCR5 is also important for the development of ELOs. It was revealed that ELOs fail to develop in the absence of CXCR5 [43]. In summary, ectopic CXCL13 secreted by Tph cells plays an important role in the formation of ELOs, suggesting that Tph cells are closely related to CXCL13-driven diseases that are linked to the formation of ELOs.
Macrophages
Macrophages can initiate the immune and inflammatory responses against pathogens and play a central role in several diseases, including metabolic and autoimmune diseases, cancer, infection, and fibrosis [44]. Aside from Tph cells, macrophages produce ectopic CXCL13 in large quantities during infection or inflammatory states. In tissue-specific macrophages, there is evidence of ectopic CXCL13 production in large amounts. In lung tissues, Nessrine et al. revealed that macrophages are an important source of CXCL13 in patients with idiopathic fibrosis and are significantly associated with TNF-α. TNF-α positively regulates CXCL13 gene expression in human inflammatory monocyte-derived macrophages (MoDM) and alveolar macrophages (AM) by activation of the NF-κB pathway, while IL-10 enhances TNF-α induction through the JAK/STAT pathway [45]. In neural tissues, Nilufer et al. showed that microglia are one of the major sources of CXCL13 in the brain during virus-induced central nervous system (CNS) inflammation. Microglia cultured in vitro produce CXCL13 due to stimulation by the viral particles or synthetic toll-like receptor 9 (TLR9) ligands. Meanwhile, type-I interferon (IFN-1) specifically inhibits this process via the IFN-1 receptor signaling (IFNAR) pathway [46]. In renal tissues, Lena et al. revealed that CXCL13 can be expressed by renal macrophages in systemic lupus erythematosus (SLE) nephritis, resulting in the induction of peripheral blood monocytes in NZB/W mice through IL-1 and TNF-α, and ultimately upregulating CXCL13 [47]. In intestinal tissues, monocyte-derived macrophages are major sources of CXCL13. Carlsen’s study revealed that in inflammatory lesions of ulcerative colitis (UC), macrophages produce more CXCL13 than monocyte-derived DCs [48].
These studies indicate that in non-lymphoid tissues, ectopic CXCL13 secreted by macrophages is regulated by inflammatory cytokines such as TNF, IL, and IFN regardless of the lymphotoxin. Additionally, macrophages act as antigen-presenting cells that responsible for the recruitment of T or B cells into the inflammatory site to form ELOs. In summary, macrophages are responsible for the ectopic expression of CXCL13 in CXCL13-driven diseases related to the formation of ELOs, including chronic inflammatory and autoimmune diseases.
Role of CXCL13 in IMIDs
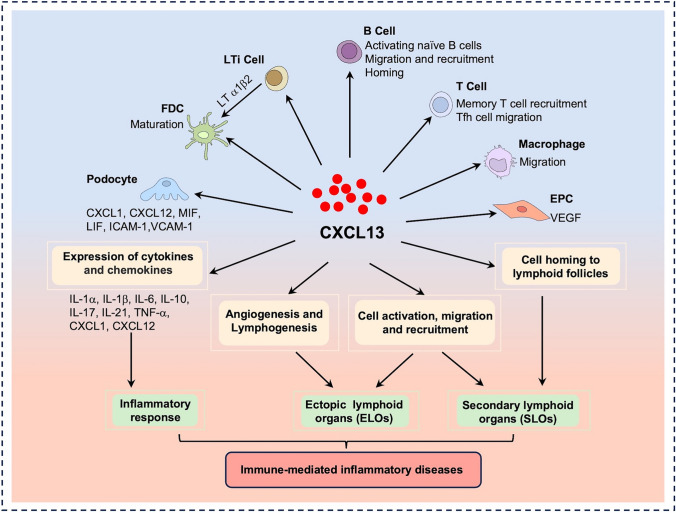
CXCL13 is involved with the development of several IMIDs, including IBD, RA, SLE, MS, psoriasis, asthma, and so on [12,49–53]. Notably, ectopic CXCL13 from various sources plays a significant role in the development of various IMIDs (Fig. 2).
Fig. 2.
CXCL13 is involved in the development and progress of IMIDs CXCL13 regulates the activation and migration of various immune cells at the site of inflammation and also mediates the expression of inflammatory mediators, thereby modulating the inflammatory response. Meanwhile, CXCL13 also plays a key role in the formation of ELOs by regulating immune cell migration and recruitment, angiogenesis, and lymphogenesis. Through these pathways, CXCL13 is involved in the pathogenesis of IMIDs
IBD
IBD is a chronic gastrointestinal IMID that includes UC and CD. Intestinal inflammation in IBD is typified by rapid infiltration and recruitment of chronic inflammatory cells that are related to an imbalance between inflammatory and anti-inflammatory factors [54,55]. Studies have revealed that CXCL13 expression is significantly upregulated in the serum of patients and mice with IBD, and that CXCL13 is positively correlated with the severity of the inflammatory response [19,56]. Similarly, mRNA expression of CXCL13 was significantly upregulated in colonic tissues of rats with dextran sulfate disodium (DSS)-induced colitis [57]. Collectively, these confirm the correlation between CXCL13 and IBD. Aligned with the significantly elevated levels of CXCL13, the number of Tph cells with CXCL13-secreting potential in inflamed bowel tissues was significantly higher than that in the blood of patients with CD and UC [42]. Monocytes and macrophages are also two of the major cells that express CXCL13 in UC [48]. This indicated that Tph cells and macrophages may be the main CXCL13-secreting cells in pathologically inflamed non-lymphoid tissues.
Ectopic CXCL13 is involved in the pathogenesis of IBD in several ways. In a mouse model of DSS-induced colitis, the absence of CXCL13 significantly downregulated the expression of inflammatory cytokines IL-21, IL-1b, IL-1a, IL-17, IL-6, and TNF-a in the mouse colon as well as attenuated the inflammatory response to colitis in mice [19]. Additionally, the number of mesenteric lymphoid B (MLB) cells in CXCL13 knockout mice was significantly reduced compared to that in wild mice, suggesting that CXCL13 may be involved in inducing the migration of MLB cells to the intestine [19]; this was confirmed in a rat model of DSS-induced colitis [57]. Studies have also revealed that ELO formation in the mesentery plays an important role in the disease process of IBD [58–60]. ELOs impede the efflux of cells and molecules from the gut, disrupting lymphatic transport at the intestinal site and leading to impaired immune cell transit [59]. It was also revealed that macrophage-secreted CXCL13 in UC is involved in the pathogenesis of IBD by influencing ELO formation [5,48]. In summary, ectopic CXCL13 affects the expression of inflammatory cytokines in IBD, regulates the migration of B lymphocytes in the intestines, and participates in ELO formation.
RA
RA is an autoimmune disease that causes damage to multiple joints and is the most common type of inflammatory arthritis. The main cause of joint destruction and pathology in RA is the occurrence of local infiltrative synovial [61,62]. Robust evidence suggests that CXCL13 is closely associated to the pathogenesis of RA. In mice with collagen-induced arthritis and in patients with RA, studies have showed that CXCL13 levels are significantly elevated in serum or synovial fluid and correlated with disease activity [17,63–66]. Additionally, studies have confirmed that serum CXCL13 levels correlate with synovial CXCL13 levels in individual joints, indicating that synovial tissues may be the source of serum CXCL13 [67]. Ectopic expression of CXCL13 in the synovium may be induced by various cells, including monocytes/macrophages, Tph cells, and FDCs [18,48,64,68]. Furthermore, ectopic expression of CXCL13 is linked to ELO formation in inflamed tissues in RA and is an important process in the development of chronic arthritis [69,70]. CXCL13 mediates B-cell recruitment through CXCR5, directing the migration of CXCR5+ Tfh cells and other CXCR5+ cells, suggesting that CXCL13 is not only a key driver of B lymphocyte migration and recruitment in inflamed synovium but is also a key mediator of ELO formation in patients with RA [68,71].
CXCL13 also induces the production of local autoantibodies, such as rheumatoid factor and anticitrullinated protein antibodies, by enhancing B-cell receptor-mediated B-cell activation [65,68,72]. This indicates that CXCL13 is involved in regulating autoantibody secretion during RA progression. Meanwhile, CXCL13 is also involved in angiogenesis in the early stages of RA. CXCL13 binds to CXCR5, which induces the expression of vascular endothelial growth factor and angiogenesis through the PLC, MEK, and AP-1 signaling pathways [17]. Additionally, it has been demonstrated that inhibition of CXCL13 expression significantly reduces disease severity in a CIA mouse model [66]. Inhibition of CXCL13 in mice attenuates CIA by inhibiting ERK/p38 signaling and activating miR-330-3p synthesis, which reduces the production of the pro-inflammatory cytokine TNF-α and prevents the infiltration of inflammatory cells into the synovium. Therefore, ectopic CXCL13 expression is essential in the pathogenesis of RA, making it a potential therapeutic target.
SLE
SLE is a multisystem inflammatory autoimmune disease that may have multi-organ involvement, resulting in kidney damage, neuropsychiatric symptoms, anemia, arthritis, pleurisy, or pericarditis [73]. SLE is typified by the presence of autoantibodies against nuclear antigens, immune complex deposition, and chronic inflammation in target organs [74]. In patients with SLE, CXCL13 is being considered as a potential biomarker of disease activity. Several studies have confirmed that CXCL13 levels are significantly elevated in the serum of patients and positively correlated with disease activity [75–77]. Additionally, the number of CXCL13-producing cTfh and Tph cells is also significantly elevated in patients with clearly active disease [78–81]. As CXCL13 is expressed by Tfh cells and acts primarily in SLOs, we inferred that CXCL13 secreted by Tph cells may be a crucial source of function in inflammatory tissues. Meanwhile, high expression of CXCL13 was noted in renal macrophages in SLE and lupus nephritis (LN) mice [47,82].
Upregulated expression of CXCL13 and mRNA at sites of renal inflammatory lesions were noted in both patients with SLE and MRL/Lpr mice [21,83–86]. LN is a serious complication of SLE, and significant elevation of CXCL13 levels is a unique early event in LN. CXCL13 is also related to the accumulation of inflammatory cells in the kidneys of patients with SLE. Ectopic CXCL13 binds to CXCR5 and activates several signaling pathways, leading to the accumulation of B cells in the kidney, promoting the secretion of multiple pro-inflammatory factors (CXCL1, CXCL12, MIF, LIF, ICAM-1, and VCAM-1) by podocytes, and enhancing albuminuria and organ damage [87–89]. Meanwhile, inhibiting CXCL13 significantly reduces urinary protein and serum creatinine levels and significantly attenuates pathological damage to kidneys. Anti-CXCL13 antibody treatment also decreased serum anti-dsDNA levels and attenuated renal immune complex deposition and inflammatory cytokine secretion [90]. In contrast, ectopic CXCL13 induces ELO development in the kidneys of lupus-susceptible mice and patients with LN, suggesting that elevated CXCL13 levels may be linked to renal impairment and ELO formation in LN [20]. Neuropsychiatric lupus (NPSLE) is the neurological manifestation of SLE, and a potential factor contributing to disease progression is ELOs in the brain. Abnormally high CXCL13 expression in the brains of MRL/Lpr mice is an important chemokine for ELO formation and may play a role in the progression of NPSLE [91]. CXCL13 is also being considered a marker for evaluating the severity of autoimmune hemolytic anemia. Patients with primary and SLE-associated autoimmune hemolytic anemia have significantly elevated levels of plasma CXCL13 that correlates negatively with hemoglobin levels [92]. In summary, ectopic CXCL13 expression in different organs plays an important role in the pathogenesis of SLE and its complications. Meanwhile, inhibition of CXCL13 can effectively attenuate inflammatory damage caused, thereby reducing the severity of SLE.
MS
MS is a chronic inflammatory disease of the CNS that is mediated by T cells and typified by inflammation, leading to myelin destruction and axonal degeneration [93,94]. Studies have showed that there is abnormal elevation of CXCL13 levels in the serum and cerebrospinal fluid (CSF) of patients with MS. Intrathecal CXCL13 production in the CNS has diagnostic and prognostic value in MS and is used to determine disease activity in patients [95–100]. Intrathecal CXCL13 is produced only in lesions of patients with active MS and not in chronic inactive lesions, non-inflammatory neurological disorders, or in the CNS of health individuals [95,96]. The differential expression of CXCL13 in active versus inactive lesions was revealed by quantitative PCR and immunohistology, confirming that CXCL13 is localized to the infiltrating immune cells, parenchyma, and perivascular macrophages highly enriched in CD68 in active MS lesions [96,101,102]. This indicates that the primary source of intrathecal CXCL13 in the CNS of patients with MS may be macrophages infiltrated by inflammatory lesions.
Elevated levels of intrathecal CXCL13 in patients with MS are associated with inflammatory activity and are involved in the recruitment of B and T cells. CXCL13 mediates the recruitment of B, memory T, and Tfh cells to inflammatory sites via CXCR5 [95,96]. Several studies have showed that CXCL13 levels in the CSF of patients with MS correlate with the presence of B cells, plasma blasts, and T cells [98,103–105]. Additionally, CXCL13 is essential for B-cell recruitment, survival, maturation, and function, thereby affecting intrathecal immunoglobulin (Ig) production [103,106]. In addition to regulating immune cell migration, CXCL13 is positively correlated with IL-6 and IL-10, and it affects B-cell homeostasis in MS by regulating these cytokines [107]. More importantly, ectopic CXCL13 production in MS is related to ELO formation in the CNS [50,95,108–110]. In a mouse autoimmune encephalomyelitis (EAE) model, stimulating CXCL13 production induced ELO formation within the CNS [108]. In summary, CXCL13 is involved in immune cell recruitment, inflammatory cytokine production, and ELO formation in inflamed CNS tissues of patients with MS. Intrathecal CXCL13 production may be considered as one of the hallmarks of acute inflammation in MS. CXCL13 may be a potential target for inhibiting B and T cell recruitment or ELO formation in the CNS of patients with MS.
Other IMIDs
In addition to the IMIDs discussed above, there are also a few studies showing that CXCL13 is also involved in the pathogenesis of other IMIDS, such as psoriasis, asthma, primary Sjogren’s syndrome (PSS), systemic sclerosis (SSc), idiopathic arthritis, idiopathic inflammatory myositis (IIM), leukodystrophy (BD), and uveitis.
Psoriasis is an immune-mediated chronic inflammatory disease that mainly presents with skin erythema and joint damage in some patients [111,112]. Inflammation in psoriasis is characterized by hyperproliferative keratinocytes and persistent inflammatory immune cell infiltrates [113]. Increased CXCL13 levels are presented in the plasma of patients with psoriasis, and in situ hybridization staining of skin lesions revealed the presence of ectopic CXCL13 mRNA that is positively correlated with disease severity [51,113]. Studies also showed a positive correlation between CXCL13 levels and CXCR3−CCR6 Tph cell frequency. This may be attributed to the fact that CXCR3−CCR6 Tph cells secrete CXCL13. As such, plasma CXCL13 levels increase with increased numbers of CXCR3-CCR6 Tph cells [51]. Additionally, ectopic CXCL13 expression in inflamed synovial tissues and synovial ELOs is closely related in patients with psoriatic arthritis (PsA) [114]. This indicates that CXCL13 is involved in the pathogenesis of psoriasis and may be a potentially novel immunotherapeutic target.
Asthma is a chronic inflammatory disease of the lower respiratory tract that is characterized by airway infiltration of inflammatory cells, including lymphocytes, mast cells, macrophages, and granulocytes [115]. A significant increase in CXCL13 mRNA levels was detected in a mouse model of OVA-induced allergic inflammation and in human patients with asthma. Additionally, immunohistochemistry revealed ectopic CXCL13 expression in the airway epithelium and perivascular and lung interstitium in allergic mice as well as significant inflammatory cell infiltration [52]. Furthermore, ectopic CXCL13 plays an essential role in B-cell infiltration and the formation of induced bronchial-associated lymphoid tissues (iBALT, a type of ELO) [52,116]. These findings confirm the involvement of ectopic CXCL13 in the airway inflammatory response in asthma. Targeting CXCL13 may be a novel approach in the treatment and management of asthma.
Primary Sjogren’s syndrome (PSS) is a chronic inflammatory autoimmune disease characterized by lymphocytic infiltration and B-cell hyperactivity in exocrine glands, particularly the salivary or lacrimal glands [117]. CXCL13 has been suggested as a potential biomarker for monitoring and diagnosing PSS. Significant elevations of CXCL13 have been detected in serum, saliva, and salivary glands of both PSS patients and mice [118–121]. More importantly, serum and saliva CXCL13 levels increased with disease progression [122]. Particularly, CXCL13 is expressed relatively early in the disease, even before lymphocytes infiltrate the glandular tissue [123]. This suggests the value of CXCL13 for early diagnosis of PSS. Further studies also showed that macrophages significantly expressed CXCL13 in the salivary glands of mice with late PSS [123]. Ectopic expression of CXCL13 by macrophages may be important in the pathogenesis of PSS, which also deserves more investigation. In addition, ectopic high expression of CXCL13 promotes migration and recruitment of marginal B-zone cells into salivary glands, which plays a key role in the formation and maintenance of ELOs in salivary glands in PSS [124–126]. These evidences suggest that CXCL13 may be important target for early intervention in PSS before irreversible gland destruction.
Accumulated clinical studies have also identified a remarkable clinical phenomenon, a significant elevated CXCL13 levels in serum or inflammatory lesion tissues of patients with SSc, idiopathic arthritis, IIM, BD, and uveitis [127–131]. However, there are few mechanistic studies on the role of CXCL13 in these diseases. Only one research on SSc found a positive correlation between elevated serum levels of CXCL13 and scores of diffuse skin involvement, suggesting that CXCL13 may be a specific biomarker for SSc [127]. This also indicates that the association of CXCL13 with the severity of these disorders, as well as the specific mechanism, remains to be more explored.
Therapeutic potential of targeting CXCL13
Current therapeutic strategies for targeting CXCL13 are mainly direct blockade, and these approaches can be categorized into two levels: (1) At the genetic level, knockout or inhibition of the CXCL13 gene in mice or rats attenuates the inflammatory response [19,132–134]. In a DSS-induced colitis model, CXCL13 knockout mice manifested with reduced distal colonic inflammation and tissue damage, and levels of pro-inflammatory cytokines, including IL-1β, IL-17α, IL-6, IL-11, and TNF- α, were significantly reduced [19]. In an EAE model, CXCL13 knockout mice presented with reduced inflammatory infiltration and demyelination in the CNS as well as reduced gliosis and fibrosis [134]. It is well-known that gene knockouts are not feasible in humans. Therefore, some researchers have also tried gene suppression methods. Inhibition of CXCL13 gene expression by short hairpin RNA, microRNA, or zinc finger protein 382 can attenuate neuroinflammation by suppressing ERK-mediated inflammatory mediator (TNF-a, IL-1β, CCL2, and CXCL1) production, thereby alleviating neuropathic pain [132,133,135]. This also lays a certain foundation for gene therapy for CXCL13 to enter the clinical trial stage in the future. (2) At the protein level, anti-CXCL13 antibody treatment also attenuated the inflammatory response, as evidenced in several animal models of IMIDs disease [52,91,136,137]. In an MRL/lpr mouse model, anti-CXCL13 neutralizing antibody treatment decreased neuroinflammation in lupus-susceptible mice by altering the gene expression of inflammatory mediators (IL-6, JAK1 and BCL2, PTPN11 and EPHA1) [91]. In a model of allergic airway inflammation, infiltration of CD4+ T cells, neutrophils, and eosinophils was reduced in anti-CXCL13-treated mice, resulting in attenuation of inflammatory infiltration in the airways and absence of iBALT formation [52]. In a CIA model, polyclonal/monoclonal anti-CXCL13 antibody treatment significantly upregulated the production of the anti-inflammatory cytokine IL-10 and inhibited the follicular response and lymphoid cell neogenesis at the sites of synovial inflammation, thereby decreasing the severity of arthritis in mice [136–138]. Interestingly, the monoclonal anti-CXCL13 antibody (Mab5261) also plays a therapeutic role in the Th17-mediated EAE model. It attenuated the severity of passive-induced encephalomyelitis in mice by inhibiting CXCL13-mediated migration of Th17 cells to the CNS [137].
Overall, these studies demonstrate the broad therapeutic potential of CXCL13 in IMIDs. In particular, drugs targeting CXCL13 may have advantages over therapeutic drugs targeting one IMID alone when treating patients with a combination of multiple IMIDs. Of course, there are some limitations to CXCL13 as a therapeutic target for IMIDs. For example, in the NPSLE disease model, current approaches have not resulted in therapeutic efficacy [91]. This may be because knockout or absence of CXCL13 affects the SLO development, thereby compromising therapeutic efficacy 13,91. And the effect on the development of SLOs may further limit the clinical application of CXCL13. Based on the above discussion, blocking CXCL13 may proceed in two directions in the future. The first direction is that cell-specific targeted inhibition of CXCL13 based on the source of CXCL13 in different conditions. The other direction is tissue-specific targeted inhibition by local injection of anti-CXCL13 antibody at sites wherein there is ectopic CXCL13 expression. These might enable targeting CXCL13 to be therapeutic without affecting the development of SLOs.
Conclusions
Herein, we focused on the regulatory role of CXCL13, which is a B-cell chemotaxis agent, in IMIDs. Ectopic CXCL13 expression is involved in the pathogenesis several various IMIDs, resulting in the activation of inflammatory pathways, production of inflammatory cytokines, and formation of ELOs. Therefore, targeting CXCL13 may be a potential and promising therapeutic approach in the treatment and management of IMIDs. Also, due to the existence of some common pathogenic mechanisms, IMID patients often have multiple IMIDs. However, long-term clinical practice has found that most biologics are effective against only one or a few diseases. This indicates that targets for a single disease do not completely address the clinical needs of IMID patients. In contrast, CXCL13, as a broad target for the treatment of IMIDs, has great potential for both disease-specific precision treatment of ectopically expressed CXCL13 and in the combination treatment of patients with a combination of multiple IMIDs.
In this review, we focus on the origin of CXCL13 in different states and its regulatory mechanisms in IMIDs, as well as provide new insights for further research on targeting CXCL13 for the treatment of IMIDs. These are necessary for the safe and effective treatment of IMIDs.
Acknowledgements
Not applicable.
Abbreviations
- GPCRs
G-protein-coupled receptors
- CXCL13
C-X-C motif ligand 13
- BCA-1
B cell attracting chemokine 1
- BLC
B lymphocyte chemokine
- Tfh cells
Follicular helper T cells
- CXCR5
C-X-C chemokine receptor type 5
- BLR1
Burkitt's lymphoma receptor 1
- DCs
Dendritic cells
- PLC
Phospholipase C
- PKC
Protein kinase C
- MAPK
Mitogen-activated protein kinase
- NF-kB
Nuclear factor-kB
- SLOs
Secondary lymphoid organs
- ELOs
Ectopic lymphoid organs or third lymphoid organs
- LT
Lymphotoxin
- Tph cells
Peripheral helper T cells
- FDCs
Follicular dendritic cells
- MDCs
Marginal reticular cells
- FRCs
Fibroblastic reticular cells
- LTi cells
Lymphoid tissue-inducing cells
- GC
Germinal center
- IMIDs
Immune-mediated inflammatory diseases
- BC
Breast cancer
- CD
Crohn's disease
- RA
Rheumatoid synovitis
- cTph
Circulating Tph
- SLE
Systemic lupus erythematosus
- APCs
Antigen presenting cells
- IPF
Idiopathic fibrosis
- MoDM
Monocyte-derived macrophages
- AM
Alveolar macrophages
- CNS
Central nervous system
- TLR9
Toll-like receptor 9
- IFN-1
Type-I Interferon
- IFNAR
IFN-1 receptor signaling
- IL
Interleukin
- IBD
Inflammatory bowel disease
- UC
Ulcerative colitis
- DSS
Dextran sulfate disodium
- MLB cells
Mesenteric lymphoid B cells
- RA
Rheumatoid arthritis
- CIA
Collagen-induced arthritis
- RF
Rheumatoid factor
- ACPA
Anticitrullinated protein antibodies
- VEGF
Vascular endothelial growth factor
- SLE
Systemic lupus erythematosus
- LN
Lupus nephritis
- NPSLE
Neuropsychiatric lupus
- MS
Multiple sclerosis
- CSF
Cerebrospinal fluid
- Ig
Immunoglobulin
- PSS
Primary Sjogren’s syndrome
- SSc
Systemic sclerosis
- IIM
Idiopathic inflammatory myositis
- BD
Leukodystrophy
Author contributions
Conceptualization was contributed by LH, TL, and YMJ; methodology was contributed by LH, YL, TL, and YMJ; formal analysis was contributed by LH, YL, TL, and YMJ; investigation was con tributed by LH, YL, and MKH; data curation was contributed by LH and YL; writing—original draft preparation was contributed by LH and YL; writing—review and editing was contributed by LH, YL, and MKH; funding acquisition was contributed by TL and YMJ; supervision was contributed by TL and YMJ. All authors reviewed the manuscript.
Funding
This work was supported by Sichuan Science and Technology Program (2023YFS0222) and Sichuan Provincial Medical Research Youth Innovation Project (202407050112).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lu Hui and Ye Li have contributed equally to this work and share first authorship.
Contributor Information
Yong-mei Jiang, Email: jiangym_SCU@163.com.
Ting Liu, Email: liuting88823@163.com.
References
- 1.Trivedi PJ, Adams DH. Chemokines and chemokine receptors as therapeutic targets in inflammatory bowel disease pitfalls and promise. J Crohns Colitis. 2018;12:1508. 10.1093/ecco-jcc/jjy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mello JDC, Gomes LEM, Silva JF, et al. The role of chemokines and adipokines as biomarkers of Crohn’s disease activity: a systematic review of the literature. Am J Transl Res. 2021;13:8561–74. [PMC free article] [PubMed] [Google Scholar]
- 3.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 5.Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51:364–71. 10.1136/gut.51.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–60. 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain M, Adah D, Tariq M, Lu Y, Zhang J, Liu J. CXCL13/CXCR5 signaling axis in cancer. Life Sci. 2019;227:175–86. 10.1016/j.lfs.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 8.Kain MJ, Owens BM. Stromal cell regulation of homeostatic and inflammatory lymphoid organogenesis. Immunology. 2013;140:12–21. 10.1111/imm.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–63. 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 10.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Wang M, Ao D, Wei X. CXCL13-CXCR5 axis: regulation in inflammatory diseases and cancer. Biochim Biophys Acta Rev Cancer. 2022;1877:188799. 10.1016/j.bbcan.2022.188799. [DOI] [PubMed] [Google Scholar]
- 12.Pan Z, Zhu T, Liu Y, Zhang N. Role of the CXCL13/CXCR5 axis in autoimmune diseases. Front Immunol. 2022;13:850998. 10.3389/fimmu.2022.850998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–47. 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 14.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9302–7. 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 16.Gudowska-Sawczuk M, Mroczko B. Chemokine ligand 13 (CXCL13) in neuroborreliosis and neurosyphilis as selected spirochetal neurological diseases: a review of its diagnostic significance. Int J Mol Sci. 2020. 10.3390/ijms21082927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai CH, Chen CJ, Gong CL, et al. CXCL13/CXCR5 axis facilitates endothelial progenitor cell homing and angiogenesis during rheumatoid arthritis progression. Cell Death Dis. 2021;12:846. 10.1038/s41419-021-04136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542:110–4. 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Liu Y, Liu CX, Jiang YM. CXCL13 is elevated in inflammatory bowel disease in mice and humans and is implicated in disease pathogenesis. Front Immunol. 2022;13:997862. 10.3389/fimmu.2022.997862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Rajkumar S, Lai Y, et al. Tertiary lymphoid structures as local perpetuators of organ-specific immune injury: implication for lupus nephritis. Front Immunol. 2023;14:1204777. 10.3389/fimmu.2023.1204777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiffer L, Kümpers P, Davalos-Misslitz AM, et al. B-cell-attracting chemokine CXCL13 as a marker of disease activity and renal involvement in systemic lupus erythematosus (SLE). Nephrol Dial Transplant. 2009;24:3708–12. 10.1093/ndt/gfp343. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi S, Watanabe T, Suzuki R, et al. TGF-β induces the differentiation of human CXCL13-producing CD4(+) T cells. Eur J Immunol. 2016;46:360–71. 10.1002/eji.201546043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteleone G, Moscardelli A, Colella A, Marafini I, Salvatori S. Immune-mediated inflammatory diseases: common and different pathogenic and clinical features. Autoimmun Rev. 2023;22:103410. 10.1016/j.autrev.2023.103410. [DOI] [PubMed] [Google Scholar]
- 24.Beyaert R, Beaugerie L, Van Assche G, et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol Cancer. 2013;12:98. 10.1186/1476-4598-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081. 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezzio C, Della Corte C, Vernero M, Di Luna I, Manes G, Saibeni S. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at the less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. 10.1177/17562848221115312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl. 2010;85:2–10. 10.3899/jrheum.091461. [DOI] [PubMed] [Google Scholar]
- 28.Williams JP, Meyers JA. (2002) Immune-mediated inflammatory disorders (I.M.I.D.s): the economic and clinical costs. Am J Manag Care 8:S664–81; quiz S82–5 [PubMed]
- 29.McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21:680–6. 10.1038/s41577-021-00603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cyster JG, Ansel KM, Reif K, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–93. 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 31.Buckley CD, Barone F, Nayar S, Bénézech C, Caamaño J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. 2015;33:715–45. 10.1146/annurev-immunol-032713-120252. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–29. 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Mod Pathol. 2006;19:1101–7. 10.1038/modpathol.3800625. [DOI] [PubMed] [Google Scholar]
- 34.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 35.Gu-Trantien C, Migliori E, Buisseret L, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017. 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Müller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–903. 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 37.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–49. 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–42. 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–60. 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 41.Yoshitomi H, Ueno H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell Mol Immunol. 2021;18:523–7. 10.1038/s41423-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin SJS, Bai L, Haileselassie Y, et al. Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nat Commun. 2019;10:2686. 10.1038/s41467-019-10387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo S, Zhu R, Yu T, et al. Chronic inflammation: a common promoter in tertiary lymphoid organ neogenesis. Front Immunol. 2019;10:2938. 10.3389/fimmu.2019.02938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–55. 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellamri N, Viel R, Morzadec C, et al. TNF-α and IL-10 control CXCL13 expression in human macrophages. J Immunol. 2020;204:2492–502. 10.4049/jimmunol.1900790. [DOI] [PubMed] [Google Scholar]
- 46.Esen N, Rainey-Barger EK, Huber AK, Blakely PK, Irani DN. Type-I interferons suppress microglial production of the lymphoid chemokine, CXCL13. Glia. 2014;62:1452–62. 10.1002/glia.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiffer L, Bethunaickan R, Ramanujam M, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–47. 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–7. 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 49.Haugen M, Frederiksen JL, Degn M. B cell follicle-like structures in multiple sclerosis-with focus on the role of B cell activating factor. J Neuroimmunol. 2014;273:1–7. 10.1016/j.jneuroim.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Aloisi F, Columba-Cabezas S, Franciotta D, et al. Lymphoid chemokines in chronic neuroinflammation. J Neuroimmunol. 2008;198:106–12. 10.1016/j.jneuroim.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Zhou X, Wang A, Ma J, Bai Y. Increased peripheral helper T cells type 17 subset correlates with the severity of psoriasis vulgaris. Immunol Lett. 2021;229:48–54. 10.1016/j.imlet.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Baay-Guzman GJ, Huerta-Yepez S, Vega MI, et al. Role of CXCL13 in asthma: novel therapeutic target. Chest. 2012;141:886–94. 10.1378/chest.11-0633. [DOI] [PubMed] [Google Scholar]
- 53.Liu S, Liu X, Xiong H, et al. CXCL13/CXCR5 signaling contributes to diabetes-induced tactile allodynia via activating pERK, pSTAT3, pAKT pathways and pro-inflammatory cytokines production in the spinal cord of male mice. Brain Behav Immun. 2019;80:711–24. 10.1016/j.bbi.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 54.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–55. 10.1016/s0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 55.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–70. 10.1016/s0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh UP, Singh NP, Murphy EA, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–9. 10.1016/j.cyto.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Wu Z, Zhao Q, et al. Mesenteric lymphatic B cells migrate to the intestine and aggravate DSS-induced colitis via the CXCR5-CXCL13 Axis. Biology. 2024. 10.3390/biology13050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez-Nguyen A, Gupta N, Sanaka H, et al. Chronic stress induces colonic tertiary lymphoid organ formation and protection against secondary injury through IL-23/IL-22 signaling. Proc Natl Acad Sci U S A. 2022;119:e2208160119. 10.1073/pnas.2208160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Czepielewski RS, Erlich EC, Onufer EJ, et al. Ileitis-associated tertiary lymphoid organs arise at lymphatic valves and impede mesenteric lymph flow in response to tumor necrosis factor. Immunity. 2021;54:2795-811.e9. 10.1016/j.immuni.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lochner M, Ohnmacht C, Presley L, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208:125–34. 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–11. 10.1016/s0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 62.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38. 10.1016/s0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 63.Bao YQ, Wang JP, Dai ZW, et al. Increased circulating CXCL13 levels in systemic lupus erythematosus and rheumatoid arthritis: a meta-analysis. Clin Rheumatol. 2020;39:281–90. 10.1007/s10067-019-04775-z. [DOI] [PubMed] [Google Scholar]
- 64.Masneri S, Piantoni S, Galoppini G, et al. CXCL-13 serum levels in patients with rheumatoid arthritis treated with abatacept. Clin Exp Rheumatol. 2022;40:2103–8. 10.55563/clinexprheumatol/sk0rx7. [DOI] [PubMed] [Google Scholar]
- 65.Greisen SR, Mikkelsen C, Hetland ML, et al. CXCL13 predicts long-term radiographic status in early rheumatoid arthritis. Rheumatology. 2022;61:2590–5. 10.1093/rheumatology/keab763. [DOI] [PubMed] [Google Scholar]
- 66.Achudhan D, Lai YL, Lin YY, et al. CXCL13 promotes TNF-α synthesis in rheumatoid arthritis through activating ERK/p38 pathway and inhibiting miR-330-3p generation. Biochem Pharmacol. 2024;221:116037. 10.1016/j.bcp.2024.116037. [DOI] [PubMed] [Google Scholar]
- 67.Rosengren S, Wei N, Kalunian KC, Kavanaugh A, Boyle DL. CXCL13: a novel biomarker of B-cell return following rituximab treatment and synovitis in patients with rheumatoid arthritis. Rheumatology. 2011;50:603–10. 10.1093/rheumatology/keq337. [DOI] [PubMed] [Google Scholar]
- 68.Bugatti S, Manzo A, Vitolo B, et al. High expression levels of the B cell chemoattractant CXCL13 in rheumatoid synovium are a marker of severe disease. Rheumatology. 2014;53:1886–95. 10.1093/rheumatology/keu163. [DOI] [PubMed] [Google Scholar]
- 69.Shi K, Hayashida K, Kaneko M, et al. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166:650–5. 10.4049/jimmunol.166.1.650. [DOI] [PubMed] [Google Scholar]
- 70.Corsiero E, Bombardieri M, Manzo A, Bugatti S, Uguccioni M, Pitzalis C. Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol Lett. 2012;145:62–7. 10.1016/j.imlet.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Armas-González E, Domínguez-Luis MJ, Díaz-Martín A, et al. Role of CXCL13 and CCL20 in the recruitment of B cells to inflammatory foci in chronic arthritis. Arthritis Res Ther. 2018;20:114. 10.1186/s13075-018-1611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamada H. Adaptive immunity in the joint of rheumatoid arthritis. Immunol Med. 2022;45:1–11. 10.1080/25785826.2021.1930371. [DOI] [PubMed] [Google Scholar]
- 73.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 74.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 75.Lindblom J, Beretta L, Borghi MO, Alarcón-Riquelme ME, Parodis I. Serum profiling identifies CCL8, CXCL13, and IL-1RA as markers of active disease in patients with systemic lupus erythematosus. Front Immunol. 2023;14:1257085. 10.3389/fimmu.2023.1257085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schiffer L, Kielstein JT, Haubitz M, et al. Elevation of serum CXCL13 in SLE as well as in sepsis. Lupus. 2011;20:507–11. 10.1177/0961203310383301. [DOI] [PubMed] [Google Scholar]
- 77.Fang C, Luo T, Lin L. The correlational research among serum CXCL13 levels, circulating plasmablasts and memory B cells in patients with systemic lupus erythematosus: A STROBE-compliant article. Medicine. 2017;96:e8675. 10.1097/md.0000000000008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sagrero-Fabela N, Ortíz-Lazareno PC, Salazar-Camarena DC, et al. BAFFR expression in circulating T follicular helper (CD4(+)CXCR5(+)PD-1(+)) and T peripheral helper (CD4(+)CXCR5(-)PD-1(+)) cells in systemic lupus erythematosus. Lupus. 2023;32:1093–104. 10.1177/09612033231189804. [DOI] [PubMed] [Google Scholar]
- 79.Ezzat M, El-Gammasy T, Shaheen K, Shokr E. Elevated production of serum B-cell-attracting chemokine-1 (BCA-1/CXCL13) is correlated with childhood-onset lupus disease activity, severity, and renal involvement. Lupus. 2011;20:845–54. 10.1177/0961203311398513. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki T, Bracero S, Keegan J, et al. Longitudinal immune cell profiling in patients with early systemic lupus erythematosus. Arthritis Rheumatol. 2022;74:1808–21. 10.1002/art.42248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanemura S, Seki N, Tsujimoto H, et al. Role of interferons (IFNs) in the differentiation of T peripheral helper (Tph) cells. Int Immunol. 2022;34:533–44. 10.1093/intimm/dxac032. [DOI] [PubMed] [Google Scholar]
- 82.Nomura A, Mizuno M, Noto D, et al. Different spatial and temporal roles of monocytes and monocyte-derived cells in the pathogenesis of an imiquimod induced lupus model. Front Immunol. 2022;13:764557. 10.3389/fimmu.2022.764557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kong J, Li L, Lu Z, et al. MicroRNA-155 suppresses mesangial cell proliferation and TGF-β1 production via Inhibiting CXCR5-ERK signaling pathway in lupus nephritis. Inflammation. 2019;42:255–63. 10.1007/s10753-018-0889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong CK, Wong PT, Tam LS, Li EK, Chen DP, Lam CW. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J Clin Immunol. 2010;30:45–52. 10.1007/s10875-009-9325-5. [DOI] [PubMed] [Google Scholar]
- 85.Dorraji SE, Hovd AK, Kanapathippillai P, et al. Mesenchymal stem cells and T cells in the formation of tertiary lymphoid structures in lupus nephritis. Sci Rep. 2018;8:7861. 10.1038/s41598-018-26265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng Y, Zhang Y, Lin Y, et al. The CXCL13 chemokine serves as a potential biomarker to diagnose systemic lupus erythematosus with disease activity. Clin Exp Med. 2021;21:611–9. 10.1007/s10238-021-00707-x. [DOI] [PubMed] [Google Scholar]
- 87.Schiffer L, Worthmann K, Haller H, Schiffer M. CXCL13 as a new biomarker of systemic lupus erythematosus and lupus nephritis - from bench to bedside? Clin Exp Immunol. 2015;179:85–9. 10.1111/cei.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He N, Chen WL, Long KX, Zhang X, Dong GF. Association of serum CXCL13 with intrarenal ectopic lymphoid tissue formation in lupus nephritis. J Immunol Res. 2016;2016:4832543. 10.1155/2016/4832543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Worthmann K, Gueler F, von Vietinghoff S, et al. Pathogenetic role of glomerular CXCL13 expression in lupus nephritis. Clin Exp Immunol. 2014;178:20–7. 10.1111/cei.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu X, Guo J, Ding R, Lv B, Bi L. CXCL13 blockade attenuates lupus nephritis of MRL/lpr mice. Acta Histochem. 2015;117:732–7. 10.1016/j.acthis.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Huang MW, Stock AD, Putterman C. CXCL13 neutralization attenuates neuropsychiatric manifestations in lupus-prone mice. Front Immunol. 2021;12:763065. 10.3389/fimmu.2021.763065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu B, Wang W, Zhan Y, et al. CXCL13, CCL4, and sTNFR as circulating inflammatory cytokine markers in primary and SLE-related autoimmune hemolytic anemia. J Transl Med. 2015;13:112. 10.1186/s12967-015-0474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Faissner S, Plemel JR, Gold R, Yong VW. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019;18:905–22. 10.1038/s41573-019-0035-2. [DOI] [PubMed] [Google Scholar]
- 94.Plemel JR, Liu WQ, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov. 2017;16:617–34. 10.1038/nrd.2017.115. [DOI] [PubMed] [Google Scholar]
- 95.DiSano KD, Gilli F, Pachner AR. Intrathecally produced CXCL13: a predictive biomarker in multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6:2055217320981396. 10.1177/2055217320981396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iwanowski P, Losy J, Kramer L, Wójcicka M, Kaufman E. CXCL10 and CXCL13 chemokines in patients with relapsing remitting and primary progressive multiple sclerosis. J Neurol Sci. 2017;380:22–6. 10.1016/j.jns.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 97.Sellebjerg F, Börnsen L, Ammitzbøll C, et al. Defining active progressive multiple sclerosis. Mult Scler. 2017;23:1727–35. 10.1177/1352458517726592. [DOI] [PubMed] [Google Scholar]
- 98.Lucchini M, De Arcangelis V, Piro G, et al. CSF CXCL13 and chitinase 3-like-1 levels predict disease course in relapsing multiple sclerosis. Mol Neurobiol. 2023;60:36–50. 10.1007/s12035-022-03060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindén M, Khademi M, Lima Bomfim I, et al. Multiple sclerosis risk genotypes correlate with an elevated cerebrospinal fluid level of the suggested prognostic marker CXCL13. Mult Scler. 2013;19:863–70. 10.1177/1352458512463482. [DOI] [PubMed] [Google Scholar]
- 100.Erhart DK, Klose V, Schäper T, Tumani H, Senel M. CXCL13 in cerebrospinal fluid: clinical value in a large cross-sectional study. Int J Mol Sci. 2023. 10.3390/ijms25010425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–11. 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 102.Khademi M, Kockum I, Andersson ML, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler. 2011;17:335–43. 10.1177/1352458510389102. [DOI] [PubMed] [Google Scholar]
- 103.Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine. 2016;77:227–37. 10.1016/j.cyto.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 104.Holm Hansen R, Talbot J, Højsgaard Chow H, et al. Increased intrathecal activity of follicular helper t cells in patients with relapsing-remitting multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2022. 10.1212/nxi.0000000000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schropp V, Chunder R, Dietel B, Tacke S, Kuerten S. The presence of cerebellar B cell aggregates is associated with a specific chemokine profile in the cerebrospinal fluid in a mouse model of multiple sclerosis. J Neuroinflammation. 2023;20:18. 10.1186/s12974-023-02695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Magliozzi R, Mazziotti V, Montibeller L, et al. Cerebrospinal fluid IgM levels in association with inflammatory pathways in multiple sclerosis patients. Front Cell Neurosci. 2020;14:569827. 10.3389/fncel.2020.569827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ragheb S, Li Y, Simon K, et al. Multiple sclerosis: BAFF and CXCL13 in cerebrospinal fluid. Mult Scler. 2011;17:819–29. 10.1177/1352458511398887. [DOI] [PubMed] [Google Scholar]
- 108.Negron A, Stüve O, Forsthuber TG. Ectopic lymphoid follicles in multiple sclerosis: centers for disease control? Front Neurol. 2020;11:607766. 10.3389/fneur.2020.607766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eggers EL, Michel BA, Wu H, et al. Clonal relationships of CSF B cells in treatment-naive multiple sclerosis patients. JCI Insight. 2017. 10.1172/jci.insight.92724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mitsdoerffer M, Peters A. Tertiary lymphoid organs in central nervous system autoimmunity. Front Immunol. 2016;7:451. 10.3389/fimmu.2016.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Langley RG, Krueger GG, Griffiths CE. (2005) Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 64 Suppl 2:ii18–23; discussion ii24–5.10.1136/ard.2004.033217 [DOI] [PMC free article] [PubMed]
- 112.Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983–94. 10.1016/s0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 113.Liu J, Chang HW, Huang ZM, et al. Single-cell RNA sequencing of psoriatic skin identifies pathogenic Tc17 cell subsets and reveals distinctions between CD8(+) T cells in autoimmunity and cancer. J Allergy Clin Immunol. 2021;147:2370–80. 10.1016/j.jaci.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cañete JD, Santiago B, Cantaert T, et al. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann Rheum Dis. 2007;66:720–6. 10.1136/ard.2006.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamid Q, Tulic MK, Liu MC, Moqbel R. Inflammatory cells in asthma: mechanisms and implications for therapy. J Allergy Clin Immunol. 2003. 10.1067/mai.2003.22. [DOI] [PubMed] [Google Scholar]
- 116.Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 2010;3:537–44. 10.1038/mi.2010.52. [DOI] [PubMed] [Google Scholar]
- 117.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–31. 10.1016/s0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 118.Loureiro-Amigo J, Franco-Jarava C, Perurena-Prieto J, Palacio C, Martínez-Valle F, Soláns-Laqué R. Serum CXCL13, BAFF, IL-21 and IL-22 levels are related to disease activity and lymphocyte profile in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2021;39(Suppl 133):131–9. 10.55563/clinexprheumatol/fp741f. [DOI] [PubMed] [Google Scholar]
- 119.He J, Xu C, Zhu Y, et al. Clinical significance of the expression levels of serum transforming growth factor-β and CXC type chemokine ligand 13 in primary Sjogren’s syndrome patients. Clin Rheumatol. 2023;42:3283–8. 10.1007/s10067-023-06783-6. [DOI] [PubMed] [Google Scholar]
- 120.Wang R, Yang Y, Liu X, Lei L, Qi X. Abnormal expression of CXCL13, MIF and IL-35 in patients with primary Sjögren’s syndrome and its relationship with disease severity. Cent Eur J Immunol. 2023;48:144–9. 10.5114/ceji.2023.127536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blokland SLM, Hillen MR, van Vliet-Moret FM, et al. Salivary gland secretome: a novel tool towards molecular stratification of patients with primary Sjögren’s syndrome and non-autoimmune sicca. RMD Open. 2019;5:e000772. 10.1136/rmdopen-2018-000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu T, Pan Z, Zhang N. Elevated CXCL13 in primary Sjögren’s syndrome and its correlation with disease activity: a systematic review and meta-analysis. Clin Rheumatol. 2022;41:2791–802. 10.1007/s10067-022-06210-2. [DOI] [PubMed] [Google Scholar]
- 123.Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjögren’s syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol. 2013;94:1079–89. 10.1189/jlb.0113036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barone F, Bombardieri M, Rosado MM, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren’s syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180:5130–40. 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 125.Wu H, Chen X, Gu F, et al. CP-25 alleviates antigen-induced experimental Sjögren’s syndrome in mice by inhibiting JAK1-STAT1/2-CXCL13 signaling and interfering with B-cell migration. Lab Invest. 2021;101:1084–97. 10.1038/s41374-020-0453-0. [DOI] [PubMed] [Google Scholar]
- 126.Peck AB, Nguyen CQ, Ambrus J. Early covert appearance of marginal zone B cells in salivary glands of sjögren’s syndrome-susceptible mice: initiators of subsequent overt clinical disease. Int J Mol Sci. 2021. 10.3390/ijms22041919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mathes AL, Christmann RB, Stifano G, et al. Global chemokine expression in systemic sclerosis (SSc): CCL19 expression correlates with vascular inflammation in SSc skin. Ann Rheum Dis. 2014;73:1864–72. 10.1136/annrheumdis-2012-202814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pay S, Musabak U, Simsek I, et al. Synovial lymphoid neogenetic factors in Behçet’s synovitis: do they play a role in self-limiting and subacute course of arthritis? Clin Exp Rheumatol. 2007;25:S21–6. [PubMed] [Google Scholar]
- 129.De Paepe B, Creus KK, De Bleecker JL. Role of cytokines and chemokines in idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2009;21:610–6. 10.1097/BOR.0b013e3283317b31. [DOI] [PubMed] [Google Scholar]
- 130.Abu El-Asrar AM, Berghmans N, Al-Obeidan SA, et al. The cytokine interleukin-6 and the chemokines CCL20 and CXCL13 are novel biomarkers of specific endogenous uveitic entities. Invest Ophthalmol Vis Sci. 2016;57:4606–13. 10.1167/iovs.16-19758. [DOI] [PubMed] [Google Scholar]
- 131.Tomé C, Oliveira-Ramos F, Campanilho-Marques R, et al. Children with extended oligoarticular and polyarticular juvenile idiopathic arthritis have alterations in B and T follicular cell subsets in peripheral blood and a cytokine profile sustaining B cell activation. RMD Open. 2023. 10.1136/rmdopen-2022-002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang BC, Cao DL, Zhang X, et al. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest. 2016;126:745–61. 10.1172/jci81950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma L, Yu L, Jiang BC, et al. ZNF382 controls mouse neuropathic pain via silencer-based epigenetic inhibition of Cxcl13 in DRG neurons. J Exp Med. 2021. 10.1084/jem.20210920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bagaeva LV, Rao P, Powers JM, Segal BM. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176:7676–85. 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- 135.Shen Y, Zhang Y, Chen L, et al. Chemokine CXCL13 acts via CXCR5-ERK signaling in hippocampus to induce perioperative neurocognitive disorders in surgically treated mice. J Neuroinflammation. 2020;17:335. 10.1186/s12974-020-02013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng B, Ozen Z, Zhang X, et al. CXCL13 neutralization reduces the severity of collagen-induced arthritis. Arthritis Rheum. 2005;52:620–6. 10.1002/art.20768. [DOI] [PubMed] [Google Scholar]
- 137.Klimatcheva E, Pandina T, Reilly C, et al. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16:6. 10.1186/s12865-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Finch DK, Ettinger R, Karnell JL, Herbst R, Sleeman MA. Effects of CXCL13 inhibition on lymphoid follicles in models of autoimmune disease. Eur J Clin Invest. 2013;43:501–9. 10.1111/eci.12063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.