Abstract
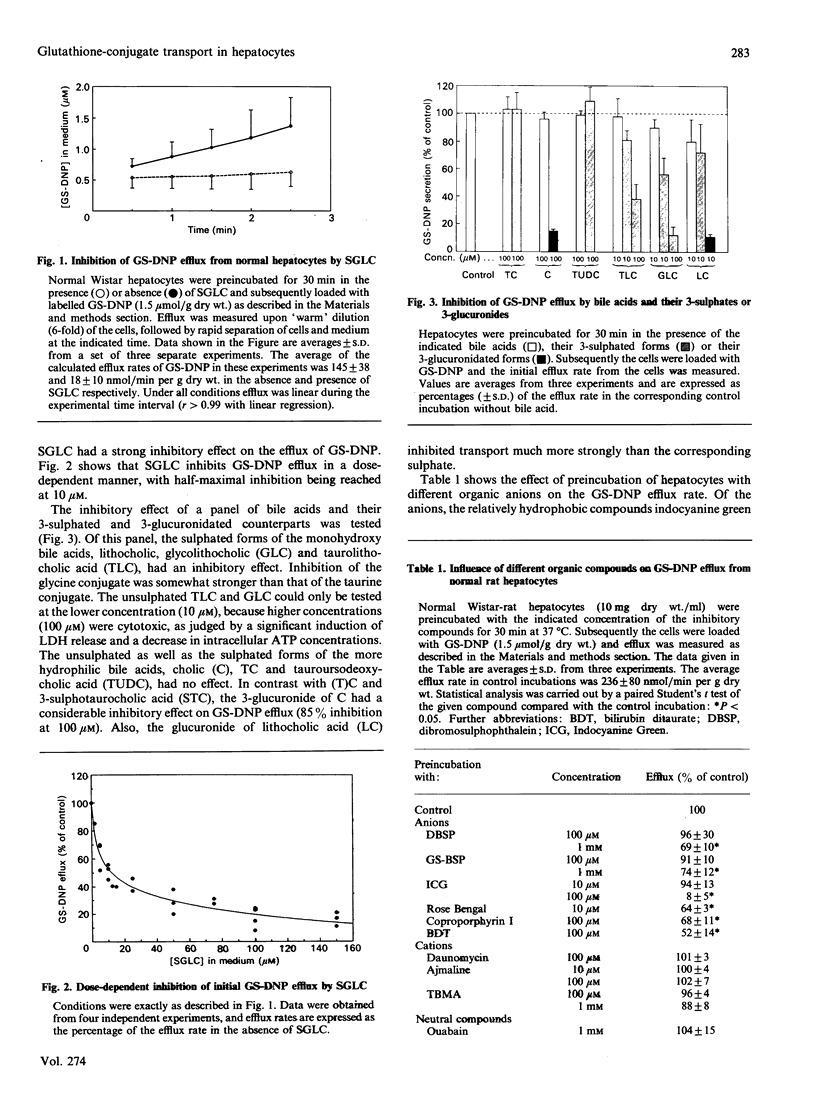
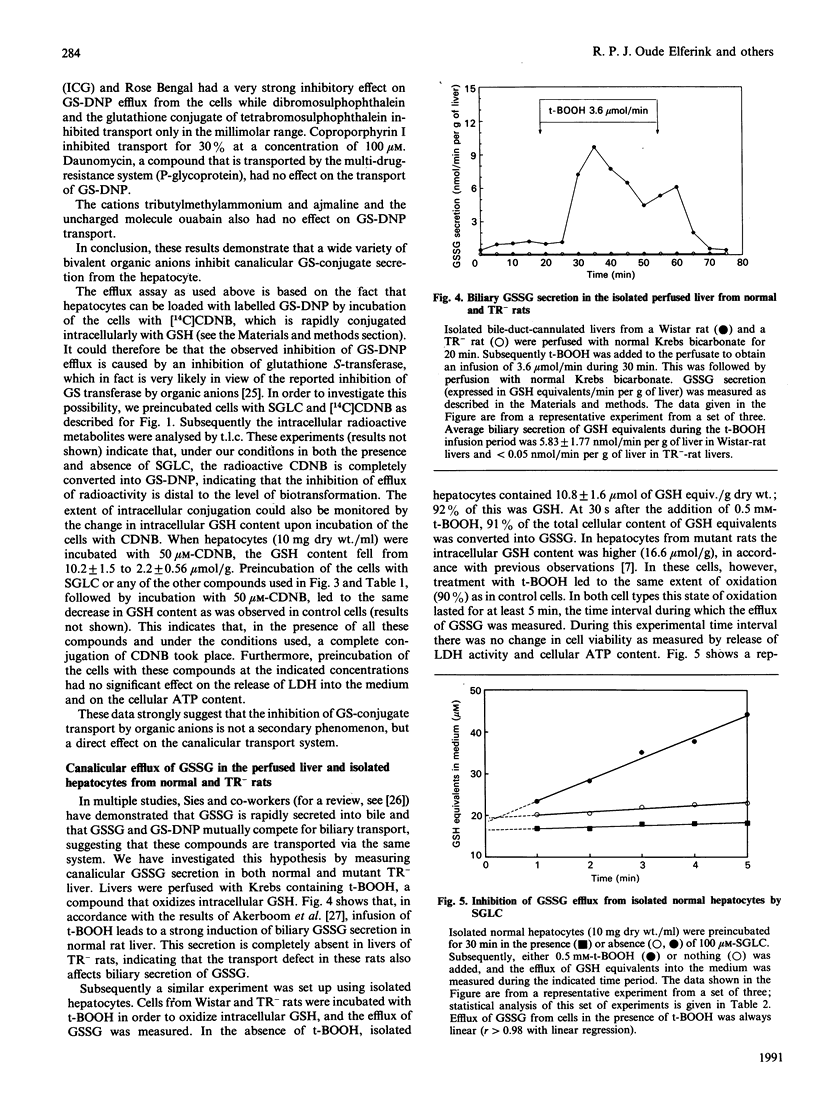
The effect of a spectrum of organic compounds on the secretion of a model organic anion, dinitrophenylglutathione (GS-DNP), by hepatocytes was tested. Previous experiments have demonstrated that the secretion of GS-DNP from isolated rat hepatocytes is predominantly mediated by a canalicular transport system for this compound. Preincubation of isolated rat hepatocytes with the bile acids cholic acid (C), taurocholic acid (TC), tauroursodeoxycholic acid (TUDC) and glyco- or tauro-lithocholic acid (GLC or TLC) had no effect on the initial efflux rate of GS-DNP. In contrast, the 3-sulphates of GLC (SGLC) and TLC (STLC) did inhibit GS-DNP efflux; half-maximal inhibition with SGLC was reached with 10 microM. The 3-O-glucuronides of both cholate and lithocholate (GlucLC) were even more potent inhibitors of transport; 10 microM-GlucLC inhibited GS-DNP transport by 89%. Other cholephilic organic anions also inhibited GS-DNP secretion, albeit at higher concentrations; at 100 microM, bilirubin ditaurate, an analogue of bilirubin diglucuronide, inhibited transport by 48%. On the other hand, a number of cholephilic cationic and neutral compounds had no effect on GS-DNP efflux. The hepatobiliary secretion of oxidized glutathione (GSSG) was also investigated. In normal isolated perfused rat liver, extensive biliary secretion of GSSG was observed upon intracellular oxidation of reduced glutathione (GSH). GSSG was also actively secreted from isolated normal hepatocytes, and this secretion could be inhibited by 95% by incubation of the cells with 100 microM-SGLC. In contrast, biliary secretion was absent in the isolated perfused liver and in isolated hepatocytes from TR- mutant rats with a hereditary conjugated hyperbilirubinaemia. These results show that the canalicular efflux of GSSG and GS conjugates can be inhibited by a wide variety of polyvalent organic anions, but not by cations, neutral compounds and unianionic bile acids. This suggests that a multispecific organic-anion transporter is responsible for transport of these polyvalent anions, which is in close agreement with the fact that the biliary transport of all these compounds is defective in the mutant TR4 rat.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bilzer M., Sies H. Competition between transport of glutathione disulfide (GSSG) and glutathione S-conjugates from perfused rat liver into bile. FEBS Lett. 1982 Apr 5;140(1):73–76. doi: 10.1016/0014-5793(82)80523-1. [DOI] [PubMed] [Google Scholar]
- Akerboom T. P., Sies H. Transport of glutathione, glutathione disulfide, and glutathione conjugates across the hepatocyte plasma membrane. Methods Enzymol. 1989;173:523–534. doi: 10.1016/s0076-6879(89)73036-6. [DOI] [PubMed] [Google Scholar]
- Balistreri W. F., Suchy F. J., Farrell M. K., Heubi J. E. Pathologic versus physiologic cholestasis: elevated serum concentration of a secondary bile acid in the presence of hepatobiliary disease. J Pediatr. 1981 Mar;98(3):399–402. doi: 10.1016/s0022-3476(81)80702-0. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Truong A. T. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am J Physiol. 1989 Jan;256(1 Pt 1):G22–G30. doi: 10.1152/ajpgi.1989.256.1.G22. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. Biochemistry of bile secretion. Biochem J. 1987 Jun 1;244(2):249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cradock J. C., Egorin M. J., Bachur N. R. Daunorubicin biliary excretion and metabolism in the rat. Arch Int Pharmacodyn Ther. 1973 Mar;202(1):48–61. [PubMed] [Google Scholar]
- Elferink R. P., Ottenhoff R., Liefting W., de Haan J., Jansen P. L. Hepatobiliary transport of glutathione and glutathione conjugate in rats with hereditary hyperbilirubinemia. J Clin Invest. 1989 Aug;84(2):476–483. doi: 10.1172/JCI114189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto J., Kato H., Hasegawa F., Nambara T. Synthesis of monosulfates of unconjugated and conjugated bile acids. Chem Pharm Bull (Tokyo) 1979 Jun;27(6):1402–1411. doi: 10.1248/cpb.27.1402. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Groothuis G. M., Peters W. H., Meijer D. F. Selective hepatobiliary transport defect for organic anions and neutral steroids in mutant rats with hereditary-conjugated hyperbilirubinemia. Hepatology. 1987 Jan-Feb;7(1):71–76. doi: 10.1002/hep.1840070116. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Oude Elferink R. P. Hereditary hyperbilirubinemias: a molecular and mechanistic approach. Semin Liver Dis. 1988 May;8(2):168–178. doi: 10.1055/s-2008-1040537. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Peters W. H., Lamers W. H. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985 Jul-Aug;5(4):573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Klaassen C. D., Watkins J. B., 3rd Mechanisms of bile formation, hepatic uptake, and biliary excretion. Pharmacol Rev. 1984 Mar;36(1):1–67. [PubMed] [Google Scholar]
- Kuipers F., Bijleveld C. M., Kneepkens C. M., van Zanten A., Fernandes J., Vonk R. J. Sulphated lithocholic acid conjugates in serum from children with hepatic and intestinal diseases. Scand J Gastroenterol. 1985 Dec;20(10):1255–1261. doi: 10.3109/00365528509089286. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Enserink M., Havinga R., van der Steen A. B., Hardonk M. J., Fevery J., Vonk R. J. Separate transport systems for biliary secretion of sulfated and unsulfated bile acids in the rat. J Clin Invest. 1988 May;81(5):1593–1599. doi: 10.1172/JCI113493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers F., Radominska A., Zimniak P., Little J. M., Havinga R., Vonk R. J., Lester R. Defective biliary secretion of bile acid 3-O-glucuronides in rats with hereditary conjugated hyperbilirubinemia. J Lipid Res. 1989 Dec;30(12):1835–1845. [PubMed] [Google Scholar]
- Kupferberg H. J., Schankl L. S. Biliary secretion of ouabain-3H and its uptake by liver slices in the rat. Am J Physiol. 1968 May;214(5):1048–1053. doi: 10.1152/ajplegacy.1968.214.5.1048. [DOI] [PubMed] [Google Scholar]
- Meier P. J., St Meier-Abt A., Barrett C., Boyer J. L. Mechanisms of taurocholate transport in canalicular and basolateral rat liver plasma membrane vesicles. Evidence for an electrogenic canalicular organic anion carrier. J Biol Chem. 1984 Aug 25;259(16):10614–10622. [PubMed] [Google Scholar]
- Mia A. S., Gronwall R. R., Cornelius C. E. Unconjugated bilirubin transport in normal and mutant Corriedale sheep with Dubin-Johnson syndrome. Proc Soc Exp Biol Med. 1970 Oct;135(1):33–37. doi: 10.3181/00379727-135-34981. [DOI] [PubMed] [Google Scholar]
- Okudaira K., Sawada Y., Sugiyama Y., Iga T., Hanano M. Effects of basic drugs on the hepatic transport of cardiac glycosides in rats. Biochem Pharmacol. 1988 Aug 1;37(15):2949–2955. doi: 10.1016/0006-2952(88)90280-8. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Ottenhoff R., Liefting W. G., Schoemaker B., Groen A. K., Jansen P. L. ATP-dependent efflux of GSSG and GS-conjugate from isolated rat hepatocytes. Am J Physiol. 1990 May;258(5 Pt 1):G699–G706. doi: 10.1152/ajpgi.1990.258.5.G699. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., de Haan J., Lambert K. J., Hagey L. R., Hofmann A. F., Jansen P. L. Selective hepatobiliary transport of nordeoxycholate side chain conjugates in mutant rats with a canalicular transport defect. Hepatology. 1989 Jun;9(6):861–865. doi: 10.1002/hep.1840090612. [DOI] [PubMed] [Google Scholar]
- Ruetz S., Hugentobler G., Meier P. J. Functional reconstitution of the canalicular bile salt transport system of rat liver. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6147–6151. doi: 10.1073/pnas.85.16.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetta P., Di Cola D., Federici G. Alkaline hydrolysis of N-ethylmaleimide allows a rapid assay of glutathione disulfide in biological samples. Anal Biochem. 1986 Apr;154(1):205–208. doi: 10.1016/0003-2697(86)90516-6. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Klein P. D. Formylated bile acids: improved synthesis, properties, and partial deformylation. Steroids. 1977 May;29(5):635–648. doi: 10.1016/0039-128x(77)90015-0. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Klein P. D. Synthesis of sulfate esters of lithocholic acid, glycolithocholic acid, and taurolithocholic acid with sulfur trioxide-triethylamine. J Lipid Res. 1977 Jul;18(4):491–495. [PubMed] [Google Scholar]
- West I. C. What determines the substrate specificity of the multi-drug-resistance pump? Trends Biochem Sci. 1990 Feb;15(2):42–46. doi: 10.1016/0968-0004(90)90171-7. [DOI] [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]
- de Vries M. H., Redegeld F. A., Koster A. S., Noordhoek J., de Haan J. G., Oude Elferink R. P., Jansen P. L. Hepatic, intestinal and renal transport of 1-naphthol-beta-D-glucuronide in mutant rats with hereditary-conjugated hyperbilirubinemia. Naunyn Schmiedebergs Arch Pharmacol. 1989 Nov;340(5):588–592. doi: 10.1007/BF00260615. [DOI] [PubMed] [Google Scholar]


