Abstract
Introduction
Vesatolimod is a Toll-like receptor-7 (TLR7) agonist in clinical development as part of a combination regimen for human immunodeficiency virus (HIV) cure. Influenza-like symptoms associated with TLR7-mediated immune activation have been reported in clinical trials of vesatolimod. Therefore, a broader understanding of the safety profile of vesatolimod and association with dose and mechanism of action will help inform future clinical studies.
Methods
In this analysis, data on flu-like adverse events of interest (AEIs) were pooled from eight clinical studies in which 606 participants either received single or multiple doses of vesatolimod (0.3–12 mg; n = 505) or placebo (n = 101). Vesatolimod pharmacokinetics, inflammatory responses, and pharmacodynamics were assessed.
Results
The incidence of flu-like AEIs was higher with vesatolimod versus placebo (19% [96/505] vs. 8% [8/101]) and increased with vesatolimod dose and exposure. Most flu-like AEIs with vesatolimod were grade 1 or 2 severity (55% [53 of 96] grade 1; 35% [34 of 96] grade 2) with onset primarily after the first and second dose. Occurrence of flu-like AEIs after doses 1–3 was predictive of reoccurrence after later doses. Dose-dependent elevations of pharmacodynamic biomarkers (interferon-stimulated gene 15, 2′-5′-oligoadenylate synthetase 1, myxovirus resistance-1, interferon-α, interleukin-1 receptor antagonist, interferon-γ-induced protein 10, interferon-inducible T-cell-α chemoattractant) observed in participants with flu-like AEIs suggest a link with vesatolimod mechanism of action.
Conclusions
Flu-like AEIs associated with vesatolimod administration were typically mild but increased with exposure, which may be predicted by the response to initial doses. The data suggest that adaptive clinical monitoring could help maximize pharmacodynamic responses and balance adverse events in future clinical trials of vesatolimod.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-01034-w.
Keywords: Toll-like receptor-7 agonist, Vesatolimod, Safety, Influenza-like adverse events
Key Summary Points
| Why carry out this study? |
| Several clinical studies are evaluating the efficacy of vesatolimod as an immunomodulator in combination with other modalities such as broadly neutralizing antibodies and therapeutic vaccines for human immunodeficiency virus (HIV) remission. |
| As an immunomodulator, flu-like symptoms are known to occur with vesatolimod; therefore, a broader understanding of the safety profile of vesatolimod and its relationship with dose and mechanism of action will help inform future clinical studies. |
| We conducted a pooled analysis of data on flu-like adverse events in participants who received vesatolimod or placebo in eight clinical trials. |
| What was learned from the study? |
| Flu-like adverse events associated with vesatolimod administration were typically mild but increased with exposure and were predicted by the response to initial doses. |
| Dose-dependent elevations of pharmacodynamic biomarkers observed in participants with flu-like adverse events suggested a link with vesatolimod mechanism of action. |
| The data suggest that adaptive clinical monitoring could help maximize pharmacodynamic responses and balance adverse events in future clinical trials of vesatolimod in people with HIV. |
Introduction
Vesatolimod (VES; formerly known as GS-9620) is an orally administered investigational Toll-like receptor-7 (TLR7) agonist previously studied as a therapeutic agent for chronic hepatitis B infection and currently in development as part of a combination regimen aimed at inducing antiretroviral therapy (ART)-free remission of human immunodeficiency virus (HIV)-1 infection.
In clinical trials, VES was generally safe and well tolerated in healthy participants, people with hepatitis B virus (PWHBV), treatment-naïve people with hepatitis C virus, and people with HIV (PWH) on ART [1–4]. Data from human ex vivo and in vivo studies have shown that VES induces interferon (IFN)-stimulated gene (ISG) expression, increases circulating cytokines and chemokines, and activates immune cells that are critical to control HIV-1 infection [2, 3, 5, 6]. In experiments using peripheral blood mononuclear cells from virally suppressed PWH, VES promoted a dose-dependent increase in CD8+ T-cell activation, expansion of HIV-specific CD8+ T cells, HIV-1 reactivation, and inhibited viral replication ex vivo [7–9]. VES in combination with a therapeutic vaccine and/or broadly neutralizing antibodies (bNAbs) showed preliminary efficacy in virological control after ART discontinuation in a subset of early treated rhesus macaques infected with simian immunodeficiency virus [10–12]. Moreover, multiple-dose VES resulted in a modest increase in time to viral rebound and a decrease in viral setpoint in HIV controllers, highlighting its potential as part of a combination strategy aimed at HIV remission [6]. More recently, VES was studied in combination with a therapeutic vaccine in a phase 2 clinical trial (AELIX-003) [13]. Although there was no difference in viral load dynamics post-ART, the vaccine/VES regimen was highly immunogenic, vaccine immunogenicity correlated with improved viral outcomes, and VES consistently induced a pharmacodynamic (PD) response. Additionally, there are two ongoing clinical trials (NCT05281510 and NCT06071767) evaluating VES as an immunomodulator in combination with other modalities such as bNAbs and therapeutic vaccine for HIV remission. NCT05281510 is a phase 2a clinical trial that is investigating VRC07-523LS, CAP256V2LS, and in early antiretroviral-treated women living with HIV-1 clade C in South Africa [14]; NCT06071767 is a phase 1/2a study that aims to evaluate the safety, immunogenicity, and efficacy of a triple immune regimen in adults initiated on ART during acute HIV-1 [15].
VES results in immune activation and upregulation of IFNα and ISGs [16]. Type I IFN responses can elicit a complex cascade of events in response to viral infection [17], and influenza-like (flu-like) symptoms are common side effects associated with type I IFN therapy [18]. Therefore, flu-like symptoms or adverse events (AEs) are expected after VES administration and have, in fact, been previously reported in clinical studies of VES [3, 6]. However, the associations between the pharmacokinetics (PK) of TLR7 agonists and the mechanism by which they may contribute to AEs has not been systemically studied. Here, we undertook a detailed analysis of the relationships between flu-like AEs and the mechanism of action (MOA) and PK of VES from eight independent clinical studies.
Methods
We analyzed pooled safety, PK, and PD data from eight clinical studies of VES in three populations: healthy participants (two studies), PWHBV (four studies), and PWH (two studies). The analysis included 606 participants; 101 who received placebo and 505 who received VES at doses ranging from 0.3–12 mg. Eight VES doses were evaluated: 0.3 mg (n = 27), 1 mg (n = 136), 2 mg (n = 139), 4 mg (n = 140), 6 mg (n = 24), 8 mg (n = 34), 10 mg (n = 6), and 12 mg (n = 12); 13 participants underwent dose escalations. Among these eight clinical studies, 58 participants from two studies of healthy participants received a single dose of VES; 447 participants were enrolled in multiple-dose studies and received up to 13 VES administrations. Data from the AELIX-003 study (NCT04364035) in which participants received VES plus vaccine were not available at the time of this analysis. The clinical studies and detailed dosing strategy for the pooled analysis are summarized in the supplementary materials (Tables S1–S3). Each study in this analysis was conducted in accordance with the protocol and ethical principles derived from international guidelines including the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonization (ICH) guidelines for Good Clinical Practice (GCP), and all applicable laws, rules and regulations. The protocols for each study were approved by an institutional review board or ethics committee. Written informed consent was obtained from all participants.
Safety Analysis
AEs were recorded and graded per the Gilead grading scale for severity of AEs and the laboratory abnormalities toxicity grading scale (Table S4). A search for flu-like AEs was performed across the eight clinical studies, according to the following MedDRA preferred terms: cytokine release syndrome, hemophagocytic lymphohistiocytosis, cytokine storm, capillary leak syndrome, pyrexia, chills, headache, fatigue, cancer fatigue, arthralgia, nausea, vomiting, systemic inflammatory response syndrome, hypotension, hypoxia, influenza-like illness, myalgia, influenza, and malaise (Table S5). A subset of influenza-like AEs (collectively termed “flu-like AEs of interest” [flu-like AEIs]) was selected for analysis based on clinical significance and potential association with the MOA of VES. Flu-like AEIs included the following preferred terms: any grade of pyrexia, chills, influenza-like illness, influenza, and cytokine release syndrome (CRS), and grade ≥ 2 myalgia, headache, fatigue, and malaise (Table S5).
Food intake can reduce VES exposure [2]; therefore, only participants (n = 492) who received VES fasted or with an empty stomach (no food 2 h before and 2 h after dose; hereafter referred to as fasted state) were included in analyses by VES dose.
Pharmacokinetic and Pharmacodynamic Analyses
Plasma concentration of VES was determined by high-performance liquid chromatography–tandem mass spectroscopy. Maximal plasma concentration (Cmax) and area under the concentration–time curve to infinity after a single dose (AUCinf) were estimated by standard noncompartmental methods using Phoenix (Certara, Princeton, NJ, USA).
The PD response to VES was assessed through evaluation of whole-blood ISGs (ISG15, myxovirus resistance-1 [MX1], and 2′-5′-oligoadenylate synthetase 1 [OAS1]), and circulating biomarkers downstream of TLR7 stimulation, including several cytokines (IFNα, IFN-inducible T-cell-α chemoattractant [ITAC], interleukin-1 receptor antagonist [IL-1RA], and IFNγ-induced protein [IP-10]). Markers of inflammation included interleukin-6 (IL-6), IFNγ, tumor necrosis factor-α (TNFα), and C-reactive protein (CRP). The mRNA expression of ISGs and TLR7 single-nucleotide polymorphisms (SNPs; rs179008 and rs3853839) were measured using real-time quantitative polymerase chain reaction (Covance, Indianapolis, IN, USA). Assay details for evaluating cytokine and immune cell phenotyping are summarized in Table S6. Fold changes from baseline for each biomarker analyte were calculated before consolidating the data from the eight studies for further analysis.
To identify circulating biomarkers associated with flu-like AEIs, we compared 24-h changes from baseline after the first dose in ISGs (ISG15, MX1, OAS1), PD biomarkers (IFNα, ITAC, IL-1RA, IP-10), soluble biomarkers of CRS (IL-6, IFNγ, TNFα, CRP), and frequency of activated (CD69+) natural killer (NK) cells in participants with or without flu-like AEIs in both placebo and VES arms.
Statistical Analysis
The Wilcoxon rank-sum test was used to compare differences in the data obtained between early and later flu-like AEIs, whereas the chi-square test was used to analyze associations between those two groups. The Wilcoxon rank-sum test was used to compare fold changes in each biomarker between participants with and without flu-like AEI. A generalized linear mixed-effect model and Tukey trend test were used to evaluate if any biomarkers could be used as prognostic or predictive baseline biomarkers for any flu-like AEI after the first VES dose, regardless of age, sex at birth, race, or study population (e.g., healthy participants, PWHBV, or PWH) (additional details provided in the supplementary materials).
Results
This analysis included 606 adults (73 PWH, 454 PWHBV, and 79 healthy volunteers). The median age was 43 years (interquartile range, 34–51), 442 (73%) of participants were male, 333 (55%) Asian or Native Hawaiian, and 205 (34%) were white (Table S7).
Safety and Incidence of Flu-Like AEIs across Eight Clinical Studies
The incidence of flu-like AEIs was 8% (8/101) in participants who received placebo, 19% (96/505) in participants who received VES (fed or fasted state), and 20% (96/492) in participants who received VES (fasted state). Of the flu-like AEIs in participants who received VES (fasted state), 55% (53/96) were grade 1, 35% (34/96) were grade 2, and 9% (9/96) were grade 3. There were no grade 4 AEIs. The most common flu-like AEIs were pyrexia (9%; n = 46), chills (7%; n = 35), and headache (4%; n = 20) (Table 1).
Table 1.
Summary of influenza-like adverse events of interest by dose (fasted state)
| Participants, n (%) | Placebo n = 101 | 0.3 mg n = 27 |
1 mg n = 136 |
2 mg n = 139 |
4 mg n = 140 |
6 mg n = 24 |
8 mg n = 21 |
10/12 mg n = 12 |
All VES n = 492 |
|---|---|---|---|---|---|---|---|---|---|
| Any flu-like AEI | 8 (8%) | 1 (4%) | 23 (17%) | 21 (15%) | 33 (24%) | 2 (8%) | 9 (43%) | 7 (58%) | 96 (20%) |
| Grade 1 | 3 (3%) | 1 (4%) | 10 (7%) | 11 (8%) | 19 (14%) | 1 (4%) | 7 (33%) | 4 (33%) | 53 (11%) |
| Grade 2 | 5 (5%) | 0 | 11 (8%) | 8 (6%) | 12 (9%) | 1 (4%) | 1 (5%) | 1 (8%) | 34 (7%) |
| Grade 3 | 0 | 0 | 2 (2%) | 2 (1%) | 2 (1%) | 0 | 1 (5%) | 2 (17%) | 9 (2%) |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CRS (grade 3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 0 | 1 (< 1%) |
| Pyrexia | 3 (3%) | 1 (4%) | 9 (7%) | 8 (6%) | 18 (13%) | 1 (4%) | 3 (14%) | 6 (50%) | 46 (9%) |
| Grade 1 | 3 (3%) | 1 (4%) | 5 (4%) | 6 (4%) | 13 (9%) | 1 (4%) | 3 (14%) | 3 (25%) | 32 (7%) |
| Grade 2 | 0 | 0 | 2 (2%) | 1 (1%) | 3 (2%) | 0 | 0 | 1 (8%) | 7 (1%) |
| Grade 3 | 0 | 0 | 2 (2%) | 1 (1%) | 2 (1%) | 0 | 0 | 2 (17%) | 7 (1%) |
| Myalgia (grade 2)a | 0 | 0 | 1 (1%) | 1 (1%) | 4 (3%) | 1 (4%) | 1 (5%) | 1 (8%) | 9 (2%) |
| Chills | 2 (2%) | 0 | 3 (2%) | 9 (7%) | 13 (9%) | 0 | 4 (19%) | 6 (50%) | 35 (7%) |
| Grade 1 | 2 (2%) | 0 | 3 (2%) | 8 (6%) | 11 (8%) | 0 | 4 (19%) | 5 (42%) | 31 (6%) |
| Grade 2 | 0 | 0 | 0 | 0 | 2 (1%) | 0 | 0 | 1 (8%) | 3 (1%) |
| Grade 3 | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 1 (< 1%) |
| Influenza | 0 | 0 | 2 (2%) | 0 | 1 (1%) | 0 | 0 | 0 | 3 (1%) |
| Grade 1 | 0 | 0 | 1 (1%) | 0 | 1 (1%) | 0 | 0 | 0 | 2 (< 1%) |
| Grade 2 | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 1 (< 1%) |
| Influenza-like illness | 0 | 0 | 7 (5%) | 1 (1%) | 9 (6%) | 0 | 0 | 0 | 17 (4%) |
| Grade 1 | 0 | 0 | 5 (4%) | 1 (1%) | 7 (5%) | 0 | 0 | 0 | 13 (3%) |
| Grade 2 | 0 | 0 | 2 (2%) | 0 | 2 (1%) | 0 | 0 | 0 | 4 (1%) |
| Malaise (grade 2)a | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 1 (< 1%) |
| Headache | 3 (3%) | 0 | 6 (4%) | 6 (4%) | 6 (4%) | 0 | 1 (5%) | 1 (8%) | 20 (4%) |
| Grade 2 | 3 (3%) | 0 | 6 (4%) | 5 (4%) | 6 (4%) | 0 | 1 (5%) | 1 (8%) | 19 (4%) |
| Grade 3 | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 1 (< 1%) |
| Fatigue (grade 2)a | 2 (2%) | 0 | 3 (2%) | 2 (1%) | 4 (3%) | 0 | 0 | 0 | 9 (2%) |
| Joint pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Flu-like AEIs included ≥ grade 2 fatigue, headache, malaise, myalgia; ≥ grade 3 joint pain; any grades of chills, cytokine release syndrome, influenza, influenza-like illness and pyrexia
Fasted state excluded participants who received VES with a high-fat meal, moderate-fat meal, or 4 h after a high-fat meal. Six participants received placebo with a meal. Eighteen participants were treated with VES 8 mg immediately after a high-fat or moderate-fat meal, or 4 h after a high-fat meal (n = 6 per group). Among those, five participants crossed over from the fasted group to the high-fat meal group after a 1-month washout. Four participants in GS-US-382-3961 crossed over from VES 4 mg to 6 mg and three participants in GS-US-382-3961 crossed over from VES 6 mg to 8 mg after a washout
Multiple adverse events were counted only once per participant for the highest severity grade for each preferred term
AEI adverse events of interest, CRS cytokine release syndrome, flu-like AEIs influenza-like adverse events of interest, VES vesatolimod
aAll events of myalgia, malaise and fatigue were grade 2 severity
A summary of all flu-like AEs in the fasted state, including AEIs, is shown in Table S8. Among the eight studies, there was one grade 3 serious AE of CRS in a healthy participant who received VES. This participant received VES 8 mg with elipovimab (formerly named GS-9722) 250 mg, an investigational effector-enhanced broadly neutralizing antibody targeting the HIV envelope. The participant developed tachycardia, fever, chills, nausea, vomiting, myalgia, labile blood pressure, and hypoxemia, requiring hospitalization for intravenous fluids, corticosteroids, and diphenhydramine. Although this serious AE was reported as grade 3 by the investigator, according to National Cancer Institute CRS grading this serious AE was consistent with grade 2, as the associated hypotension responded to fluids [19]. There were two serious cases of flu-like symptoms involving accidental overdoses of VES in PWHBV. One participant received an accidental overdose of VES 10 mg and developed chills, hot flashes, and fever that resolved spontaneously. Another participant received an accidental overdose of VES 20 mg and was hospitalized for tachycardia, fever, headache, blurred vision, nausea, vomiting, myalgia, and full-body tremors, which resolved within 1 day.
Flu-Like AEIs Increased with VES Dose and Exposure
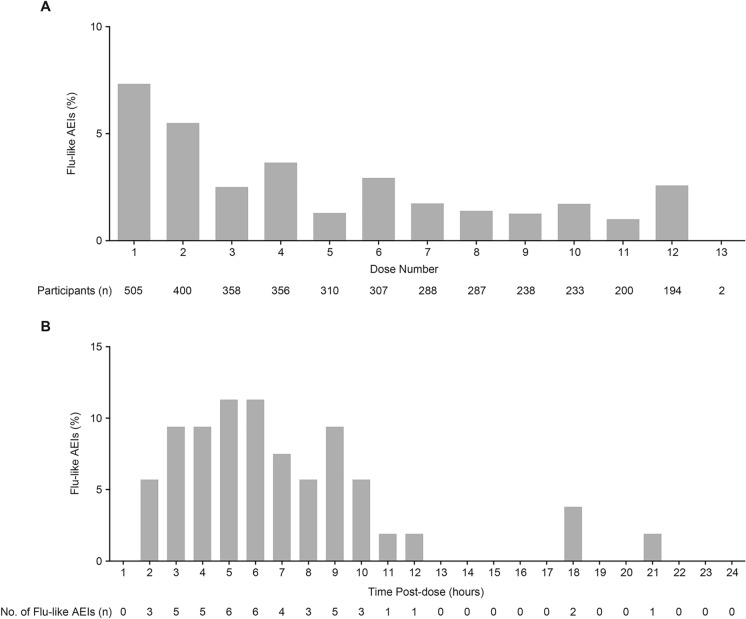
The incidence of flu-like AEIs increased with VES dose and was highest in the 8 mg (43% [9/21]) and 10/12 mg (58% [7/12]) groups (Table 1). Among all participants who received VES (any dose, fed or fasted state), the incidence of flu-like AEIs was 7% (37/505) after the first dose, 5.5% (22/400) after the second dose, and decreased to < 4% after the third dose (Fig. 1A). Additionally, there was an association between the early development of flu-like AEIs after doses 1 to 3 and later recurrence of flu-like AEI after dose 4 (P < 0.001 for dose 1, P < 0.01 for dose 2, and P < 0.001 for dose 3 by chi-square test; Table 2). Of the 52 participants with onset of any flu-like AEI after dose 4 and beyond, 12 (23%) had previously had a flu-like AEI after dose 1 and before dose 2. Most events of flu-like AEIs had onset within 12 h after the first VES dose, with the highest incidence within 5–6 h post dose (Fig. 1B).
Fig. 1.
Incidence of influenza-like adverse events of interest stratified by vesatolimod dose number and time of onset. A Percentages of flu-like AEI in all participants who received VES across all eight studies according to VES dose number. B Percentages of flu-like AEI according to time of onset of flu-like AEIs after first VES dose. Percentages were calculated based on the total number of flu-like AEIs (N = 53). AEI adverse events of interest, flu-like AEIs influenza-like adverse events of interest, VES vesatolimod
Table 2.
Association between early development of influenza-like adverse events of interest and later recurrence
| VES treatment | Onset of flu-like AEI before dose 4 | No flu-like AEI after dose 4 and beyond, N | Any flu-like AEI after dose 4 and beyond, N | P value |
|---|---|---|---|---|
| Received dose 1, participants [N = 363] | 311 | 52 | ||
| Participants with any flu-like AEI after dose 1 and before dose 2 | 14/311 (5%) | 12/52 (23%) | P < 0.001 | |
| Received dose 2, participants [N = 360] | 308 | 52 | ||
| Participants with any flu-like AEI after dose 2 and before dose 3 | 14/308 (5%) | 8/52 (15%) | P < 0.01 | |
| Received dose 3, participants [N = 358] | 306 | 52 | ||
| Participants with any flu-like AEI after dose 3 and before dose 4 | 6/306 (2%) | 7/52 (14%) | P < 0.001 |
Nominal P values generated from chi-square test
AEI adverse events of interest, flu-like AEIs influenza-like adverse events of interest, VES vesatolimod
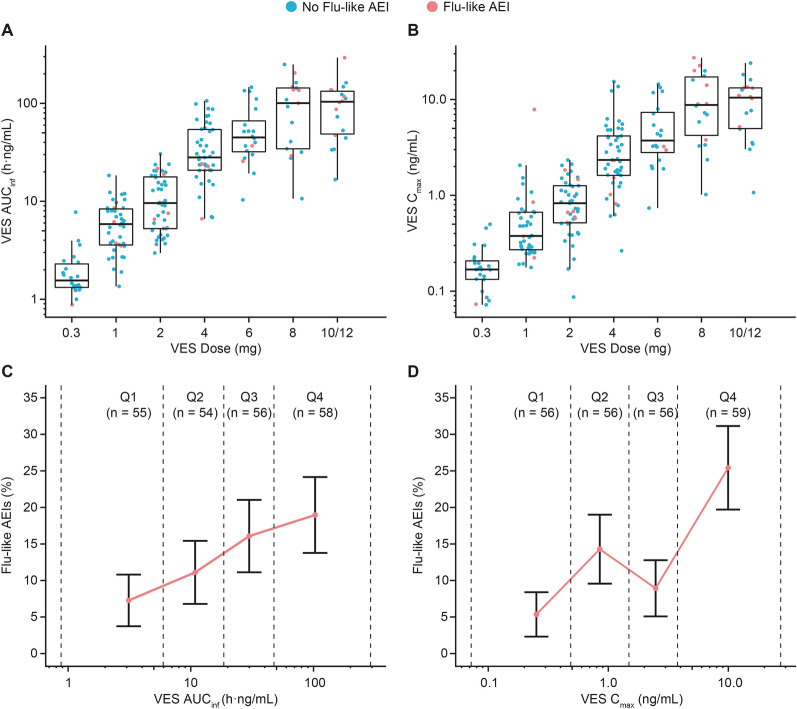
The AUCinf and Cmax of VES increased with VES dose, although there was a substantial overlap in exposures between different dose levels due to the large variability in VES PK (Fig. 2A, B). The percentages of participants with flu-like AEIs increased from the lowest to the highest quartiles of VES AUCinf and Cmax in all participants with valid PK measurements (Fig. 2C, D). The median VES dose among participants who developed flu-like AEIs was higher compared with participants without flu-like AEIs (P < 0.001; Fig. S1).
Fig. 2.
Incidence of influenza-like adverse events of interest by vesatolimod exposure. A, B Distribution of VES AUCinf and Cmax by VES dose level administered in fasted state or with empty stomach to participants with pharmacokinetic measurements across eight clinical studies. Dots represent individual data; boxes represent 25th to 75th percentiles; whiskers represent 1.5 times the interquartile range not exceeding the minimum/maximum values. Participants who experienced flu-like AEIs are highlighted in red dots. C, D Percentages of participants with flu-like AEIs are plotted by quartiles of VES AUCinf and Cmax in all participants with pharmacokinetic measurements across eight clinical studies. Error bars represent standard errors. Rates of flu-like AEIs were plotted against mean AUCinf and Cmax in each quartile. In all panels, dashed line indicates range of AUCinf or Cmax within each quartile (Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile). AUCinf area under the concentration–time curve to infinity after a single dose, Cmax maximal concentration, flu-like AEIs influenza-like adverse events of interest, Q quartile, VES vesatolimod
VES PD Responses Increased 24 Hours after VES Administration in a Dose-Dependent Manner
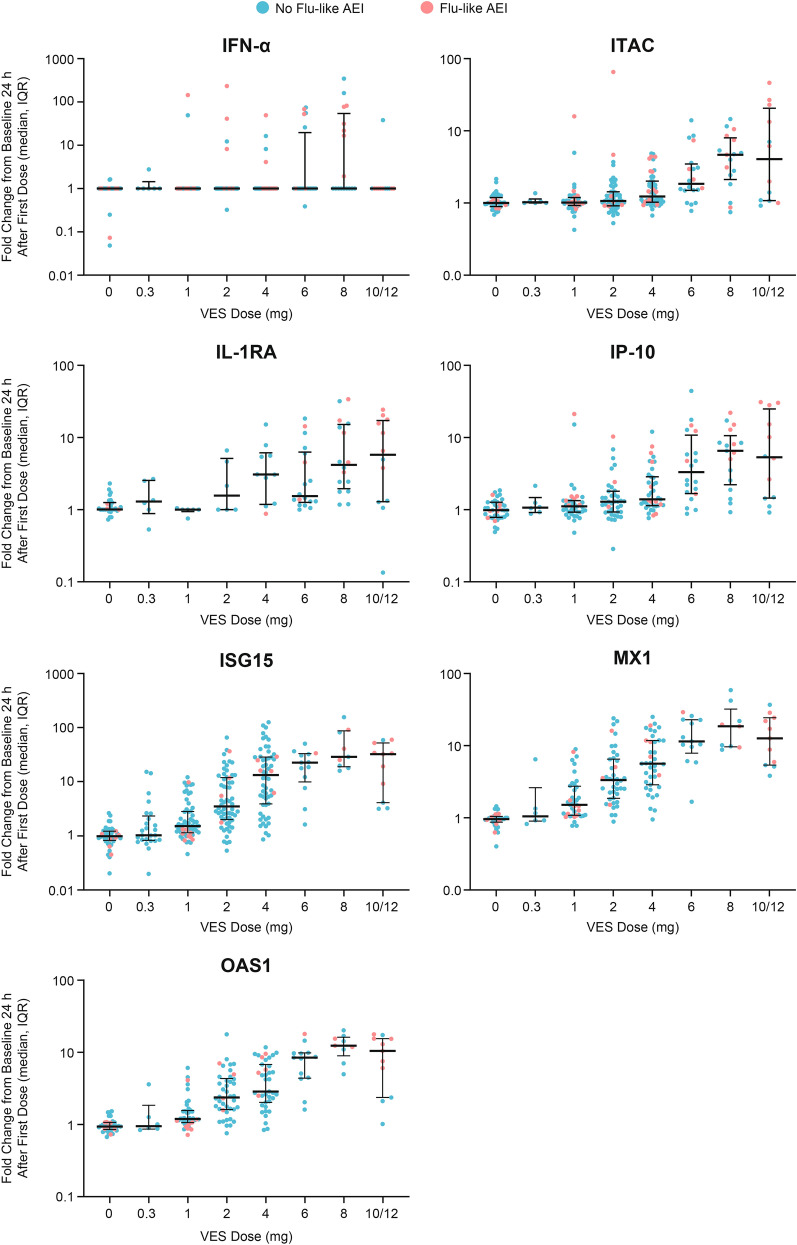
Increases in VES PD biomarkers were observed 24 h after the first dose administration at VES doses ≥ 4 mg for IFNα, ITAC, IL-1RA, and IP-10, and at VES doses ≥ 1 mg for ISG15, MX1, and OAS1 (Fig. 3). There appeared to be a dose-dependent increase of IFNα, ITAC, IL-1RA, IP-10, ISG15, MX1, and OAS1, but with high variability at the 10/12 mg dose. The highest fold change of PD biomarkers was achieved with VES doses ≥ 8 mg. However, variability in the lower limit of quantification (LLOQ) across studies contributed to lower sensitivity to detect upregulation of IFNα than the other PD biomarkers.
Fig. 3.
Changes in pharmacodynamic biomarkers 24 h after first dose administration in participants with and without influenza-like adverse events of interest. Fold changes from baseline 24 h after first dose for VES pharmacodynamic biomarkers from all eight VES studies are summarized by VES dose. Participants who presented with flu-like AEIs are highlighted in red dots. AEI adverse events of interest, flu-like AEIs influenza-like adverse events of interest, IFNα interferon-α, IL-1RA interleukin-1 receptor antagonist, IP-10 interferon-γ-induced protein 10 kDa, ISG15 interferon-stimulated gene 15, ITAC interferon-inducible T-cell-α chemoattractant, MX1 myxovirus resistance-1, OAS1 2′-5′-oligoadenylate synthetase 1, VES vesatolimod
No dose-dependent changes were observed 24 h after VES administration in the levels of IL-6, IFNγ, TNFα, and CRP—markers frequently elevated in the context of CRS (Fig. S2).
Associations of Flu-Like AEIs with PD Biomarkers
PD biomarkers were significantly increased from baseline in participants with flu-like AEIs in both placebo and VES arms, including IFNα (P < 0.001), ITAC (P < 0.01), IL-1RA (P < 0.001), and IP-10 (P < 0.001) (Fig. S3). In addition, IL-6 and CD69+ NK cells were significantly higher in participants with flu-like AEIs (P < 0.01). No significant differences were observed in IFNγ, TNFα, CRP, ISG15, MX1, and OAS1 between participants with and without flu-like AEIs (Fig. S4).
Using a post hoc Tukey test, baseline levels of IFNα (P < 0.01), ITAC (P = 0.03), IL-6 (P = 0.01), and TNFα (P < 0.01) were identified as predictive biomarkers of flu-like AEIs after the first VES dose (fasted state) (Table S9). Baseline levels of IFNα (P < 0.01), ITAC (P = 0.01), IL-6 (P = 0.02), TNFα (P < 0.01), and IFNγ (P = 0.04) were identified as prognostic biomarkers of flu-like AEIs after the first VES dose (fasted state) (Table S10).
Associations of Flu-Like AEIs with TLR7 SNPs
The rs3853839 and rs179008 SNPs in the 3' untranslated region of TLR7, which can be associated with elevated TLR7 signaling and protective immune responses [20–23], were sequenced in 422 participants. The overall distribution of the TLR7 SNPs varied by sex and ethnicity (Tables S11 and S12). The rs179008 SNPs were absent in the Asian population. Most rs179008 A/T heterozygotes were white female participants (10/11). The incidence of flu-like AEs in participants with rs179008 SNPs was 26% (27/103) for female participants and 19% (54/290) for male participants carrying A/A homozygous alleles, 45% (5/11) for female participants carrying A/T heterozygous alleles, and 11% (2/18) for male participants carrying T/T homozygous alleles (Table S11). In participants with rs3853839 SNPs, the incidence of flu-like AEs was 54% (13/24) for female participants and 19% (25/134) for male participants carrying C/C homozygous alleles, 18% (7/40) for female participants carrying C/G heterozygous alleles, 24% (12/50) for female participants carrying G/G homozygous alleles, and 18% (31/174) for male participants carrying G/G homozygous alleles (Table S12).
Discussion
VES is an immune-modulating TLR7 agonist that is under clinical development as part of a combination strategy for HIV cure [3, 6, 24–26]. Nonclinical and clinical studies have established preliminary safety and efficacy of VES, alone or in combination with other agents [6, 10–12]. VES treatment results in immune activation and upregulation of IFNα and ISGs [16]. Flu-like symptoms such as fever, chills, myalgia, headache, and nausea, which are similar to those induced by IFNα therapy [18], are likely to be associated with TLR7-mediated immune activation and have been reported in clinical trials of VES [3, 6]. The findings of this pooled analysis confirmed that a subset of participants developed VES-related flu-like AEs which were typically mild and increased with exposure and may be predicted by the response to initial doses.
Flu-like AEIs, selected based on grade, clinical relevance, and potential association with MOA, were more common with VES versus placebo. The most common reported flu-like AEIs were chills, pyrexia, and headache. A dose-dependent increase in the incidence of flu-like AEIs was also observed. This is consistent with the hypothesis that flu-like AEIs may be directly linked to the VES MOA and that the development of flu-like AEIs may be an indication of VES activity in vivo.
Analyses of PK and PD data revealed that the incidence of flu-like AEIs increased with VES exposure. Additionally, VES induced a dose-dependent elevation of the PD biomarkers IFNα, ITAC, IL-1RA, and IP-10, cytokines that are typically produced in response to direct stimulation of TLR7 [2, 3, 6]. The direct link between TLR7 stimulation and flu-like AEIs is further supported by the finding that IFNα, ITAC, IL-1RA, and IP-10, as well as IL-6 levels and frequency of activated (CD69+) NK cells, were elevated in participants who experienced flu-like AEIs.
TLR7 expression and signaling, and consequently its effect on immune responses, can be influenced by genetic factors, such as the presence of specific TLR7 SNPs [20–23]. In this study, the incidence of flu-like AEIs was higher in white female participants who carried rs179008 A/T heterologous alleles. This could potentially be explained by an increase in TLR7 signaling, as TLR7 is encoded on the X chromosome and can escape X inactivation in immune cells [27, 28]. The incidence of flu-like AEIs was also higher in white participants who carried rs3853839 hemizygous G allele or G homozygous alleles. The rs3853839 SNP has been associated with autoimmune diseases such as systemic lupus erythematous and Behçet disease [21–23, 29], also suggesting a potential link between TLR7 engagement and a heightened immune response. Overall, these findings support the hypothesis that TLR7 signaling may be directly linked to the development of flu-like AEIs in participants who receive VES.
Baseline levels of several inflammatory and PD biomarkers were identified as potentially predictive (IFNα, ITAC, IL-6, and TNFα) or prognostic (IFNα, ITAC, IL-6, TNFα, and IFNγ) of flu-like AEIs. More specifically, participants with higher baseline values of these biomarkers were less likely to develop flu-like AEIs after receiving the first VES dose. This suggests that higher inflammation levels at baseline could affect the magnitude of TLR7-associated responses and the subsequent development of flu-like AEIs. The mechanisms underlying this observation remain to be established, however it is well known that activation of TLRs can lead to upregulation of inflammatory mediators and that TLR signaling is negatively controlled by multiple mechanisms [30] to avoid immunopathology. It is therefore possible that the induction of negative signaling regulators or transcriptional downregulation could contribute to our findings.
VES-induced elevation of PD response and immune cell activation was previously identified as a potential contributor to the efficacy of VES in a study of virologically suppressed PWH on ART [6]. Stimulation of cytokine production resulting from TLR7 signaling has, however, the potential to result in severe or life-threatening AEs. General markers of inflammation (IL-6, IFNγ, TNFα, and CRP) did not increase in a dose-dependent manner after VES administration, suggesting that the VES immune-modulatory effect may not necessarily result in a hyperinflammatory state. One case of grade 2 CRS (per CTCAE grading) [31] and two serious cases of flu-like symptoms have, however, been observed in clinical trials to date. These cases were noted in participants who received either high doses of VES (10 and 20 mg) or VES 8 mg in combination with elipovimab, an effector-enhanced bNAb. These events resolved completely without any long-term sequelae, and the data suggest that clinical monitoring is warranted in patients who receive VES, especially when administered in combination with other agents. In ongoing and future studies VES dosing is staggered by ≥ 1 week relative to partner agents. Notably, the incidence of flu-like AEIs in the pooled analysis was highest after the first two doses of VES and more likely to recur in participants who experienced flu-like AEIs with earlier doses. This finding supports the implementation of an adaptive clinical monitoring strategy in future clinical studies, with closer monitoring with the first two doses.
Our study has limitations. First, this was a heterogeneous pooled population including healthy participants, PWHBV, and PWH; however, VES was investigated with full dose range (0–12 mg) only in healthy participants and virologically suppressed PWH. PWHBV received lower VES doses (0–4 mg) and ART-treated HIV controllers received higher doses (4–8 mg). Second, although a dose-dependent increase in PD biomarkers was observed and the data variability was limited, the data were probably confounded by the small sample size for the VES 10/12 mg groups and the insufficient dose range in specific subpopulations (e.g., PWHBV and ART-treated HIV controllers). Moreover, data were collected from different assay platforms, laboratories, and cohorts with variations in the lower limit of detection, which could increase experimental variability. Third, even though TLR7 can recognize RNA oligonucleotides from RNA viruses, the exact viral ligand that triggers TLR7 remains unknown. Therefore, even though PWH in the study had viral suppression, residual viral activities in the tissue cannot be excluded as it may modulate TLR7 activation, impacting the results. Last, the gender, race, and TLR7 SNP distribution among the different dose groups may have impacted the analysis of TLR7 response on VES stimulation. The data used for this analysis were collected from a total of 442 male participants (73%) and 164 female participants (27%), with < 20% female participants in the PWH population. Since TLR7 is located on the X-chromosome, its expression can be confounded by X-linked genetic factors and gender-specific hormones. Hence, the analysis might be under-representative for the female population. Future studies with expanded sample size in PWH and reduced covariates will likely facilitate the use of VES as part of combination treatment in advancing HIV cure and remission strategies.
In conclusion, flu-like AEIs occurred in a subset of participants following VES exposure and were typically mild in severity and more common after early doses. Flu-like AEIs increased with VES exposure and elevated PD biomarkers, suggesting a direct link between flu-like AEIs and the VES MOA of TLR7 activation. VES is currently in development as an immune modulator for HIV cure/remission with clinical studies evaluating its efficacy in combination with other modalities such as bNAbs and therapeutic vaccines (NCT05281510 and NCT06071767). As such, understanding the predictors and mechanisms of flu-like symptoms after VES exposure provides important context for researchers in the HIV cure field. Even though VES alone is not sufficient to induce a measurable increase in plasma viremia or long-term delay in viral rebound [10, 32], it is evident from the studies in PWH that VES increases immune cell activation, which has the capacity to enhance the combination strategy for HIV cure/remission [3, 6]. Flu-like AEIs may be predicted by the response to initial doses, and the maximum tolerable flu-like AEIs may be used as a guide to select the optimal VES dose for the HIV cure strategy, supporting adaptive clinical monitoring. All participants enrolled in the ongoing VES clinical trials are carefully monitored for early signs of CRS, and for hematologic and hepatic AEs and laboratory abnormalities, as some participants in early phase trials experienced graded elevations. The relationship between VES PD and efficacy in the setting of HIV remission strategies requires further investigation in expanded populations in future trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the individuals who participated in these trials and their families, the principal investigators and their staff, and the Gilead study staff (including, but not limited to, Peter Shweh, Meghna Shroff, Susan Guo, and Mary Wire).
Medical Writing/Editorial Assistance
Editorial support was provided by Mandakini Singh and Jean Turner of Parexel International and was funded by Gilead.
Author Contributions
Sharon A. Riddler, Constance A. Benson, Cynthia Brinson, Steven G. Deeks, Edwin DeJesus, Anthony Mills, Michael F. Para, and Moti N. Ramgopal participated in the data acquisition, patient data collection, and data analysis and interpretation. Devi SenGupta, Elena Vendrame, and Yanhui Cai participated in the study design, manuscript concept development, data analysis and interpretation, and drafting and reviewing the report. Yanan Zheng, Liao Zhang, Wendy Jiang, Xiaopeng Liu, Donovan Verrill, Daina Lim, Christiaan R. de Vries, and Jeffrey J. Wallin participated in the data analysis and interpretation and drafting and reviewing the report. Daina Lim, Yanhui Cai, and Liao Zhang verified the data. All authors reviewed and approved the final manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
This work was supported by Gilead Sciences, Inc. Gilead Sciences, Inc. covered the publication costs of the article including the Rapid Service Fee.
Data Availability
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non-conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
Declarations
Conflict of interest
Sharon A. Riddler reports grants from Gilead and NIH/NIAID during the conduct of the study, as well as a grant from Merck outside the submitted work. Constance A. Benson reports grants from Gilead and NIH/NIAID during the conduct of the study and honoraria for educational lectures and personal fees for meeting travel from the IAS-USA, and has held leadership positions on the IAS-USA Board and the CROI Foundation Board. Cynthia Brinson is on the Speaker’s Bureau for Gilead and reports personal fees from Gilead for meeting travel. Steven G. Deeks reports grants from Gilead, consulting fees from AbbVie, GlaxoSmithKline, Hookipa, and Immunocore, and stock or stock options in BryoLogyx, Enochian BioSciences, and Trendel. Anthony Mills and Michael F. Para declare no competing interests. Edwin DeJesus reports grants and speaker and/or advisory fees from Gilead, Janssen, Abbott, Bristol Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Merck, Sangamo, TaiMed, Theraco Technologies, Vertex and ViiV Healthcare, both during the conduct of the study and outside the submitted work. Moti N. Ramgopal reports consulting fees and/or honoraria from Gilead, AbbVie, Janssen, ViiV Healthcare, and Merck. Yanhui Cai, Yanan Zheng, Liao Zhang, Wendy Jiang, Xiaopeng Liu, Donovan Verrill, Daina Lim, Christiaan R. de Vries, Jeffrey J. Wallin, Elena Vendrame, and Devi SenGupta are employees and shareholders of Gilead.
Ethical approval
The studies included in this analysis were conducted in accordance with the protocol and ethical principles derived from international guidelines including the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical guidelines, applicable International Conference on Harmonization Good Clinical Practice guidelines, and all applicable laws, rules and regulations. The protocols for each study were approved by an institutional review board or ethics committee. Written informed consent was obtained from all participants.
Footnotes
Prior Presentation
This work was previously presented, in part, at HIV Glasgow 2022; October 23–26; Glasgow, UK. Poster P112.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lawitz E, Gruener D, Marbury T, et al. Safety, pharmacokinetics and pharmacodynamics of the oral Toll-like receptor-7 agonist GS-9620 in treatment-naive patients with chronic hepatitis C. Antivir Ther. 2015;20(7):699–708. [DOI] [PubMed] [Google Scholar]
- 2.Lopatin U, Wolfgang G, Tumas D, et al. Safety, pharmacokinetics and pharmacodynamics of GS-9620, an oral Toll-like receptor-7 agonist. Antivir Ther. 2013;18(3):409–18. [DOI] [PubMed] [Google Scholar]
- 3.Riddler SA, Para M, Benson CA, et al. Vesatolimod, a Toll-like receptor-7 agonist, induces immune activation in virally suppressed adults living with human immunodeficiency virus-1. Clin Infect Dis. 2021;72(11):e815–24. [DOI] [PubMed] [Google Scholar]
- 4.Janssen HLA, Brunetto MR, Kim YJ, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68(3):431–40. [DOI] [PubMed] [Google Scholar]
- 5.Rebbapragada I, Birkus G, Perry J, Xing W, Kwon H, Pflanz S. Molecular determinants of GS-9620-dependent TLR7 activation. PLoS ONE. 2016;11(1): e0146835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SenGupta D, Brinson C, DeJesus E, et al. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci Transl Med. 2021;13(599):eabg3071. [DOI] [PubMed] [Google Scholar]
- 7.Bam RA, Hansen D, Irrinki A, et al. TLR7 agonist GS-9620 is a potent inhibitor of acute HIV-1 infection in human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2017;61(1):e01369-e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ram RR, Duatschek P, Margot N, et al. Activation of HIV-specific CD8(+) T-cells from HIV+ donors by vesatolimod. Antivir Ther. 2020;25(3):163–9. [DOI] [PubMed] [Google Scholar]
- 9.Tsai A, Irrinki A, Kaur J, et al. Toll-like receptor-7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J Virol. 2017;91(8):e02166-e2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borducchi EN, Liu J, Nkolola JP, et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;563(7731):360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker-Sperling VEK, Mercado NB, Chandrashekar A, et al. Therapeutic efficacy of combined active and passive immunization in ART-suppressed, SHIV-infected rhesus macaques. Nat Commun. 2022;13(1):3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borducchi EN, Cabral C, Stephenson KE, et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540(7632):284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mothe B, Curran A, López Bernardo de Quirós JC, et al. A placebo-controlled randomized trial of the HTI immunogen vaccine and vesatolimod. Presented at: 30th Conference on Retroviruses and Opportunistic Infections (CROI); February 19–23, 2023; Seattle, WA, USA. Poster 433.
- 14.ClinicalTrials.gov. NCT05281510. https://www.clinicaltrials.gov/study/NCT05281510. Accessed 12 Aug 2024.
- 15.ClinicalTrials.gov. NCT06071767. https://www.clinicaltrials.gov/study/NCT06071767. Accessed 12 Aug 2024.
- 16.Fosdick A, Zheng J, Pflanz S, et al. Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel Toll-like receptor-7 agonist, demonstrate interferon-stimulated gene induction without detectable serum interferon at low oral doses. J Pharmacol Exp Ther. 2014;348(1):96–105. [DOI] [PubMed] [Google Scholar]
- 17.Ng CT, Mendoza JL, Garcia KC, Oldstone MB. Alpha and beta type 1 interferon signaling: passage for diverse biologic outcomes. Cell. 2016;164(3):349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sleijfer S, Bannink M, Van Gool AR, Kruit WH, Stoter G. Side effects of interferon-alpha therapy. Pharm World Sci. 2005;27(6):423–31. [DOI] [PubMed] [Google Scholar]
- 19.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buschow SI, Biesta PJ, Groothuismink ZMA, et al. TLR7 polymorphism, sex and chronic HBV infection influence plasmacytoid DC maturation by TLR7 ligands. Antiviral Res. 2018;157:27–37. [DOI] [PubMed] [Google Scholar]
- 21.Shen N, Fu Q, Deng Y, et al. Sex-specific association of X-linked Toll-like receptor-7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci USA. 2010;107(36):15838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song GG, Choi SJ, Ji JD, Lee YH. Toll-like receptor polymorphisms and vasculitis susceptibility: meta-analysis and systematic review. Mol Biol Rep. 2013;40(2):1315–23. [DOI] [PubMed] [Google Scholar]
- 23.Wang C-M, Chang S-W, Wu Y-JJ, et al. Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci Rep. 2014;4(1):3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hvilsom CT, Søgaard OS. TLR-agonist mediated enhancement of antibody-dependent effector functions as strategy for an HIV-1 cure. Front Immunol. 2021;12: 704617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Offersen R, Nissen SK, Rasmussen TA, et al. A novel Toll-like receptor 9 agonist, MGN1703, enhances HIV-1 transcription and NK cell-mediated inhibition of HIV-1-infected autologous CD4+ T cells. J Virol. 2016;90(9):4441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vibholm LK, Konrad CV, Schleimann MH, et al. Effects of 24-week Toll-like receptor 9 agonist treatment in HIV type 1+ individuals. AIDS. 2019;33(8):1315–25. [DOI] [PubMed] [Google Scholar]
- 27.Souyris M, Cenac C, Azar P, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19):eaap8855. [DOI] [PubMed] [Google Scholar]
- 28.Souyris M, Mejía JE, Chaumeil J, Guéry JC. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin Immunopathol. 2019;41(2):153–64. [DOI] [PubMed] [Google Scholar]
- 29.Raafat II, El Guindy N, Shahin RMH, Samy LA, El Refai RM. Toll-like receptor-7 gene single nucleotide polymorphisms and the risk for systemic lupus erythematosus: a case–control study. Z Rheumatol. 2018;77(5):416–20. [DOI] [PubMed] [Google Scholar]
- 30.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33(9):449–58. [DOI] [PubMed] [Google Scholar]
- 31.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published May 28, 2009 (v4.03: June 14, 2010).
- 32.Del Prete GQ, Alvord WG, Li Y, et al. TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight. 2019;4(11): e127717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non-conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.