Abstract
Background
Oral corticosteroids (OCS) are effective anti-inflammatory agents used across a range of conditions. However, substantial evidence associates their use with increased risks for adverse events (AEs), causing high burden on healthcare resources. Emerging biologics present as alternative agents, enabling the reduction of OCS use. However, current modelling approaches may underestimate their effects by not capturing OCS-sparing effects. In this study, we present a modelling approach designed to capture the health economic benefits of OCS-sparing regimens and agents.
Methods
We developed a disease-agnostic model using a UK health technology assessment (HTA) perspective, with discounting of 3.5% for costs and outcomes, a lifetime horizon, and 4-week cycle length. The model structure included type 2 diabetes mellitus, established cardiovascular disease, and osteoporosis as key AEs and drivers of morbidity and mortality, as well as capturing transient events. Quality-adjusted life-years (QALYs), life-years, and costs were determined for OCS-only and OCS-sparing treatment arms. Outcomes were determined using baseline 50% OCS-sparing, considering several OCS average daily doses (5, 10, 15 mg).
Results
A treatment regimen with 50% OCS dose-sparing led to lifetime incremental cost savings per patient of £1107 (95% confidence interval £1014–£1229) at 5 mg, £2403 (£2203–£2668) at 10 mg, and £19,501 (£748–£51,836) at 15 mg. Patients also gained 0.033 (0.030–0.036) to 0.356 (0.022–2.404) QALYs dependent on dose. The benefits of OCS sparing were long-term, plateauing after 35–40 years of treatment.
Conclusions
We present a modelling approach that captures additional long-term health economic benefits from OCS sparing that would otherwise be missed from current modelling approaches. These results may help inform future decision making for emerging OCS-sparing therapeutics by comparing them against the cost of such treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-024-00520-8.
Key Points for Decision Makers
| Oral corticosteroids (OCS) are associated with a range of adverse events, whose costs are not captured in current modelling approaches; as such, we designed a modelling approach that considers three major chronic adverse events associated with OCS (type 2 diabetes mellitus, established cardiovascular disease, and osteoporosis) and a range of transient adverse events. |
| This approach demonstrated the long-term benefits of OCS-sparing, capturing cost savings associated with avoiding chronic and transient OCS-related adverse events. |
| The modelling approach described in this study allows for more accurate predictions and quantification of the value of OCS sparing and therefore avoiding OCS-related complications, providing crucial insights needed to inform future healthcare decision making in this area. |
Introduction
Oral corticosteroids (OCS) are highly effective anti-inflammatory treatments that have been widely used for decades to treat a range of inflammatory conditions, including autoimmune disorders, allergic reactions, chronic obstructive pulmonary disease (COPD), asthma, dermatitis, and many more. However, increasing evidence has associated the use of OCS with increased risk of serious adverse outcomes, ranging from chronic conditions such as type 2 diabetes mellitus (T2DM), osteoporosis, and cardiovascular disease (CVD), to transient events such as pneumonia and peptic ulcers [1–4]. OCS-related adverse events (AEs) place a high burden on healthcare systems, with OCS treatment shown to be one of the most common causes of hospitalisation due to adverse drug events [5, 6]. Risks are increased even from short-term OCS use [7–9], with cumulative lifetime OCS dose being the main driving factor responsible for increased risk of AEs [10–13], supporting recent calls for stewardship on the use of OCS [14, 15].
Despite these risks, OCS continue to be used worldwide today for a range of indications. Up to 60% of patients with severe asthma have received long-term OCS treatment [11, 16], and over a 20-year follow-up, 32.2% of patients with rheumatoid arthritis were prescribed long-term OCS treatment lasting 1 year or longer [17]. Treatment guidelines continue to recommend OCS as a treatment option, although they are beginning to recognise the risks associated with OCS use, recommending certain restrictions be considered. For example, the recent Global Initiative for Asthma (GINA) guidelines recommend the use of OCS only after treatment with biologic therapies, to reduce the cumulative exposure and long-term adverse effects of OCS [18]. Likewise, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 report only recommends OCS for the treatment of severe acute exacerbations of COPD, for a limited period of no more than 5 days [11]. Where possible, guidelines for OCS recommend reducing dosage; however, this does not circumvent the issues around cumulative OCS dose, and studies have shown that even at low doses, long-term OCS treatment can lead to irreversible organ damage and cumulative long-term adverse effects [11].
As such, there remains a need to reduce OCS use to avoid treatment-related AEs, and research has now turned to alternative anti-inflammatory agents, such as biologics. Biologics include antibodies that target inflammatory cytokines or their receptors, or recombinant proteins, and have shown promise in reducing the need for OCS [19, 20]. Reducing OCS use is a key factor considered by National Institute for Health and Care Excellence (NICE) technology appraisals for emerging therapies [21, 22], and avoiding OCS-related AEs may have a substantial impact on economic evaluations of these treatments. Despite this, OCS-sparing effects are frequently not captured in economic modelling for health technology appraisals (HTAs) or reimbursement, with many models ignoring OCS-related AEs or implementing a simplified impact of OCS [23].
In the recent NICE technology appraisals for tezepelumab and benralizumab, biologics recommended for the treatment of severe asthma and severe eosinophilic asthma, respectively, the effects of long-term OCS maintenance are considered to impact quality of life and resource utilisation through the risk of developing comorbidities [21, 23]. In these models, 10 AEs commonly associated with OCS exposure were included as transient AEs with incident rates based on the proportion of patients’ average daily dose in that model cycle. However, during the tezepelumab appraisal, expert review noted that the full effects of OCS-sparing are unlikely to be fully realised within current models [23]. This underestimation is primarily due to the interpretation that all AEs are transient events incurring a one-off incidence cost and quality-adjusted life-year (QALY) loss, which underestimates the long-term costs and QALY losses from conditions such as T2DM, CVD, chronic kidney disease (CKD), and osteoporosis.
As such, current HTAs may undervalue OCS-sparing treatments, and therefore a new modelling approach is required that captures the broader impacts of OCS-sparing agents. To address this evidence gap, and better inform future healthcare decision making, we designed a modelling approach to capture the health economic outcomes related to reducing OCS use.
Methods
Model Overview
We have developed a disease agnostic model in Microsoft Excel® 365 (Microsoft Corporation, Redmond, WA, USA) that captures the costs and health economic outcomes associated with OCS AEs and estimates the potential benefits of OCS sparing. The model uses a UK HTA perspective, with discounting of 3.5% for costs and outcomes and a lifetime time horizon to capture the long-term burden of OCS-related AEs on the UK population [24]. To balance the requirement to follow changes in patient outcomes over time with a practical model run time, a model cycle length of 4 weeks was selected. Treatment arms include a control arm of OCS alone versus an intervention arm of a hypothetical OCS-sparing scenario, for example comprising OCS in combination with an OCS-sparing agent.
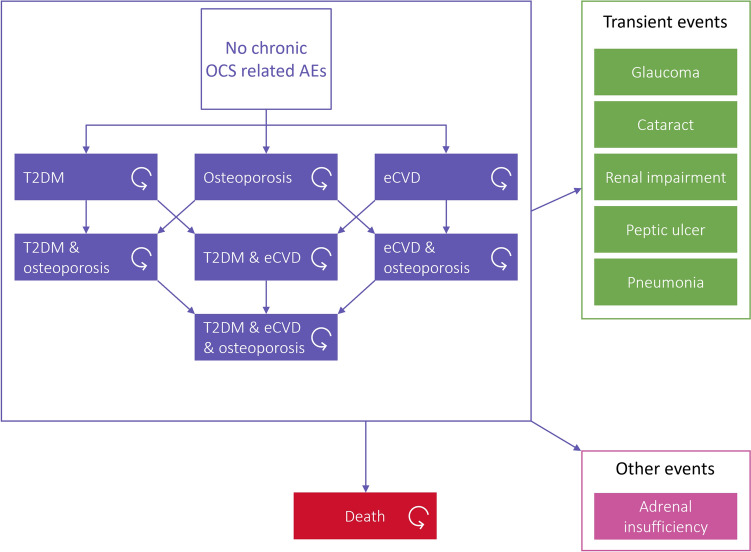
The model structure includes three key AEs related to OCS treatment: T2DM, osteoporosis, and established CVD (eCVD), including heart failure, chronic impacts of myocardial infarction and stroke. These AEs were selected as they are key drivers of morbidity and mortality from OCS AEs [25, 26]. Additional AEs that are commonly associated with OCS in the literature but are considered as one-off events are captured in a simplified manner as transient events, driven either by incidence, for acute events, or prevalence, for chronic events. These include glaucoma, cataract, renal impairment, peptic ulcer and pneumonia [24]. The model structure is presented in Fig. 1.
Fig. 1.
Model structure. AEs adverse events, eCVD established cardiovascular disease, OCS oral corticosteroids. T2DM type 2 diabetes mellitus
All patients start at baseline with no OCS-related AEs, and as time progresses, patients may experience AEs. Patients who have previously experienced one type of AE may go on to experience others, with interactions between the three main events—T2DM, osteoporosis, and eCVD—influencing each patient’s risk of experiencing a future event. All analyses were run for both deterministic and probabilistic analysis using 1000 randomised runs; complete information on the distributions assumed and maximum upper and lower bounds applied are detailed in the input summary tables in the electronic supplementary material (ESM). Probabilistic results are presented where available, with confidence intervals to capture uncertainty. If the results of the probabilistic analysis show significant skew, both probabilistic and deterministic results are presented.
Patient Characteristics and Oral Corticosteroid (OCS) Dose Dynamics
In order to demonstrate the ability of the model to capture the benefits of OCS sparing, the model compares a scenario with all patients treated with OCS with a hypothetical OCS-sparing scenario, in a patient population that aligns to an Observational and Pragmatic Research Institute (OPRI) real-world evidence (RWE) study. The OPRI RWE study is a matched historical cohort study conducted using the Optimum Patient Care Research Database (OPCRD) and the Clinical Practice Research Datalink (CPRD) database [1, 27]. In brief, the OPRI study investigated the impact of OCS exposure on disease onset, burden and healthcare resource use over a minimum 2-year timescale in a broad patient population: patients diagnosed with conditions where OCS may be prescribed. Patient characteristics in the model (age 57.0 years, 58.6% female) were defined based on an alternative published UK RWE study [28].
The model assumes that all patients are taking OCS at initiation, with no prior exposure and no prior background rates for any AE. Arbitrary reductions in OCS dose were tested to demonstrate the potential sensitivity of model outcomes to the effectiveness of OCS sparing. Baseline OCS sparing is set at 50% following clinician feedback that a biologic would be considered OCS sparing if it resulted in a > 50% reduction in OCS use. A baseline daily dose of 10 mg/day was used as a plausible mid-range dose scenario among trials of patients with severe asthma, where observed doses range from 2 to 40 mg/day [29, 30]. Different doses and OCS-sparing proportions are explored in sensitivity analyses, with a low OCS dose of 5 mg/day and a high OCS dose of 15 mg/day.
Patients may discontinue the OCS-sparing scenario, switching to OCS alone, through natural attrition. Upon discontinuation, the OCS dose immediately returns to that of the ‘OCS only’ arm. The OCS dose is captured using cumulative OCS dose over time, reflecting the progressive and cumulative nature of OCS AEs, which do not resolve upon OCS discontinuation.
Adverse Event Risk
OCS AE risk is captured using adjusted regression hazard ratios for each AE, applied to a baseline ‘non-OCS’ population risk. This is informed by the OPRI RWE study [1]. The calculation is presented in Eq. (1). The same risk equation is applied across both arms. The value of OCS-sparing in avoiding events accumulates over time, dependent on the extent that the patient’s cumulative exposure to OCS is reduced.
| 1 |
Event risk per model cycle. The AE event risk for patients is defined as 1 minus the exponential to the power of the baseline risk multiplied by an exponent of the additional risk per gram to the power of the cumulative dosage in grams, where c is the cycle length adjustment, is the general population risk for patients with no prior exposure to OCS, is the additional hazard per cumulative gram of OCS, as an HR, and is the cumulative OCS dose.
The relationship between OCS dose and beta values, informing hazard ratios, is assumed to be linear.
As previously described, AEs that are chronic in nature, such as T2DM, are modelled using prevalence, reflecting that these events continue for the remainder of the patient’s life. AEs that are acute in nature are modelled using incidence, reflecting the ‘one-off’ nature of these events.
Adrenal insufficiency is a well-recognised AE of treatment with OCS and is also captured, despite not being included in the OPRI RWE study [31, 32]. Patients experiencing adrenal insufficiency remain dependent on OCS and are unable to lower their OCS dose, and therefore their cumulative OCS dose increases regardless of any attempts to avoid or reduce OCS (e.g. treatment with OCS-sparing agents). At a population level, because exposure to OCS increases a patient’s likelihood of experiencing adrenal insufficiency, at high cumulative OCS doses the effectiveness of OCS sparing is reduced, potentially creating an ‘adrenal insufficiency trap’ where OCS-sparing regimens are ineffective at reducing cumulative exposure to OCS.
Interdependencies between the three key OCS-related AEs—T2DM, osteoporosis, and CVD—are captured by applying hazard ratios where appropriate and where data are available. This includes between the chronic events and the risk of additional AEs, but not the interdependencies between these additional AEs. Tables detailing the hazard ratios applied to patients experiencing AEs are provided in the ESM.
Costs
Costs are applied per AE, either reflecting an ongoing cost for the remainder of the patient’s life, or within a single cycle for acute events. Costs for AEs primarily reflect the associated healthcare resource use. Where patients experience multiple AEs, a simplifying conservative assumption is made that costs align to whichever AE has a greater cost. Treatment costs are minimal for OCS but are included for completeness, using the cost of prednisolone 5 mg tablets, sourced from the electronic market information tool (eMIT) database as reported in TA880 [23, 33]. Intervention arm treatment costs are not included for this analysis; therefore, this analysis should be interpreted as the cost savings from OCS sparing to health care systems. Cost inputs included in the model are detailed in the ESM. Costs were sourced from the literature; where necessary, costs were inflated to 2022 values using Personal Social Services Research Unit (PSSRU) inflation indices [34].
Utilities
A disutility per event is applied on top of baseline utility, reflecting a population with inflammatory conditions requiring OCS. Baseline utility is calculated using a weighted average of disease areas from a RWE study and utility values from a UK population where an average baseline utility of 0.6519 was applied [28, 35]. Utility values are capped at age-dependent levels from the study by Ara and Brazier [36], estimated using EQ-5D responses from Health Survey for England and using time trade-off (TTO) values from the study by Dolan et al. [37]. Multiple comorbid AEs are able to occur simultaneously, however where patients experience multiple comorbid AEs, a conservative simplifying assumption is made that the disutility applied aligns to whichever AE disutility is greatest. Utility decrements for OCS complications are sourced from the literature, using EQ-5D responses from the Medical Expenditure Panel Survey and UK specific value set, as described by Sullivan et al. [35].
Mortality
Health state-based AEs are associated with an increased mortality from baseline. Additional mortality from OCS-related AEs is applied based on hazard ratios obtained from the literature, as described in ESM Table 8, comparing patients with and without the OCS-related AE.
It is assumed that AE-related mortality also captures the additional mortality associated with transient events, although this is a conservative simplifying assumption.
Where patients experience multiple comorbid AEs, hazard ratios are applied where available from the general literature to estimate the additional risk of having multiple comorbid AEs. Where these data are not available, as a conservative assumption, the additional risk of other AEs and mortality are assumed to be equal to the largest risk for patients with at least one prior OCS-related AE. Hazard ratios applied to patients with multiple comorbidities are outlined in the tables in the ESM.
Results
In order to demonstrate the ability of the model to capture the benefits of OCS sparing, a hypothetical OCS-sparing treatment was compared with treatment with OCS alone, in a patient population that aligns to the OPRI RWE study [27, 38]. The model estimated the difference in costs, QALYs, and life-years when treating patients with a hypothetical OCS-sparing regimen compared with OCS alone.
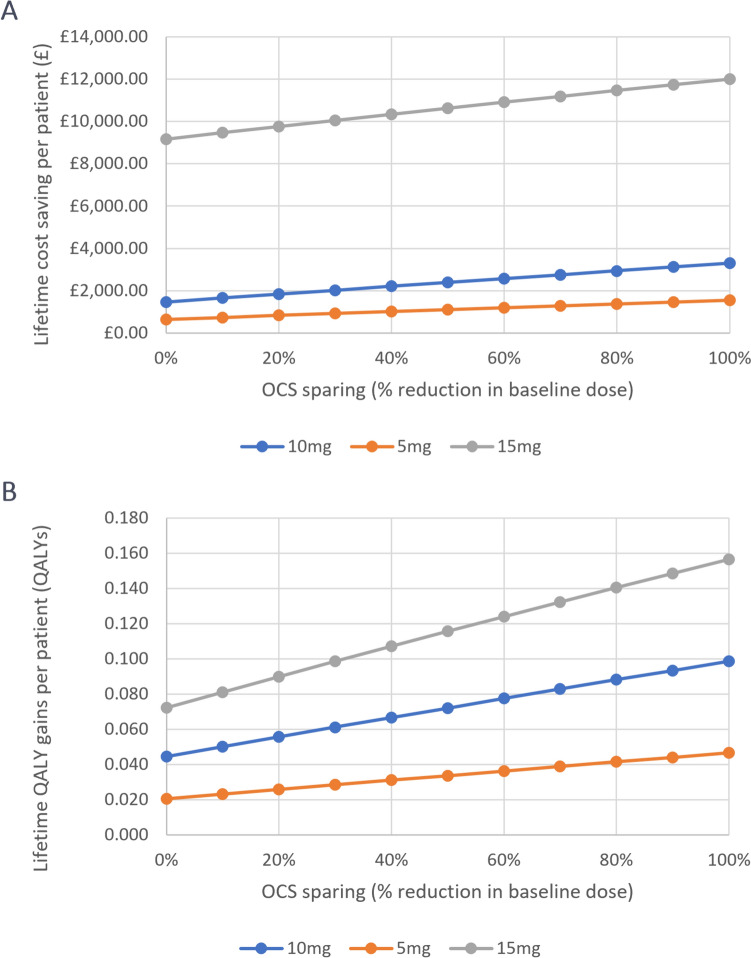
Treating patients with an OCS-sparing regimen resulted in cost savings that increased with dose (Fig. 2a). Using the probabilistic base-case scenario of a 10 mg daily dose of OCS and a 50% dose-sparing effect from the intervention, the addition of an OCS-sparing treatment demonstrated lifetime incremental cost savings of £2403 (£2203–£2668) (Table 1). OCS-sparing also demonstrated increases in QALYs and life-years gained compared with treatment with OCS alone (Fig. 2b). In the base-case, 0.071 (0.064–0.077) QALYs and 0.205 (0.197–0.213) life-years were gained over a patient’s lifetime. The life expectancy in patients receiving only OCS was 24.769 (24.201–25.329) years compared with 24.974 (24.401–25.544) years when in the hypothetical OCS-sparing arm. These health gains result from the substantial decrease in the average lifetime cumulative OCS exposure, which was 14.0 g over the lifetime of patients receiving OCS only compared with 5.6 g over the lifetime of patients in the OCS-sparing arm.
Fig. 2.
Deterministic cost savings and QALYs across patient populations with baseline OCS daily doses of 5, 10 and 15 mg. OCS oral corticosteroids, QALY quality-adjusted life-year
Table 1.
Base-case probabilistic analysis results, OCS-only daily dose of 10 mg, hypothetical 50% dose sparing from the OCS-sparing regimen scenario
| Outcome | Intervention arm (95% CI) | OCS-only arm (95% CI) | Incremental (95% CI) |
|---|---|---|---|
| Costs | £26,566 (£24,284–£29,342) | £28,969 (£26,477–£32,010) | −£2403 (−£2203 to −£2668) |
| QALYs | 10.197 (8.761–11.389) | 10.126 (8.761–11.389) | 0.071 (0.064–0.077) |
| Life years | 24.975 (24.401–25.544) | 24.769 (24.201–25.329) | 0.205 (0.197–0.213) |
CI confidence interval, OCS oral corticosteroids, QALYs quality-adjusted life-years
Additional analyses were carried out to consider the effects of higher or lower daily doses of OCS. When considering a 15 mg daily dose, lifetime incremental cost savings increased substantially to £10,627 (deterministic) and £19,501 (£51,836 to −£748, probabilistic), and for a lower daily dose of 5 mg, lifetime incremental cost savings were £1107 (£1014–£1229) (Fig. 2a). Similar results were seen with respect to QALYs, with a 15 mg daily dose resulting in a gain of either 0.356 (−0022 to 2.404, probabilistic analysis) or 0.116 (deterministic analysis, presented due to skew in probabilistic analysis) QALYs, compared with 0.033 (0.030–0.036) QALYs for the 5 mg daily dose (Fig. 2b).
The cost-saving effects of OCS-sparing resulted from the reduction in OCS-related AEs. Of the events considered, renal impairment accrued the highest costs of £17,537 (£16,615–£18,492) in the OCS-only arm in the base-case scenario (Table 2). The avoidance of this event demonstrated the greatest incremental cost saving with OCS sparing at £1403 (£1330–£1481). Notably, avoidance of adrenal insufficiency demonstrated the next-highest incremental cost savings, with the greatest relative difference between the OCS only and the intervention arms (£113 [£73–159] and £1 [£0–£1], respectively). These cost savings were due to a reduction in the time spent with chronic AEs and a reduction in the number of events experienced per patient. In the deterministic base case, a total of 0.668 years (8.0 months) spent with renal impairment was avoided in the OCS-sparing arm.
Table 2.
Breakdown of costs, probabilistic analysis
| Outcome | Intervention arm (95% CI) | OCS-only arm (95% CI) | Incremental (95% CI) |
|---|---|---|---|
| Health state costs | |||
| No OCS-related AEs | £0 (£0–£0) | £0 (£0–£0) | £0 (£0–£0) |
| T2DM | £3114 (£2992–£3231) | £3217 (£3093–£3336) | −£102 (−£105 to −£100) |
| Osteoporosis | £129 (£123–£135) | £148 (£141–£154) | −£18 (−£19 to −£18) |
| eCVD | £4558 (£2980–£6482) | £4814 (£3149–£6845) | −£256 (−£363 to −£169) |
| Following multiple events | £1659 (£1369–£2246) | £2020 (£1663–£2734) | −£362 (−£491 to −£295) |
| Treatment | |||
| OCS | £15 (£9–£21) | £37 (£23–53) | −£22 (−£31 to −£14) |
| Transient events | |||
| Glaucoma | £35 (£23−£50) | £39 (£26−£26) | −£4 (£3−£6) |
| Cataract | £129 (£84−£183) | £152 (£99−£215) | −£22 (−£32 to −£15) |
| Renal impairmenta | £16,134 (£15,871−£17,623) | £17,537 (£16,615−£18,492) | −£1403 (−£1481 to −£1330) |
| Peptic ulcer | £18 (£3−£51) | £20 (£3−£56) | −£2 (−£5 to £0) |
| Pneumonia | £411 (£403–418) | £468 (£459−£477) | −£57 (£56–£58) |
| Adrenal insufficiency | £1 (£0−£1) | £113 (£73−£159) | −£112 (−£159 to −£73) |
| Other | |||
| Death | £363 (£137−£722) | £405 (£153−£804) | −£42 (−£83 to −£16) |
| Subtotals | |||
| Total health state costs | £9460 (£7553−£11,984) | £10,199 (£8,132−£12,957) | −£730 (−£979 to −£585) |
| Total transient event costs | £16,728 (£15,871−£17,623) | £18,329 (£17,404−£19,294) | −£1601 (−£1696 to −£1516) |
| Grand total | £26,566 (£24,284−£29,342) | £28,969 (£26,477−£32,010) | −£2,403(−£2668 to −£2203) |
AEs adverse events, CI confidence interval, eCVD established cardiovascular disease, OCS oral corticosteroids, T2DM type 2 diabetes mellitus
aRenal impairment is captured per cycle but is based on prevalence and can therefore be considered as a chronic event
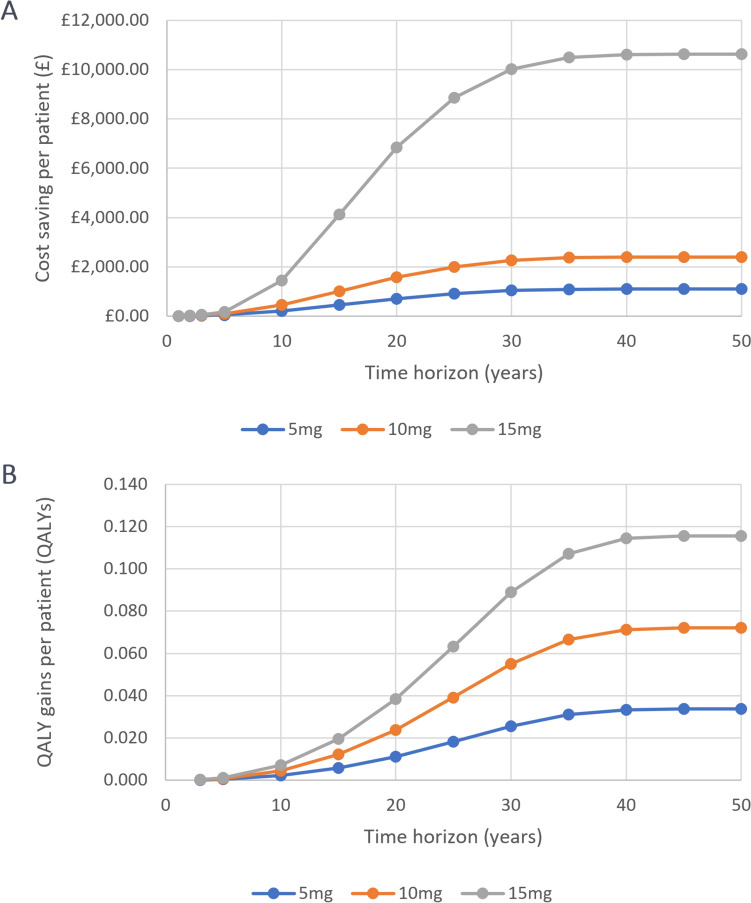
The benefits of OCS sparing accumulated over time; typically, full cost savings only plateaued after 35 years across all three dose scenarios, and QALY gains continued to accumulate for 40 years after treatment initiation (Fig. 3, Table 3). As such, the benefits gained from OCS-sparing can be considered long-term and require modelling over a lifetime horizon to fully capture them.
Fig. 3.
Cost savings and QALYs across patient populations with baseline OCS daily doses of 5, 10 and 15 mg (deterministic, by time horizon). OCS oral corticosteroids, QALY quality-adjusted life-year
Table 3.
QALY breakdown, probabilistic analysis
| Outcome | Intervention arm (95% CI) | OCS-only arm (95% CI) | Incremental (95% CI) |
|---|---|---|---|
| QALYs gained by health state | |||
| No OCS-related AEs | 8.176 (7.050–9.122) | 7.932 (6.840–8.856) | 0.244 (0.212–0.271) |
| T2DM | 0.615 (0.523–0.693) | 0.635 (0.540–0.715) | −0.020(−0.023 to −0.017) |
| Osteoporosis | 0.302 (0.259–0.340) | 0.346 (0.296–0.388) | −0.043(−0.049 to −0.037) |
| eCVD | 0.840 (0.714–0.949) | 0.887 (0.755–1.002) | −0.047(−0.054 to −0.040) |
| Following multiple events | 0.295 (0.250–0.333) | 0.360 (0.304–0.406) | −0.065 (−0.073 to −0.055) |
| Treatment | |||
| OCS | 0.000 | 0.000 | 0.000 |
| Transient events | |||
| Glaucoma | 0.000090 (0.000057–0.000129) | 0.000101 (0.000064–0.000145) | −0.000011 (−0.000016 to −0.000007 |
| Cataract | 0.000327 (0.000198–0.000487) | 0.000384 (0.000233–0.000571) | −0.000056 (−0.000084 to −0.000035) |
| Renal impairmenta | 0.030018 (0.022816–0.037427) | 0.032594 (0.024798–0.040678) | −0.002608 (−0.002007 to −0.003265) |
| Peptic ulcer | 0.000097 (0.000054–0.000146) | 0.000106 (0.000059–0.000160) | −0.000010 (−0.000015 to −0.000006 |
| Pneumonia | 0.000593 (0.000143–0.001295) | 0.000672 (0.000163–0.001476) | −0.000082 (−0.000186 to −0.000020) |
| Adrenal insufficiency | 0.000000 | 0.000000 | 0.000000 |
| Totals | |||
| Total health state utilities | 10.228 (8.796–11.437) | 10.176 (8.735–11.369) | 0.066 (0.014–0.121) |
| Total transient event disutilities | −0.031 (−0.039 to −0.023) | −0.034 (−0.042 to −0.026) | 0.003 (0.0021–0.0034) |
| Grand total | 10.197 (8.761–11.389) | 10.126 (8.761–11.389) | 0.071 (0.064–0.077) |
AEs adverse event, CI confidence interval, eCVD established cardiovascular disease, OCS oral corticosteroids, T2DM type 2 diabetes mellitus, QALY quality-adjusted life-year
aRenal impairment is captured per cycle but is based on prevalence and can therefore can be considered as a chronic event
Discussion
Summary of Key Results
The prevalence of AEs in patients taking OCS is widespread; studies report that up to 93% of patients using long-term OCS experience at least one condition linked to their use [3, 13, 39]. OCS continue to be used globally despite the well-demonstrated associations with severe adverse effects. This is in part due to their widespread availability and affordability, making these treatments particularly favoured in low- and middle-income countries [40–42]. However, in the long-term, the AEs associated with OCS treatment lead to significant healthcare costs that outweigh the initial lower treatment costs [43, 44]. As such, reducing the need for OCS treatment and offering alternative anti-inflammatories is vital. Current HTAs do not fully capture the potential benefits of OCS sparing, and therefore risk undervaluing new therapies [23]. In this study, we describe a modelling approach that allows more accurate predictions of the value of emerging treatments with OCS-sparing capabilities, which is crucial to inform future decision making. Using this model, we demonstrate that avoidance of OCS and OCS-related AEs led to improvements in patient quality of life and reductions in AE-related costs.
In the model base-case, assuming a patient population taking an average dose of 10 mg of OCS per day and an OCS-sparing agent capable of sparing 50% of that dose resulted in lifetime incremental cost savings of £2403 (£2203–£2668) and 0.071 (0.064–0.077) QALYs gained. These cost savings increased significantly when considering a population with an average daily OCS dose of 15 mg at baseline, with cost savings of £10,627 (deterministic) or £19,501 (−£51,836 to −£748, probabilistic) and QALY gains of 0.116 (deterministic) or 0.356 (−0.022 to 2.404, probabilistic) QALYs. Therefore, the introduction of an OCS-sparing treatment has the potential to reduce the number of transient and chronic AEs a patient experiences, resulting in cost savings and QALY and life-year gains. While these estimated gains may appear small, they would be incremental to the additional effects of a new treatment and would otherwise have been missed using previous modelling approaches. Therefore, they should be considered in the context of the benefits of the treatment itself. Likewise, considering all disease indications where OCS treatment is approved, almost 1% of adults in the UK are prescribed OCS, and therefore small individual cost savings have the potential to accumulate into substantial cost savings for the NHS when considering the large patient population they affect [25, 45].
Our results estimate significant additional long-term costs and QALYs incurred due to OCS exposure resulting from OCS-related AEs, especially for patients taking higher doses. This is particularly impactful for conditions such as rheumatoid arthritis or ulcerative colitis, where higher average daily doses of over 7.5 mg/day and up to 40 mg/day, respectively, are commonly administered to control symptoms [46, 47].
The cost savings modelled here are aligned with similar modelling studies considering the costs of OCS treatment. In the UK, healthcare costs for patients with asthma have been demonstrated to increase according to OCS exposure, and patients receiving maintenance OCS treatment are estimated to accrue 39% and 51% higher healthcare costs for patients with mild-moderate and severe asthma, respectively, compared with patients not receiving OCS [43, 48]. In these studies, patients receiving maintenance OCS had increased prescription costs as well as increased healthcare utilisation costs [43, 48]. This translated to an additional annual OCS-related cost per year of £224 for mild asthma and £1310 for severe asthma [43].
In addition, Asaria et al. estimated the lifetime treatment costs for the general population, defined as the expected costs of hospital admission over the average life expectancy, to be between £50,908 and £69,671 (inflated from 2011/2012 costs) [49]. Our total deterministic lifetime cost estimates due to OCS exposure (£30,277 in the OCS-only arm) represent 41.6–56.8% of these costs, demonstrating the high additional burden that OCS related-AEs represent. This additional burden is well demonstrated; in the study by Barry et al. [43], OCS-related AEs were found to increase lifetime prescription costs by 39–51% in patients with severe asthma compared with patients with moderate or no asthma.
Compared with existing models of OCS-related AEs, our model has a significant advantage in that it is the only model to include, in detail, three OCS-related chronic AEs; T2DM, eCVD, and osteoporosis, while also including other relevant AEs such as renal impairment, glaucoma, cataract, peptic ulcers, pneumonia, and adrenal insufficiency. The use of chronic health states addresses a significant weakness of prior approaches such as the previous tezepelumab and benralizumab NICE TAs [21, 23], where chronic events were considered as transient events with one-off costs and disutilities applied, resulting in an underestimation of the total cost and QALY impact from OCS-related AEs.
A direct comparison of outcomes between outcomes herein and the tezepelumab and benralizumab NICE TAs is not possible as results are redacted due to a commercial arrangement [21, 23]. The approach applied in TA278, for omalizumab for the treatment of severe persistent allergic asthma, applied the costs and health losses incurred with the excess relative risk associated with each OCS-related AE into an annual OCS cost or disability-adjusted life-year (DALY) loss per patient taking OCS per year [50]. A reanalysis by Norman et al. found incorporation of OCS AEs reduced the ICER from £50,181/QALY to £46,634/QALY [51]. Similarly, in NICE TA278, the Assessment Group found that including the adverse effects of OCS substantially reduced the potential ICER of omalizumab [50]. The outcomes from our model suggest that including the impact of avoiding OCS complications could likewise be expected to offset a significant proportion of OCS-sparing agent treatment costs (which are not considered in this model and hypothetical scenario), contribute to additional QALY gain, and therefore may have a significant impact on the cost effectiveness of OCS-sparing agents for future HTA assessments.
Limitations and Critique of Analysis
While this model accounts for several acute and chronic OCS-related transient events, it also relies on several assumptions that may be considered limitations to the analysis. For instance, mortality hazards and interactions are based on populations observed in the general literature, and therefore they may not reflect a specific OCS-eligible population. Furthermore, the cost estimates here do not take into account the treatment costs of OCS-sparing agents; in the UK, the net prices of such treatments are currently confidential and therefore could not be included in the analysis. Rather than use estimated prices, which may not reflect reality, the results are presented as the costs associated with the reduction of-related AEs, and this limitation should be taken into account when considering the cost effectiveness of OCS-sparing agents in the UK.
The population data used in this study were aligned to the OPRI RWE study, which used data from the OPCRD and CPRD databases, with patient characteristics defined based on an alternative published UK RWE study [1, 27, 28]. As such, these analyses may not be generalisable beyond the UK, and caution should be taken when comparing these results across countries. However, in a related study conducted in Italy, the costs of OCS-related AEs were predicted and were applied to epidemiological data from the Severe Asthma Network in Italy (SANI) registry [44]. In this study, the annual cost per patient for OCS-related AEs was reported at €1957.50. Averaging our costs across the life-years included, we estimate average per patient per year costs of £2183, excluding discounting. These costs align with the results reported by Canonica et al., with minor differences explained by differences in the unit costs of treating complications across countries. Together, these results demonstrate the burden of OCS-related AEs, but highlight the need for country-specific considerations for exact costings.
While the baseline characteristics of the model are likely to align well with the OCS-taking population in the UK, the OCS-related AE event incidence rates and additional risk applied to those events following OCS exposure were sourced from the OPRI study [1], which considered a younger patient population with more males. This limitation is expected to have an uncertain impact on model outcomes, as a younger population will be expected to experience a lower baseline incidence of OCS-related AEs, and may also experience higher OCS-related hazard ratios due to this. The model may therefore be underestimating the general population incidence rate of OCS-related AEs, while overestimating the impact of increasing OCS exposure.
In addition, prior cumulative dose for patients upon entering the OPRI study were unavailable [1], therefore prior OCS dose was assumed to be zero. This is not reflective of the true OCS-eligible population, as many patients would have prior OCS exposure. Similarly, in the model, patients may discontinue from the OCS-sparing scenario. Upon discontinuation, the OCS dose returns to that of the ‘OCS only’ arm immediately, whereas in reality, the OCS dose would increase more slowly. These are conservative assumptions that are likely to result in underestimation of the benefits of OCS sparing.
In the model, the OCS dose is captured using cumulative OCS dose over time, reflecting the progressive and cumulative nature of OCS-related AEs, which do not resolve upon discontinuation. However, as the risk of some AEs may decrease with OCS discontinuation, the model may overestimate the OCS-related risk for patients who have discontinued from OCS, yet are still assumed to experience the OCS-related risk driven by their cumulative OCS exposure.
The model assumes that the relationship between OCS dose and beta values (informing hazard ratios) is linear, however it may not be truly linear and this may result in an overestimation of OCS-related AEs, particularly at high cumulative doses (> 15 g), as this would represent an extrapolation beyond the OPRI study population [1]. This is an issue particularly for adrenal insufficiency, for which there is a very strong relationship between incidence and OCS exposure [31], which when applied with the linear exponential relationship and high cumulative doses results in clinically implausible incidence estimates. This limitation, along with the decision to use a skew log-normal distribution (to reflect skewed dosing observed in clinical practice) with a standard error of 20% of the mean value, explain the right-hand side skew observed in the 15 mg probabilistic scenario analyses, with higher incremental costs and QALYs observed driven by the small number of patients receiving very high OCS doses. Unfortunately, the relationship between the incidence of adrenal insufficiency and cumulative OCS exposure is unknown. However, to address this limitation, conservative assumptions have been applied where possible, and for the 15 mg scenario, both deterministic and probabilistic results are presented. These include removing patients receiving inhaled corticosteroids from the baseline incidence rate and applying costs due to adrenal insufficiency only for the duration of the model cycle (28 days) to avoid double counting where patients experience adrenal insufficiency multiple times in a single year.
A limitation of the current approach compared with previous models is the data used to inform this model, which does not always align with the OCS-receiving population in the UK. While both the tezepelumab technology appraisal and this model use the same source for OCS-related AE incidence, the modelling approach used was significantly different, with the tezepelumab model applying incidence rates for OCS-related AEs by dosage categories to estimate the incidence of transient events, whereas the model presented here used cumulative dose-estimated hazard ratios per additional gram of OCS. The approach for transient events in both models is similar. However, for chronic events, significantly more QALYs and costs are captured in our model, as the lifetime impact of incident events of chronic events are captured.
Conclusion
The AEs associated with OCS treatment place a high burden on healthcare resources. However, the benefits of avoiding these events through the use of OCS-sparing agents are not fully captured in current modelling approaches. In order to more accurately capture the broader impact of OCS treatment, we have developed a model that captures the health economic outcomes associated with avoiding OCS-related AEs. This approach to the economic modelling of the benefit of reducing OCS use reflects the chronic and cumulative nature of OCS AEs, and the clinical importance of avoiding long-term OCS use and its related AEs. These results may help to inform healthcare decision making and guide future HTAs for novel OCS-sparing agents.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing and editorial assistance were provided by Carla De Villiers and Emma Crouch-Baker of HEOR Ltd. HEOR Ltd were paid consultants to AstraZeneca in connection with the development of this manuscript.
Declarations
Funding
This work was supported by AstraZeneca, who provided research funding for this study.
Conflict of interest
Danny Gibson, Neil Branscombe, Neil Martin, Andrew Menzies-Gow, Priya Jain are employees of AstraZeneca. Katherine Padgett and Florian Yeates are employees of Health Economics and Outcomes Research (HEOR) Ltd. HEOR Ltd received fees from AstraZeneca in relation to this study.
Availability of data and material
Data are available upon request from the authors.
Ethics approval
This study was conducted on a modelled population and as such no ethical approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
The cost-effectiveness model was developed in Microsoft Excel® 365. Any additional information about model programming is available from the corresponding author upon request.
Author contributions
DG, AMG, and KP conceptualised and designed this study. KP and FY were responsible for data analysis. All authors contributed to interpretation of the results, and preparation and review of the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Price DB, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;29(11):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalitsios CV, McKeever TM, Shaw DE. Incidence of osteoporosis and fragility fractures in asthma: a UK population-based matched cohort study. Eur Respir J. 2021;57(1):2001251. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney J, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71(4):339–46. [DOI] [PubMed] [Google Scholar]
- 4.Zhao S, et al. Is there an association between peptic ulcer disease and osteoporosis: a systematic review and cumulative analysis. Eur J Gastroenterol Hepatol. 2020;33(1):9–16. [DOI] [PubMed] [Google Scholar]
- 5.Elixhauser A, Owens P. Adverse drug events in US Hospitals, 2004, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2006, Agency for Healthcare Research and Quality (US): Rockville (MD).
- 6.Weiss AJ, et al. Origin of adverse drug events in US Hospitals, 2011, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2006, Agency for Healthcare Research and Quality (US): Rockville (MD). [PubMed]
- 7.Waljee AK, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivapalan P, et al. COPD exacerbations: the impact of long versus short courses of oral corticosteroids on mortality and pneumonia: nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Respir Res. 2019;6(1): e000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saag KG, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96(2):115–23. [DOI] [PubMed] [Google Scholar]
- 10.Ryu K, et al. Frailty and muscle weakness in elderly patients with asthma and their association with cumulative lifetime oral corticosteroid exposure. Allergol Int. 2023;72(2):252–61. [DOI] [PubMed] [Google Scholar]
- 11.Global Initiative for Asthma Management and Prevention (GINA). Difficult-to-treat and severe asthma in adolescent and adult patients, diagnosis and management. GINA; 2019.
- 12.Mebrahtu TF, et al. Oral glucocorticoids and incidence of hypertension in people with chronic inflammatory diseases: a population-based cohort study. CMAJ. 2020;192(12):E295-e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis JR, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420–6. [DOI] [PubMed] [Google Scholar]
- 14.Bleecker ER, et al. Systemic corticosteroids in asthma: a call to action from World Allergy Organization and Respiratory Effectiveness Group. World Allergy Organ J. 2022;15(12):100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourdin A, Suehs C, Charriot J. Integrating high dose inhaled corticosteroids into oral corticosteroids stewardship. Eur Respir J. 2020;55(1):1902193. [DOI] [PubMed] [Google Scholar]
- 16.Bleecker ER, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crossfield SSR, et al. Changes in the pharmacological management of rheumatoid arthritis over two decades. Rheumatology (Oxford). 2021;60(9):4141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Initiative for Asthma Management and Prevention (GINA) Global strategy for asthma management and prevention (2023 update). 2023.
- 19.Rider P, Carmi Y, Cohen I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol. 2016;2016:9259646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phinyo P, et al. Efficacy and safety of biologics for oral corticosteroid-dependent asthma: a systematic review and network meta-analysis. J Allergy Clin Immunol Pract. 2023;12(2):409–20. [DOI] [PubMed]
- 21.National Institute for Health and Care Excellence Benralizumab for treating severe eosinophilic asthma (TA565). 2019.
- 22.National Institute for Health and Care Excellence Mepolizumab for treating severe eosinophilic asthma (TA671). 2021.
- 23.National Institute for Health and Care Excellence Tezepelumab for treating severe asthma (TA880). 2023.
- 24.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013 [PMG9]. 2013. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#discounting. Accessed Aug 2024. [PubMed]
- 25.Manson SC, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975–94. [DOI] [PubMed] [Google Scholar]
- 26.Rice JB, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–29. [DOI] [PubMed] [Google Scholar]
- 27.Medicines & Healthcare Products Regulatory Agency. An observational retrospective cohort study to evaluate chronic disease onset associated with long-term oral corticosteroid use and the related cost impact on patients in the OPCRD/CPRD databases. CPRD. Application Number 17_002.
- 28.Van Staa TP, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993–1000. [DOI] [PubMed] [Google Scholar]
- 29.Calzetta L, et al. Oral corticosteroids dependence and biologic drugs in severe asthma: myths or facts? A systematic review of real-world evidence. Int J Mol Sci. 2021;22(13):7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cataldo D, et al. Severe asthma: oral corticosteroid alternatives and the need for optimal referral pathways. J Asthma. 2021;58(4):448–58. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer KJ, et al. Oral and inhaled corticosteroids and adrenal insufficiency: a case-control study. Thorax. 2006;61(5):405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broersen LHA, et al. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6):2171–80. [DOI] [PubMed] [Google Scholar]
- 33.Department of Health and Social Care. eMIT national database. 2021. https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit.
- 34.Jones K, et al. Unit Costs of Health and Social Care 2022 Manual: Personal Social Services Research Unit (University of Kent) and Centre for Health Economics (University of York). 2023.
- 35.Sullivan PW, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Mak. 2011;31(6):800–4. [DOI] [PubMed] [Google Scholar]
- 36.Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18. [DOI] [PubMed] [Google Scholar]
- 37.Dolan P, et al. The time trade-off method: Results from a general population study. Health Econ. 1996;5(2):141–54. [DOI] [PubMed] [Google Scholar]
- 38.Price DB, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcocci C, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86(8):3562–7. [DOI] [PubMed] [Google Scholar]
- 40.Mortimer K, et al. Asthma management in low and middle income countries: case for change. Eur Respir J. 2022;60(3):2103179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meghji J, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet. 2021;397(10277):928–40. [DOI] [PubMed] [Google Scholar]
- 42.Mortimer K, et al. The reality of managing asthma in sub-Saharan Africa—priorities and strategies for improving care. J Pan Afr Thorac Soc. 2022;3:105–20. [Google Scholar]
- 43.Barry LE, et al. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canonica GW, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019;12(1): 100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982–90. [DOI] [PubMed] [Google Scholar]
- 46.Spinelli FR, et al. Tapering and discontinuation of glucocorticoids in patients with rheumatoid arthritis treated with tofacitinib. Sci Rep. 2023;13(1):15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masuda M, et al. Analysis of the initial dose and reduction rate of corticosteroid for ulcerative colitis in clinical practice. JGH Open. 2022;6(9):612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill S, et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registry. Thorax. 2015;70(4):376–8. [DOI] [PubMed] [Google Scholar]
- 49.Asaria M, Doran T, Cookson R. The costs of inequality: whole-population modelling study of lifetime inpatient hospital costs in the English National Health Service by level of neighbourhood deprivation. J Epidemiol Community Health. 2016;70(10):990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institute for Health and Care Excellence Omalizumab for treating severe persistent allergic asthma (TA278). 2013.
- 51.Norman G, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess. 2013;17(52):1–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Observational and Pragmatic Research Institute Pte Ltd. Oral corticosteroid exposure and chronic disease onset. Data on file.
- 53.Hippisley-Cox J, Coupland C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study. BMJ. 2017;359: j5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin HH, et al. Association between type 2 diabetes and osteoporosis risk: a representative cohort study in Taiwan. PLoS One. 2021;16(7): e0254451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gough Courtney M, Quintero Y, Godde K. Assessing the roles of demographic, social, economic, environmental, health-related, and political factors on risk of osteoporosis diagnosis among older adults. Arch Osteoporos. 2021;16(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357: j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen SJ, et al. Osteoporosis is associated with high risk for coronary heart disease: a population-based cohort study. Medicine (Baltimore). 2015;94(27): e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ene-Iordache B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307–19. [DOI] [PubMed] [Google Scholar]
- 59.Taylor KS, et al. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large UK primary care database. Diabetes Care. 2013;36(8):2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai S, et al. Bone mineral density and osteoporosis in relation to all-cause and cause-specific mortality in NHANES: A population-based cohort study. Bone. 2020;141: 115597. [DOI] [PubMed] [Google Scholar]
- 61.Hippisley-Cox J, Coupland C. Development and validation of QMortality risk prediction algorithm to estimate short term risk of death and assess frailty: cohort study. BMJ. 2017;358: j4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.England N. National Cost Collection for the NHS 2021/22. 2023. https://www.england.nhs.uk/costing-in-the-nhs/national-cost-collection/. Accessed 26 Oct 2023.
- 63.Willis M, et al. Cost-effectiveness of canagliflozin added to standard of care for treating diabetic Kidney disease (DKD) in patients with type 2 diabetes mellitus (T2DM) in England: estimates using the CREDEM-DKD model. Diabetes Ther. 2021;12(1):313–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pritchard DM, et al. Cost-effectiveness modelling of use of urea breath test for the management of Helicobacter pylori-related dyspepsia and peptic ulcer in the UK. BMJ Open Gastroenterol. 2021;8(1): e000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campling J, et al. Hospitalization costs of adult community-acquired pneumonia in England. J Med Econ. 2022;25(1):912–8. [DOI] [PubMed] [Google Scholar]
- 66.Chauhan R, Lee D. Adrenal insufficiency: burden of disease and cost of illness. Value Health. 2013;16(7):A436. [Google Scholar]
- 67.Wang HI, et al. Healthcare resource use and costs for people with type 2 diabetes mellitus with and without severe mental illness in England: longitudinal matched-cohort study using the Clinical Practice Research Datalink. Br J Psychiatry. 2022;221(1):402–9. [DOI] [PubMed] [Google Scholar]
- 68.Danese MD, et al. Estimating the economic burden of cardiovascular events in patients receiving lipid-modifying therapy in the UK. BMJ Open. 2016;6(8): e011805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alva ML, et al. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med. 2015;32(4):459–66. [DOI] [PubMed] [Google Scholar]
- 70.Cavender MA, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the reduction of atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132(10):923–31. [DOI] [PubMed] [Google Scholar]
- 71.Miyake H, Kanazawa I, Sugimoto T. Association of bone mineral density, bone turnover markers, and vertebral fractures with all-cause mortality in type 2 diabetes mellitus. Calcif Tissue Int. 2018;102(1):1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.