Abstract
Introduction
Gastroesophageal reflux disease (GERD) is a common ailment associated with troublesome symptoms. The standard of care in Italy involves initial treatment with proton pump inhibitor (PPI)-based medical management or laparoscopic Nissen fundoplication (LNF) for patients unwilling to continue or intolerant of long-term PPI therapy. RefluxStop is a novel medical device, intended for laparoscopic implantation, that has recently proven to be an efficacious and cost-effective treatment option for patients with GERD. This analysis aims to describe the short-term budget impact of introducing RefluxStop as a GERD treatment option within the Italian National Health Service (SSN).
Methods
A model adherent to international best practice recommendations was developed to estimate the budget impact of introducing RefluxStop over a 5-year time horizon. Two scenarios were considered: one without RefluxStop (i.e., comprising PPI therapy, LNF, and magnetic sphincter augmentation using the LINX system); and one with RefluxStop (i.e., addition of RefluxStop to the three treatment options previously mentioned). Clinical benefits and costs associated with each intervention were included in the analysis.
Results
Over 5 years, the introduction of RefluxStop resulted in avoidance of 95 surgical failures, 11 reoperations, and 64 endoscopic esophageal dilations. Introduction of RefluxStop resulted in an almost neutral impact on the existing budget with a 0.316% increase in the annual Italian SSN spending on GERD treatment.
Conclusion
Introduction of RefluxStop as a GERD treatment option in Italy is likely to be associated with substantial clinical benefits and a marginal budget impact.
Key Points for Decision Makers
| RefluxStop provides substantial clinical benefits at the expense of a marginal budget impact over a 5-year time horizon. |
| RefluxStop is likely to benefit patients and the Italian National Health Service. |
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal disorders worldwide, defined as a chronic condition in which retrograde flow of gastric contents to the esophagus causes troublesome symptoms on a weekly basis [1]. Typical symptoms include heartburn and regurgitation, but other manifestations include chest pain, cough, asthma, laryngitis, and impaired sleep [1, 2]. GERD also increases the risk of developing the premalignant Barrett’s esophagus and subsequent esophageal cancer [3].
In Western countries, GERD has an estimated prevalence of 10 to 30% and has been increasing since 1995, likely related to the global increase in obesity [1, 4–9]. Thus, the rising prevalence of GERD posits an important public health issue impacting quality of life while substantially contributing to the expenditure of the healthcare system [1, 10]. The costs are likely to rise with improvements in healthcare access throughout Europe, as only 25% of patients receive adequate therapy and untreated GERD already poses substantial consequences to the healthcare systems and economies of European countries [11, 12].
Practice recommendations for GERD management in Italy currently do not exist. A prominent body for practice guidelines in Europe is the National Institute for Health and Care Excellence (NICE) in the United Kingdom. According to the NICE practice guidelines, initial treatment of GERD is with proton pump inhibitor (PPI)-based medical management, with surgical operations (i.e., laparoscopic Nissen fundoplication and magnetic sphincter augmentation [MSA]), where available, reserved for patients who are intolerant to or unwilling to accept prolonged treatment with PPIs, as well as after medical therapy failure [13]. Recently, a novel implantable device (RefluxStop™, Implantica, Zug, Switzerland) was developed as a surgical treatment option for GERD patients that are eligible for laparoscopic surgery. RefluxStop aims to restore the normal anatomy of the gastroesophageal junction, gastroesophageal flap valve, and angle of His without affecting passage of food [14]. RefluxStop received a CE mark across European Union countries in 2018 based on favorable results of a prospective, single-arm, multicenter trial assessing its safety and efficacy in 50 patients with chronic GERD requiring daily PPI therapy [14, 15]. Briefly, patients treated with RefluxStop experienced significant improvements in GERD Health-Related Quality of Life score, normalization of esophageal pH values via 24-hour pH monitoring, and a favorable safety profile [14].
A recent health economic evaluation demonstrated the cost-effectiveness of RefluxStop from the perspective of the National Health Service in the United Kingdom [16]. As such, there are numerous analyses underway to determine the cost-effectiveness and impact of this novel antireflux procedure for various regions of the world. The purpose of this study is to estimate the budgetary impact of introducing RefluxStop as a therapeutic option from the Italian National Health Service perspective.
Methods
Model Overview
A budget impact model was developed in line with the recommendations of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) [17]. The model structure was intended to estimate the budget impact of introducing RefluxStop over a 5-year time horizon (beginning in 2021–2022) from the perspective of the Italian National Health Service/Servizio Sanitario Nazionale (SSN) as per ISPOR guidelines. A time horizon of 1–5 years is commonly used as per these guidelines [17]. The scenarios considered included (i) existing interventions (i.e., PPI-based medical management, laparoscopic Nissen fundoplication, and MSA using the LINX system) but without RefluxStop; and (ii) currently existing interventions as listed but with inclusion of RefluxStop.
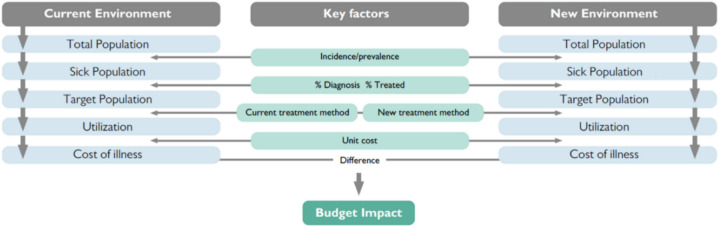
The schematic of the developed model is presented in Fig. 1 [17–19]. The population eligible for treatment with RefluxStop (i.e., patients diagnosed with GERD who currently receive PPI therapy and/or who have not undergone previous surgery but are eligible and willing surgical candidates) and the change in size of this population over the 5-year model horizon were identified. Market shares of studied treatment options were then applied to the eligible population and intervention-specific costs (including cost savings arising from benefits of each modality) were evaluated to determine the total costs associated with the two hypothetical scenarios described above. Currently in Italy, RefluxStop and MSA are reimbursed on par with fundoplication. Since all budget impact models are based on a scenario of full reimbursement, we have accounted for the same. A scenario with similar reimbursement tariffs is also discussed. The net budget impact of introducing RefluxStop is represented by the difference in these two scenarios.
Fig. 1.
Modeled Population
All those with potential to benefit from the introduction of RefluxStop (i.e., GERD patients receiving PPI therapy and/or those without previous antireflux surgery that are eligible and willing to undergo an operation) were included in the modeled population. Both prevalent patients with GERD and incident cases (i.e., patients newly developing GERD during the model time horizon) were considered in the model.
Population size and projected growth in Italy were estimated using the National Institute of Statistics (Istat) database [20]. The prevalence and incidence of GERD rates in the Italian general population and the annual incidence of laparoscopic antireflux surgery were obtained from published literature. The prevalence of GERD in Italy (23.7%) for the purposes of this study was based on an epidemiological study [21]. The literature did not discernably present an incidence rate of GERD in Italy, but it is generally believed that approximately 5 per 1,000 person-years (or 0.5%) in the Western world is an acceptable figure [22]. The proportion of GERD patients treated within the SSN was presumed to be similar to the United Kingdom (6.6%) as this information was not available for the Italian perspective [23]. This was confirmed by seven leading Italian antireflux surgery experts to be reasonable for the Italian context. Data on the number of antireflux surgeries for GERD in Italy was not available. According to a study assessing the budget impact of MSA from the Italian healthcare payer’s perspective, out of 82,955 patients eligible for antireflux surgery, <1% of surgical procedures were performed [24]. The presumption that this had progressed to 1% of patients eligible for antireflux surgery gives a surgery rate of 1.4 per 100,000 persons in the general population. Uncertainty regarding this estimate was explored using scenario analysis, as recommended by ISPOR guidelines [17].
Market Shares
The market shares of PPI-based medical management, laparoscopic Nissen fundoplication, MSA, and the projected market share value of RefluxStop were determined from market research conducted by independent vendors for Implantica (i.e., the RefluxStop manufacturer) and from leading Italian antireflux surgery expert opinion. The market share of RefluxStop was based on business development targets since the product was recently introduced into the Italian market. Market shares for model scenarios with and without RefluxStop are presented in Table 1. The uncertainty associated with real-world uptake of RefluxStop was accommodated for with the addition of two scenarios. In one scenario, the rate of RefluxStop uptake was halved, and in the other scenario, the rate was doubled. The corresponding market shares in each model year are presented in Table 1. It was assumed in the base case that increases in market share of RefluxStop would reduce the market share of Nissen fundoplication, being the standard of care for antireflux surgery. For an additional scenario, it was assumed that the market share of Nissen Fundoplication and MSA would be equally reduced.
Table 1.
Market share of interventions considered in the scenarios with and without RefluxStop
| Treatment | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Scenario without RefluxStop (all scenarios) | |||||
| Nissen fundoplication | 89.7% | 89.6% | 89.5% | 89.4% | 89.3% |
| MSA | 10.3% | 10.4% | 10.5% | 10.6% | 10.7% |
| Base case scenario with RefluxStop | |||||
| RefluxStop | 5.1% | 10.2% | 15.2% | 18.5% | 21.8% |
| Nissen fundoplication | 84.6% | 79.4% | 74.3% | 70.9% | 67.6% |
| MSA | 10.3% | 10.4% | 10.5% | 10.6% | 10.7% |
| Scenario with RefluxStop (reduced rate of RefluxStop uptake) | |||||
| RefluxStop | 2.6% | 5.1% | 7.6% | 9.3% | 10.9% |
| Nissen fundoplication | 87.2% | 84.5% | 81.9% | 80.2% | 78.4% |
| MSA | 10.3% | 10.4% | 10.5% | 10.6% | 10.7% |
| Scenario with RefluxStop (increased rate of RefluxStop uptake) | |||||
| RefluxStop | 10.3% | 20.4% | 30.4% | 37.0% | 43.6% |
| Nissen fundoplication | 79.5% | 69.3% | 59.1% | 52.4% | 45.8% |
| MSA | 10.3% | 10.4% | 10.5% | 10.6% | 10.7% |
MSA magnetic sphincter augmentation
Model calculations involved multiplying market share values by the population size to estimate the number of persons receiving each treatment option over 5 years. Given the surgical nature of most of the management strategies considered in the model, the assumption that persons who had previously undergone surgery would not switch to another treatment is likely with the relatively short timeframe of 5 years. Per-patient cost estimates included treatment switching in patients who underwent antireflux surgery following initial PPI-based medical management. Thus, the model population included all prevalent GERD patients in Year 1 and only incident cases in Years 2–5.
Costs
The cost per patient per year of each treatment reflected all costs related to each treatment option. This information was obtained by adapting a published cost-effectiveness model of RefluxStop in the United Kingdom [16] to Italian settings. Briefly, the model was a state transition (Markov) model designed to compare the cost-effectiveness of RefluxStop and three treatment options (i.e., medical management with PPIs, Nissen fundoplication, and the LINX reflux management system). Health states include ‘initial Medical Management (MM)’, ‘MM relapse’, ‘follow-on surgery’ (including surgical success and failure), ‘reoperations’, ‘MM with a higher dose’, ‘Barrett’s esophagus’, ‘esophageal cancer’, and ‘death’. The model structure also included adverse events (AEs) associated with MM and surgeries (i.e., intra- and postoperative events). Unit costs were obtained from published literature and averaged Diagnostic-Related Groups (DRGs) from five separate regions (i.e., Lombardia, Veneto, Toscana, Campania, and Emilia Romagna). Interregional tariffs were not used in the model since they reflect movement of patients from one region to another for care and not the purpose of our model. The unit costs obtained from published literature were mostly from Italian sources. However, when unit costs were not available for Italy, they were obtained from a nearby country and inflated to the 2021–2022 year. It is to be noted that most of the cost estimates were obtained from published literature. DRGs were used predominantly for procedural costs, such as for Nissen fundoplication, diagnostic endoscopy, endoscopic mucosal resection (EMR), radiofrequency ablation (RFA), therapeutic endoscopy, upper gastrointestinal tract procedures, esophagectomy, and esophageal dilation. The choice of regional DRGs used were also based on the availability of the tariffs. These were then applied to the Italian payer’s perspective at a national level rather than a regional level. DRGs are current reimbursement tariffs and were not inflated. Where data was obtained from older literature sources, cost values were inflated to 2021 using a web-based tool for cost conversion [25]. The major cost categories captured included the following:
Treatment costs, comprising costs of PPI medication, surgical treatments (with the latter including procedural costs and, for MSA and RefluxStop only, device and training costs).
Costs of diagnosing and treating Barrett’s esophagus and esophageal cancer in those developing these conditions.
Costs of managing AEs associated with PPIs (i.e., chronic kidney disease [26], cardiovascular events [27], fractures [28], pneumonia [29], Clostridium difficile infection [30], and gastric cancer [31]).
Costs of managing AEs associated with surgical management (i.e., conversion from laparoscopic to open surgery, esophageal dilation, additional surgery for major complications and, for RefluxStop and MSA only, device removal).
Table 2 presents the total annual per-patient costs associated with each modeled treatment option. First-year costs were higher for surgical management options than for medical therapy due to surgery and device costs. Surgical costs incurred during Years 2–5 were predominantly related to follow-up, and thus lower than costs for medical management. Clinical and cost parameters used in the model are provided in a Supplementary Appendix (see electronic supplementary material [ESM]).
Table 2.
Cost per patient per year for the different treatments assessed
| Treatment | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total over 5 years |
|---|---|---|---|---|---|---|
| Medical management | €408.74 | €399.07 | €367.16 | €366.59 | €306.93 | €1818.49 |
| RefluxStop | €12,171.18 | €44.12 | €42.20 | €44.15 | €45.55 | €12,347.21 |
| Nissen fundoplication | €6422.27 | €84.81 | €74.25 | €75.46 | €75.75 | €6732.54 |
| MSA | €10,384.67 | €174.88 | €150.81 | €150.13 | €147.56 | €11,008.96 |
MSA magnetic sphincter augmentation
Analysis
The means of determining the total budget required for each treatment option included multiplying the number of patients receiving each treatment by the cost per specific year associated with that treatment option. As such, patients in Year 2 of treatment would receive the Year 2 cost, and those in Year 3 of treatment would receive Year 3 costs, and so on. A summary of total costs for each year over the 5-year time horizon was then generated to determine the final healthcare system budget required for scenarios with and without RefluxStop.
Results
Clinical Outcomes
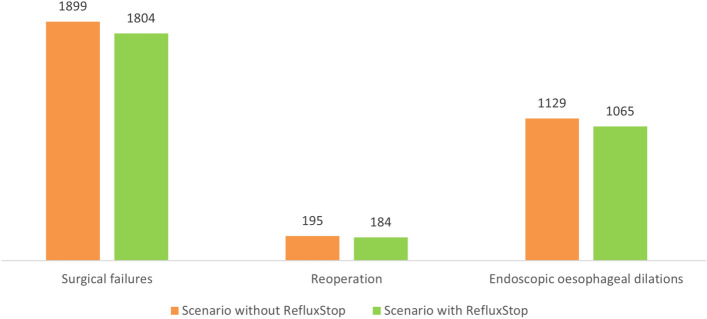
In the first year of availability, the number of patients receiving RefluxStop was estimated at 42, which then increased to 177 in the fifth year, corresponding with a decline in the annual number of patients undergoing Nissen fundoplication (from 697 in Year 1 to 550 in Year 5). These figures are delineated in Table 3. Introduction of RefluxStop was associated with improved clinical outcomes over the 5-year time horizon of the model, with 95 surgical failures, 11 reoperations, and 64 endoscopic esophageal dilations avoided relative to the scenario devoid of RefluxStop utilization (Fig. 2).
Table 3.
The number of patients receiving each treatment per model year in base case scenarios with and without RefluxStop
| Modeled year | Scenario without RefluxStop | Scenario with RefluxStop | |||||
|---|---|---|---|---|---|---|---|
| PPI-based medical management | Nissen fundoplication | MSA | PPI-based medical management | Nissen fundoplication | MSA | RefluxStop | |
| 1 | 925,749 | 739 | 84 | 925,749 | 697 | 84 | 42 |
| 2 | 18,645 | 735 | 85 | 18,645 | 651 | 85 | 84 |
| 3 | 18,682 | 732 | 86 | 18,682 | 608 | 86 | 124 |
| 4 | 18,685 | 730 | 86 | 16,685 | 579 | 86 | 151 |
| 5 | 18,689 | 727 | 87 | 18,689 | 550 | 87 | 177 |
| Total | 1,000,450 | 3663 | 428 | 1,000,450 | 3085 | 428 | 578 |
MSA magnetic sphincter augmentation, PPI proton pump inhibitor
Fig. 2.
Clinical outcomes of base case scenarios with and without RefluxStop over the 5-year model time horizon
Base Case Analysis
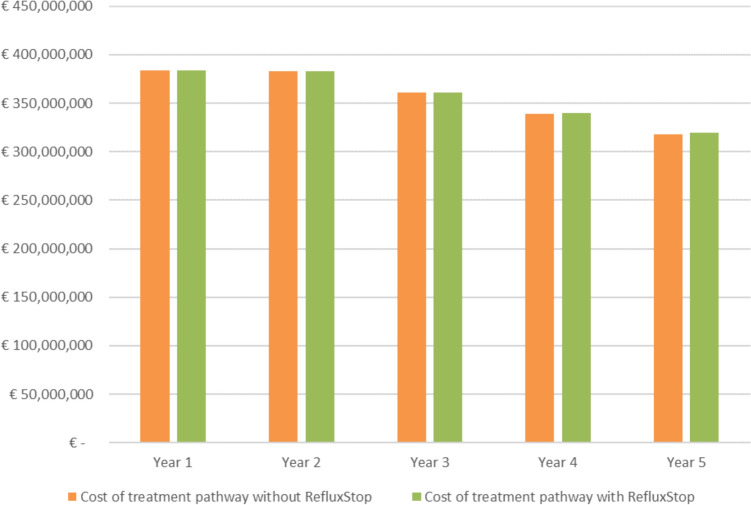
Figure 3 depicts the total costs over the 5-year model time horizon associated with scenarios with and without RefluxStop. The individual 1-year, 3-year, and 5-year budget impacts of introducing RefluxStop were €242,641, €710,651, and €1,004,946 per year, respectively. This corresponded to 0.063%, 0.197%, and 0.316% increases per year in the overall Italian SSN expenditure for GERD, respectively (Table 4).
Fig. 3.
Total costs of base case scenarios with and without RefluxStop
Table 4.
Total costs estimated in base case scenarios with and without RefluxStop and budget impact
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Eligible population | 926,573 | 946,037 | 965,537 | 985,038 | 1,004,541 |
| Population expected to receive RefluxStop | 42 | 126 | 250 | 401 | 578 |
| Cost of treatment pathway without RefluxStop | €384,012,573 | €382,736,989 | €360,706,454 | €339,332,403 | €318,224,668 |
| Cost of treatment pathway with RefluxStop | €384,255,213 | €383,215,846 | €361,417,105 | €340,191,446 | €319,229,614 |
| Nominal net budget impact | €242,641 | €478,858 | €710,651 | €859,043 | €1,004,946 |
| Net budget impact as percentage of SSN spending for GERD treatment | 0.063% | 0.125% | 0.197% | 0.253% | 0.316% |
GERD gastroesophageal reflux disease, SSN Italian National Health Service
Uptake Rate Variation
In the described scenario in which the uptake rate of RefluxStop was halved (i.e., reduced from 42 to 21 patients in Year 1, or reduced from 177 to 89 patients in Year 5, see Table 3) relative to the base case, the 5-year reductions in the number of surgical failures, reoperations, and endoscopic dilations were 48, 5, and 32, respectively. The 1-year budget impact of RefluxStop introduction in this halved scenario was €121,320, corresponding to a 0.032% increase in overall SSN expenditure for GERD treatment in Italy. The 5-year budget impact was €502,473, corresponding to a 0.158% increase in Italian SSN expenditure for GERD.
The scenario exploring a doubled rate (i.e., increased from 42 to 84 patients in Year 1, or increased from 177 to 354 patients in Year 5, see Table 3) of RefluxStop uptake observed 5-year reductions in the number of surgical failures, reoperations, and endoscopic dilations of 190, 21, and 129, respectively. The budget impact associated with this scenario was €485,281 over 1 year, corresponding to a 0.126% increase in Italian SSN spending on GERD management. The 5-year budget impact in this scenario was €2,009,893, corresponding to a 0.632% increase in SSN spending.
In the scenario in which the increase of RefluxStop market share reduced the market share of both Nissen Fundoplication and MSA equally, the budget impact associated with this scenario was €75,402 over 1 year, corresponding to a 0.020% increase. The 5-year budget impact was €819,549, corresponding to a 0.258% increase in SSN spending.
Rate of Surgery Variation
In a scenario where the surgery rate per 100,000 population was halved (0.7%), the 5-year reductions in the number of surgical failures, reoperations, and endoscopic dilations were 48, 5, and 32, respectively. The budget impact associated with an increased uptake of RefluxStop in Italy was €121,320 over 1 year, corresponding to a 0.032% increase in Italian SSN spending on GERD management, and €502,473 over 5 years, corresponding to a 0.159% increase in spending.
In a scenario where the surgery rate per 100,000 population was doubled (2.78%), the 5-year reductions in surgical failures, reoperations, and endoscopic dilations were 190, 21, and 129, respectively. The budget impact associated with increased uptake of RefluxStop in Italy was €485,281 over 1 year, corresponding to a 0.125% increase in SSN expenditure for GERD management, and €2,009,893 over 5 years, corresponding to a 0.623% increase in spending.
Discussion
The published literature provides numerous economic and budget impact analyses related to GERD management in Italy. To better grasp the general context, a review of epidemiologic study for GERD and economic analyses for existing treatment options is beneficial. In Italy, the prevalence of GERD is estimated between 10.8% and 27.7%, considered more prominent than in other European countries [1–3, 10, 11]. Thus, an estimated 6.5 million patients per year have GERD in Italy [11]. This translates to a total annual treatment cost for the population with GERD of approximately €1,759 million at a mean of €206 per patient in terms of treatment cost per year [11]. Considering the increase in prevalence among young patients [32, 33] and that 33.8% of GERD patients never receive relevant information from general practitioners, GERD is expected to have a substantial impact on the future economic sustainability of the Italian healthcare system [1, 10, 11].
RefluxStop had demonstrated a favorable safety and efficacy profile [14] and a recent cost-effectiveness analysis determined that the device is highly likely to be a cost-effective treatment option for GERD patients in the United Kingdom at the standard cost-effectiveness threshold of £20,000 per quality-adjusted life-year gained [16]. Although the lifetime cost-effectiveness of this novel device is unknown for Italy, this study focused on the short-term health and economic impact of RefluxStop introduction as a management option on the Italian SSN. Despite the time horizon of 5 years being too short to meaningfully capture some PPI-associated AEs and complications for GERD itself (i.e., Barrett’s esophagus and esophageal cancer), RefluxStop demonstrated a substantial reduction in surgical complications in this study. Surgical failures, reoperation, and postoperative esophageal dilation were significantly reduced with the introduction of RefluxStop. This favorable clinical profile resulted in cost offsets and contributed to the marginal budget impact (<1%) of RefluxStop on the Italian SSN in our current analysis.
Although no specific practice guidelines for GERD exist in Italy to the best of our knowledge, management with PPI-based medical management and laparoscopic Nissen fundoplication (in selected patients) described by a prominent European organization [13] were presumed the mainstay of treatment for the Italian SSN. Long-term PPI use has been associated with several potential AEs, including kidney disease, infections, and myocardial infarction [34]. Furthermore, up to 40% of GERD patients do not achieve adequate symptom relief with PPI therapy [35]. Although Nissen fundoplication has been reported to provide superior symptom control, this procedure is associated with an up to 5-fold increase in dysphagia risk compared with PPI therapy [36]. Furthermore, recurrence of symptoms such as heartburn and regurgitation may occur 5–10 years after Nissen fundoplication [37]. Thus, optimal control of GERD remains a challenge in some groups receiving standard-of-care management. MSA via the LINX system is a novel GERD treatment modality currently available in Italy, but the growth of utilization is not large as per market insights and research conducted by the RefluxStop manufacturer, Implantica. Hence, an annual market growth rate of 1% is assumed for MSA. Ultimately, the need for additional treatment options for GERD patients in Italy remains.
New treatments for GERD are likely to become even more important over the coming years in Italy. Although the age-standardized prevalence of GERD in Italy has not increased between 1990 and 2017 according to the Global Burden of Disease Study 2017 [3], the appreciated increase in obesity (i.e., a risk factor for GERD) expenditure of almost 30% over the last three decades may be indicative of an increasing prevalence of GERD in the future [4, 38]. As GERD patients with frequent and/or severe symptoms experience a significant impairment of both health-related quality of life and work productivity [39], and such patients are more likely to seek surgical management, the broader societal benefits of an effective novel treatment are likely substantial. RefluxStop may be a replacement modality of GERD management for patients who are potential candidates for laparoscopic fundoplication.
Limitations and Uncertainty
The results of the model in our analysis are largely driven by market share inputs. As these are associated with substantial uncertainty and the real-world uptake of RefluxStop is difficult to ascertain, scenario analyses were conducted to evaluate the effect of halving or doubling the rates of RefluxStop uptake. In both scenarios, the budget impact of introducing RefluxStop remained minute and manageable. Other inputs likely to impact model results are the size of the eligible patient population, the regional prevalence and incidence of GERD, the proportion of patients receiving PPI therapy without prior antireflux surgery, and the proportion of eligible patients willing to undergo antireflux surgery. However, these variables are applicable to all surgical interventions in the model. Variation in these variables is thus expected to affect all surgical options uniformly and are not RefluxStop-specific; thus, they were not tested via scenario analysis. Furthermore, as a new technology, it is likely that uptake of RefluxStop will be slower in the first 5 years of implementation. Therefore, the results of our model are largely driven by this slow and smaller uptake.
Averaging of available costs from regions of Italy was a limitation of this study. In Italy, healthcare is a domain of regional authorities despite our analysis focusing on the national level. Furthermore, DRG estimates are only an approximation of true costs. However, when there is no other cost estimate available, evaluation models use DRGs as a common practice. We also recommend performing this analysis at a regional level as well if regional decisions are to be made. Another issue regarding costs is that reimbursement of each antireflux procedure in Italy is the same regardless of the actual costs of each intervention. For our analysis, we included the full economic costs for each intervention (i.e., the cost of devices) as opposed to the reimbursement prices, which were equal to fundoplication (i.e., did not include any extra device costs) as per the Italian DRG. However, this does not significantly affect our conclusions. Even in a scenario in which the device costs were omitted from the analysis, the net budget impact is negative in favor of RefluxStop.
An additional source of uncertainty in the current analysis stems from the estimates of total costs, which were based on a published cost-effectiveness model of RefluxStop in the United Kingdom adapted to the Italian setting [16]. Although uncertainty was associated with some of the inputs of the UK cost-effectiveness model, it predominantly utilized well-established, standard cost sources and its results were robust to rigorous deterministic and probabilistic sensitivity analyses [16], providing additional confidence in its adaptation to the Italian context for the current budget impact analysis.
Conclusion
Introducing RefluxStop as a treatment option for GERD patients treated within the Italian SSN is likely to provide substantial clinical benefits at the expense of a marginal budget impact on the SSN over a 5-year time horizon. Considering the arguably likely increase in both medical costs and the wider impact of GERD in Italy, an effective treatment option for GERD patients that is also economically acceptable in the short term is likely to bring forth substantial benefits to patients and the Italian SSN.
Declarations
Funding
SH and SM are employed by a consultancy company that was commissioned by Implantica to develop the model.
Conflicts of interest
SH and SM are employed by a consultancy company that was commissioned by Implantica to develop the model. MK is an employee of Implantica. None have any personal conflicts to declare. MP and LB have no personal conflicts to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Code associated with this budget impact model is available upon request.
Authors’ contributions
SH, MK, and SM designed and developed the model. MP and LB contributed to the design of the model and validated the results from a clinical perspective. All authors contributed to the manuscript.
Data availability
Data used in this model is available from the corresponding author upon request.
References
- 1.Martinucci I, Natilli M, Lorenzoni V, Pappalardo L, Monreale A, Turchetti G, et al. Gastroesophageal reflux symptoms among Italian university students: epidemiology and dietary correlates using automatically recorded transactions. BMC Gastroenterol. 2018;18(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacini F, Calabrese C, Cipolletta L, Valva MD, Russo A, Savarino V, et al. Burden of illness in Italian patients with gastro-oesophageal reflux disease. Curr Med Res Opin. 2005;21(4):495–502. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Gastro-oesophageal Reflux Disease Collaborators. The global, regional, and national burden of gastro-oesophageal reflux disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(6):561-81. [DOI] [PMC free article] [PubMed]
- 4.Ayazi S, Hagen JA, Chan LS, DeMeester SR, Lin MW, Ayazi A, et al. Obesity and gastroesophageal reflux: quantifying the association between body mass index, esophageal acid exposure, and lower esophageal sphincter status in a large series of patients with reflux symptoms. J Gastrointest Surg. 2009;13(8):1440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredenoord AJ, Pandolfino JE, Smout AJ. Gastro-oesophageal reflux disease. Lancet. 2013;381(9881):1933–42. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5(1):17–26. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8(6):340–7. [DOI] [PubMed] [Google Scholar]
- 9.Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Changes in prevalence, incidence and spontaneous loss of gastro-oesophageal reflux symptoms: a prospective population-based cohort study, the HUNT study. Gut. 2012;61(10):1390–7. [DOI] [PubMed] [Google Scholar]
- 10.Bert F, Pompili E, Lo Moro G, Corradi A, Sagrawa Caro A, Gualano MR, et al. Prevalence of gastro-oesophageal reflux symptoms: an Italian cross-sectional survey focusing on knowledge and attitudes towards lifestyle and nutrition. Int J Clin Pract. 2021;75(3): e13758. [DOI] [PubMed] [Google Scholar]
- 11.Darbà J, Kaskens L, Plans P, Elizalde JI, Coma M, Cuomo R, et al. Epidemiology and societal costs of gastroesophageal reflux disease and Barrett’s syndrome in Germany, Italy and Spain. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):225–32. [DOI] [PubMed] [Google Scholar]
- 12.King A, MacDonald C, Orn C. Understanding gastro-oesophageal reflux disease: a patient-cluster analysis. Int J Clin Pract. 2008;62(12):1838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Clinical guideline [CG184]: Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. London: National Institute for Health and Care Excellence (NICE); 2019. [PubMed] [Google Scholar]
- 14.Bjelović M, Harsányi L, Altorjay Á, Kincses Z, Forsell P. Non-active implantable device treating acid reflux with a new dynamic treatment approach: 1-year results : RefluxStop™ device; a new method in acid reflux surgery obtaining CE mark. BMC Surg. 2020;20(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Implantica. Implantica receives CE Mark Approval for RefluxStop, a potential Paradigm shift in the Treatment for Acid reflux. [Available from: https://www.implantica.com/media/press-releases/2018/implantica-receives-ce-mark-approval-for-refluxstop-a-potential-paradigm-shift-in-the-treatment-for-acid-reflux/.
- 16.Harper S, Grodzicki L, Mealing S, Gemmill L, Goldsmith PJ, Ahmed AR. Cost-effectiveness of a novel, non-active implantable device as a treatment for refractory gastro-esophageal reflux disease. J Med Econ. 2023;26(1):603–13. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. [DOI] [PubMed] [Google Scholar]
- 18.Brosa M, Gisbert R, Rodríguez Barrios J, Soto J. Principios, métodos y aplicaciones del análisis del impacto presupuestario en sanidad. Span Res Articles. 2005;2:65–79. [Google Scholar]
- 19.Mauskopf JA, Sullivan SD, Annemans L, Caro J, Mullins CD, Nuijten M, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health. 2007;10(5):336–47. [DOI] [PubMed] [Google Scholar]
- 20.Istat Statistics [Available from: http://dati.istat.it/.
- 21.Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57(10):1354–9. [DOI] [PubMed] [Google Scholar]
- 22.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NHS Dorset Clinical Commissioning Group. In Focus: High-Cost PPIs [Available from: https://www.dorsetccg.nhs.uk/Downloads/aboutus/medicines-management/Other%20Guidelines/Gastroenterology%20High%20cost%20PPI%20in%20focus%200519%20%282%29.pdf?UNLID=97481392021984165.
- 24.Weickum J, Higgins A, Wright G, Qadeer R, Ferko N. PGI11 A budget impact model for medication refractory gastroesophageal reflux disease pateints treated with laparoscopic nissen fundoplication or magnetic sphincter augmentation: an Italian payer perspective. Value Health. 2019;22:S618. [Google Scholar]
- 25.Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy. 2010;6:51–60. [Google Scholar]
- 26.Nochaiwong S, Ruengorn C, Awiphan R, Koyratkoson K, Chaisai C, Noppakun K, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(2):331–42. [DOI] [PubMed] [Google Scholar]
- 27.Batchelor R, Kumar R, Gilmartin-Thomas JFM, Hopper I, Kemp W, Liew D. Systematic review with meta-analysis: risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment Pharmacol Ther. 2018;48(8):780–96. [DOI] [PubMed] [Google Scholar]
- 28.Nassar Y, Richter S. Proton-pump inhibitor use and fracture risk: an updated systematic review and meta-analysis. J Bone Metab. 2018;25(3):141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen PA, Islam M, Galvin CJ, Chang CC, An SY, Yang HC, et al. Meta-analysis of proton pump inhibitors induced risk of community-acquired pneumonia. Int J Qual Health Care. 2020;32(5):292–9. [DOI] [PubMed] [Google Scholar]
- 30.Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017;23(35):6500–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poly TN, Lin MC, Syed-Abdul S, Huang CW, Yang HC, Li YJ. Proton pump inhibitor use and risk of gastric cancer: current evidence from epidemiological studies and critical appraisal. Cancers (Basel). 2022;14(13):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonavina L, Fisichella PM, Gavini S, Lee YY, Tatum RP. Clinical course of gastroesophageal reflux disease and impact of treatment in symptomatic young patients. Ann N Y Acad Sci. 2020;1481(1):117–26. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki T, Hemond C, Eisa M, Ganocy S, Fass R. The changing epidemiology of gastroesophageal reflux disease: are patients getting younger? J Neurogastroenterol Motil. 2018;24(4):559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarpignato C, Gatta L, Zullo A, Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rettura F, Bronzini F, Campigotto M, Lambiase C, Pancetti A, Berti G, et al. Refractory gastroesophageal reflux disease: a management update. Front Med (Lausanne). 2021;8: 765061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg SK, Gurusamy KS. Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev. 2015;2015(11):3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundell L, Bell M, Ruth M. Systematic review: laparoscopic fundoplication for gastroesophageal reflux disease in partial responders to proton pump inhibitors. World J Gastroenterol. 2014;20(3):804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.d’Errico M, Pavlova M, Spandonaro F. The economic burden of obesity in Italy: a cost-of-illness study. Eur J Health Econ. 2022;23(2):177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tack J, Becher A, Mulligan C, Johnson DA. Systematic review: the burden of disruptive gastro-oesophageal reflux disease on health-related quality of life. Aliment Pharmacol Ther. 2012;35(11):1257–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this model is available from the corresponding author upon request.